Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics
Abstract
1. Introduction
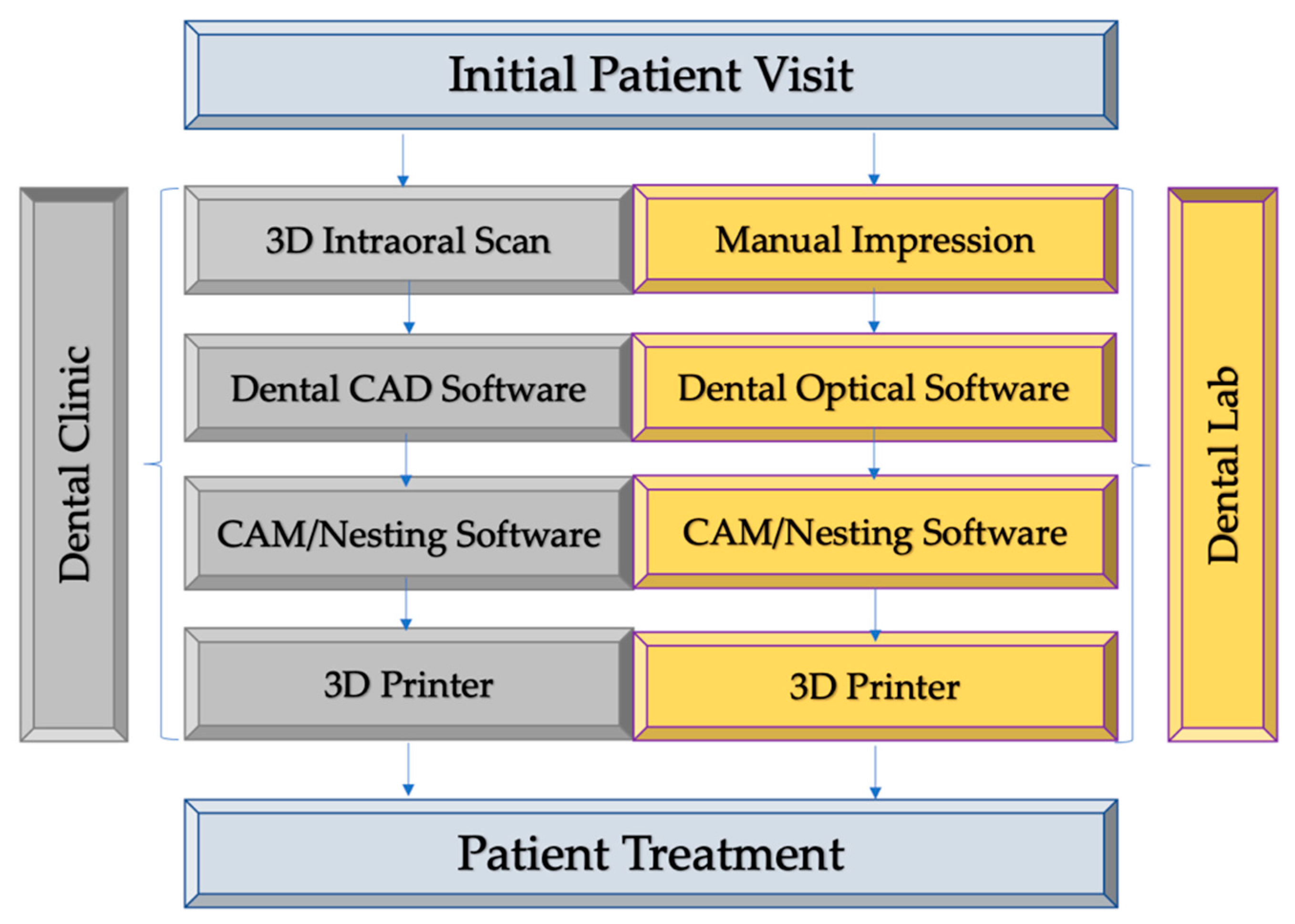
2. Digital Workflow from Clinical Diagnosis to Treatment Delivery
3. 3D Printing Techniques Used in Dentistry
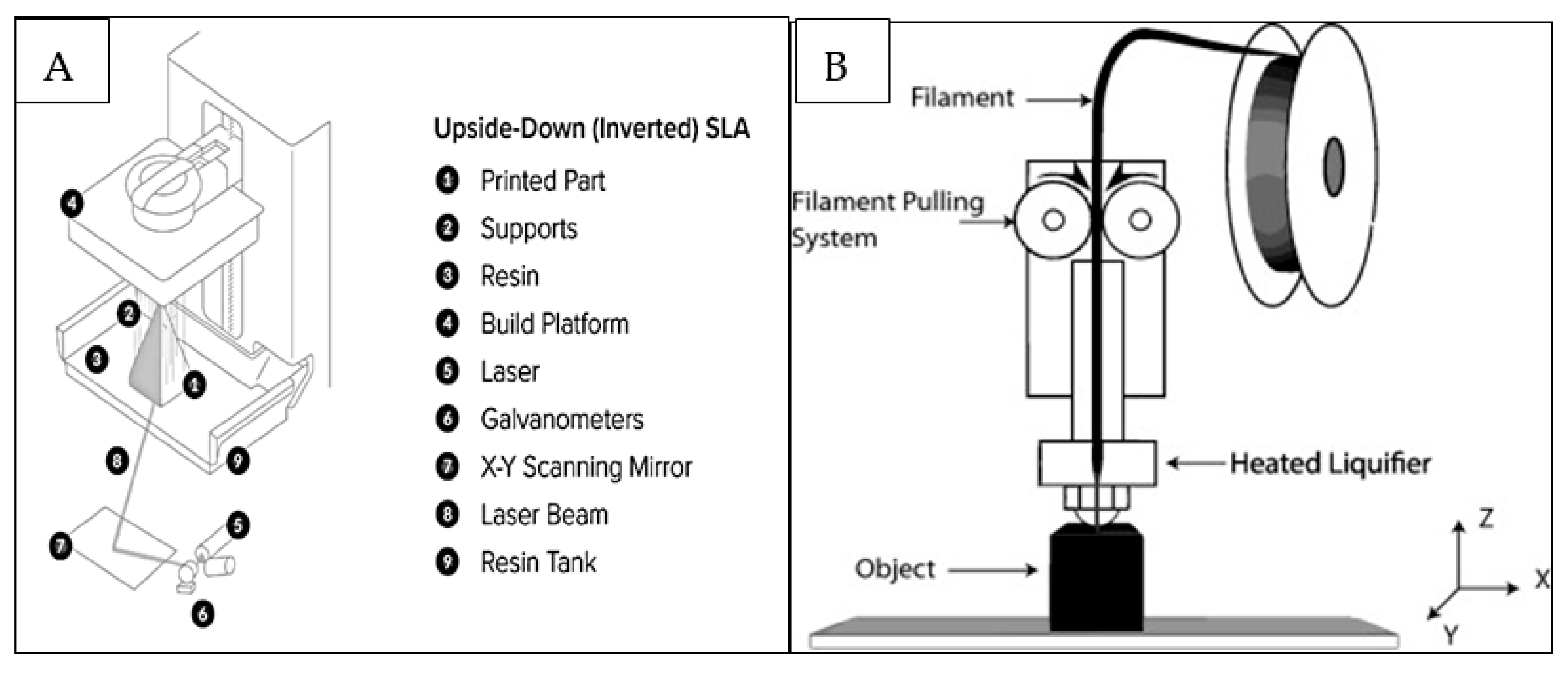
- Stereolithography (SLA/SLG) (Figure 2A) is a 3D printing process that uses mirrors and, by motorizing them, selectively moves an ultraviolet (UV) light beam to fuse surfaces that contain photoreactive liquid resin [15]. The process is followed by a wiper recoating the cured surface and another fusion step with the possibility of staining or infiltrating the specific areas [15]. The advantages and limitations of different printing techniques are displayed in Table 1.
- Extrusion-based methods rely on a continuous (no droplets) deposit of material driven out of the nozzle by either pneumatic or mechanical forces creating 3D constructs at the centimeter scale [16]. FDM, (Figure 2B) is an extrusion-based printing technique in which thermoplastic materials are subjected to melting to develop filaments which are deposited to fabricate the desired objects [17]. They are used for quick, low cost printing of basic less intricate models which are typically machined.
- Selective laser sintering (SLS) uses a high-power pulsed laser to fuse thermoplastic polymer (or metal, ceramic and glass) particles. It creates surface layers that will be refreshed using a roller or blade. A roller or blade then refreshes each sintered surface layer using powder material. One benefit of the 3D printed models using SLS is that they are thermoplastic in nature and can be autoclaved therefore can be handled safely during dental treatments [15].
- Digital light processing (DLP) uses similar concept as SLG to create 3D prints; however, instead of a set of moving mirrors, a digital micromirror device creates the cross-sectional UV image [15]. In dentistry, use of photocurable resins are highly suited an DLP utilizes this aspect for fabrication of single layer 3D objects using UV or white light. The final print properties can be modified as desired by simply manipulating the resin characteristics [19].
4. Biomaterials used in Dental 3D Printing
4.1. Hydrogels
4.2. Polymers and Thermoplastic Materials
4.3. Ceramics
4.4. Metals
5. 3D Printing in Endodontics
6. 3D Printing in Prosthodontics
6.1. Crowns and Bridges
6.2. Fabrication of 3D Printed Dentures
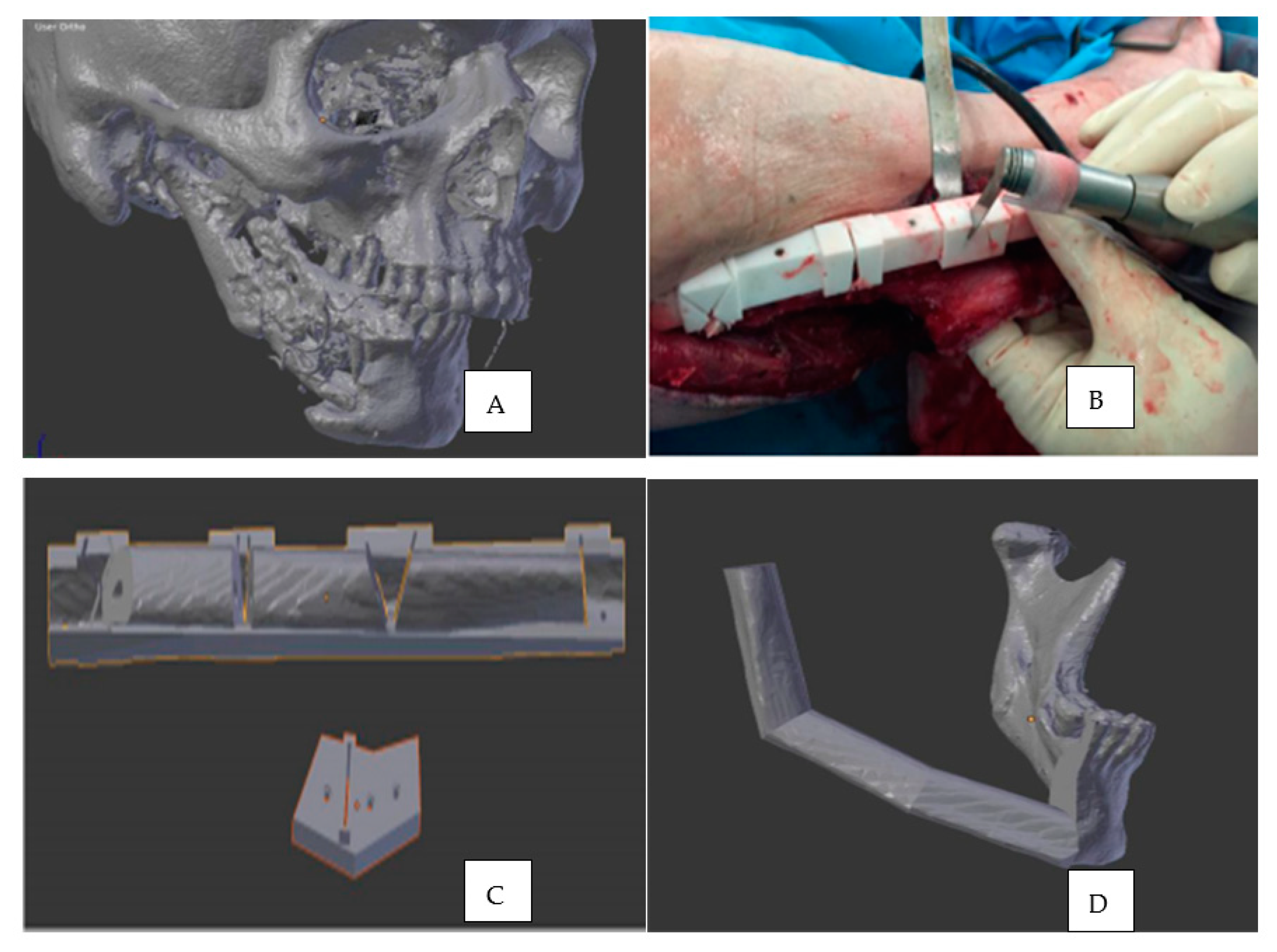
7. 3D Printing in Oral and Maxillofacial Surgery
8. 3D Printing in Periodontal Surgery
9. 3D Printing in Orthodontics and Dentofacial Orthopedics
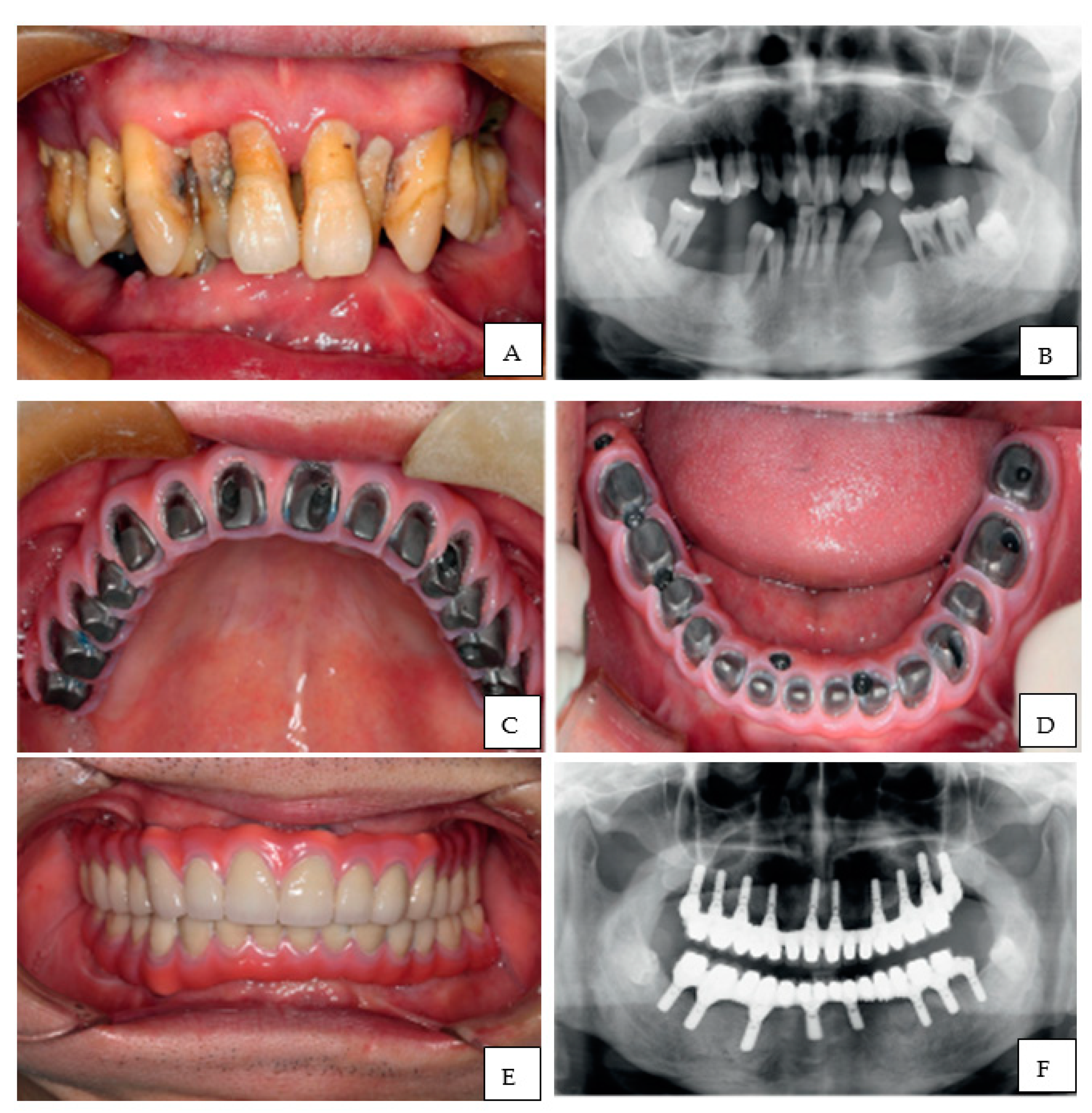
10. 3D Printing in Dental Implantology
10.1. 3D Printing Process and Dental Implantology
10.2. Pros and Cons of 3D Printed Implants
10.3. Materials Used for 3D Printing of Dental Implants
10.4. Evidence of the Success of 3D Printed Dental Implants in Various Settings
11. 3D Printing in Dental Education
12. Current Challenges and Future Directions
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torabi, K.; Farjood, E.; Hamedani, E. Rapid prototyping technologies and their applications in prosthodontics, a review of literature. J. Dent. 2015, 16, 1–9. [Google Scholar]
- Atala, A.; Forgacs, G. Three-dimensional bioprinting in regenerative medicine: Reality, hype, and future. Stem Cells Transl. Med. 2019, 8, 744–745. [Google Scholar]
- Ventola, C.L. Medical applications for 3d printing: Current and projected uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Haleem, A.; Javaid, M. Role of CT and MRI in the design and development of orthopaedic model using additive manufacturing. J. Clin. Orthop. Trauma 2018, 9, 213–217. [Google Scholar] [PubMed]
- Azari, A.; Nikzad, S. The evolution of rapid prototyping in dentistry: A review. Rapid Prototyp. J. 2009, 15, 216–225. [Google Scholar]
- Mokli, Y.; Pfaff, J.; Santos, D.P.; Herweh, C.; Nagel, S. Computer-aided imaging analysis in acute ischemic stroke—Background and clinical applications. Neurol. Res. Pract. 2019, 1, 23. [Google Scholar]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 15, 82–91. [Google Scholar]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health 2017, 17, 124. [Google Scholar]
- Mangano, F.; Shibli, J.A.; Fortin, T. Digital dentistry: New materials and techniques. Int. J. Dent. 2016, 2016, 5261247. [Google Scholar]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar]
- Güth, J.-F.; Runkel, C.; Beuer, F.; Stimmelmayr, M.; Edelhoff, D.; Keul, C. Accuracy of five intraoral scanners compared to indirect digitalization. Clin. Oral Investig. 2017, 21, 1445–1455. [Google Scholar] [PubMed]
- Zimmermanna, M.; Mehlb, A. Virtual smile design systems: A current review. Int. J. Comput. Dent. 2015, 18, 303–317. [Google Scholar]
- Stanley, M.; Paz, A.G.; Miguel, I.; Coachman, C. Fully digital workflow, integrating dental scan, smile design and CAD-CAM: Case report. BMC Oral Health 2018, 18, 134. [Google Scholar]
- Etemad-Shahidi, Y.; Qallandar, O.B.; Evenden, J.; Alifui-Segbaya, F.; Ahmed, K.E. Accuracy of 3-Dimensionally Printed Full-Arch Dental Models: A Systematic Review. J. Clin. Med. 2020, 9, 3357. [Google Scholar]
- Shah, P.; Chong, B.S. 3D imaging, 3D printing and 3D virtual planning in endodontics. Clin. Oral Investig. 2018, 22, 641–654. [Google Scholar] [PubMed]
- Chameettachal, S.; Yeleswarapu, S.; Sasikumar, S.; Shukla, P.; Hibare, P.; Bera, A.S.; Bojedla, S.S.R.; Pati, F. 3D Bioprinting: Recent Trends and challenges. J. Indian Inst. Sci. 2019, 99, 375–403. [Google Scholar]
- Carneiro, O.S.; Silva, A.; Gomes, R. Fused deposition modeling with polypropylene. Mater. Des. 2015, 83, 768–776. [Google Scholar]
- SLA vs. DLP: Guide to Resin 3D Printers. Available online: https://formlabs.com/blog/resin-3d-printer-comparison-sla-vs-dlp (accessed on 1 November 2020).
- Mu, Q.; Wang, L.; Dunn, C.K.; Kuang, X.; Duan, F.; Zhang, Z.; Qi, H.J.; Wang, T. Digital light processing 3D printing of conductive complex structures. Addit. Manuf. 2017, 18, 74–83. [Google Scholar]
- Ackerman, S.; Aguilera, F.C.; Buie, J.M.; Glickman, G.N.; Umorin, M.; Wang, Q.; Jalali, P. Accuracy of 3-dimensional-printed endodontic surgical guide: A human cadaver study. J. Endod. 2019, 45, 615–618. [Google Scholar]
- Buda, M.; Bratos, M.; Sorensen, J.A. Accuracy of 3-dimensional computer-aided manufactured single-tooth implant definitive casts. J. Prosthet. Dent. 2018, 120, 913–918. [Google Scholar]
- Zhang, Z.C.; Li, P.L.; Chu, F.T.; Shen, G. Influence of the three-dimensional printing technique and printing layer thickness on model accuracy. J. Orofac. Orthop. 2019, 80, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.F.; Choi, W.S.; Leung, Y.Y.; Curtin, J.P.; Du, R.; Zhang, C.Y.; Chen, X.S.; Su, Y.X. Three-dimensional printing of patient-specific surgical plates in head and neck reconstruction: A prospective pilot study. Oral Oncol. 2018, 78, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A. Current status and applications of additive manufacturing in dentistry: A literature-based review. J. Oral Biol. Craniofac. Res. 2019, 9, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.H.; Darwood, A.R.J.; Shaunak, S.; Kulatilake, P.; El-Hilly, A.A.; Mulki, O.; Baskaradas, A. Three-dimensional printing in surgery: A review of current surgical applications. J. Surg. Res. 2015, 199, 512–522. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Additive technology: Update on current materials and applications in dentistry. J. Prosthodont. 2017, 26, 156–163. [Google Scholar] [CrossRef]
- Vasamsetty, P.; Pss, T.; Kukkala, D.; Singamshetty, M.; Gajula, S. 3D printing in dentistry—Exploring the new horizons. Mater. Today 2020, 26, 838–841. [Google Scholar] [CrossRef]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L.E. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J. Dent. Res. 2015, 94, 143S–152S. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, R.; Peppas, N.A.; Khademhossaini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–123. [Google Scholar] [CrossRef]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.N.; Strong, R.; Gold, S.A. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H., Jr.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Abduo, J.; Lyons, K.; Bennamoun, M. Trends in computer-aided manufacturing in prosthodontics: A review of the available streams. Int. J. Dent. 2014, 2014, 783948. [Google Scholar] [CrossRef]
- Bukhari, S.; Goodacre, B.J.; AlHelal, A.; Kattadiyil, M.T.; Richardson, P.M. Three-dimensional printing in contemporary fixed prosthodontics: A technique article. J. Prosthet. Dent. 2018, 119, 530–534. [Google Scholar] [CrossRef]
- Michna, S.; Wu, W.; Lewis, J. Concentrated hydroxyapatite inks for direct-write assembly of 3-D periodic scaffolds. Biomaterials 2005, 26, 5632–5639. [Google Scholar] [CrossRef]
- Tarafder, S.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 679–690. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef]
- Khaing, M.W.; Fuh, J.Y.H.; Lu, L. Direct metal laser sintering for rapid tooling: Processing and characterisation of EOS parts. J. Mater. Process. Technol. 2001, 113, 269–272. [Google Scholar] [CrossRef]
- Osakada, K.; Shiomi, M. Flexible manufacturing of metallic products by selective laser melting of powder. Int. J. Mach. Tools Manuf. 2006, 46, 1188–1193. [Google Scholar] [CrossRef]
- Kathuria, Y.P. Microstructuring by selective laser sintering of metallic powder. Surf. Coat. Technol. 1999, 116–119, 643–647. [Google Scholar] [CrossRef]
- Frazier, W. Metal additive manufacturing: A review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Padrós, R.; Punset Fuste, M.; Molmeneu, M.; Brizuela, A.; Climent, M.; Rupérez, E.; Gil, F.J. Mechanical properties of cocr dental-prosthesis restorations made by three manufacturing processes. influence of the microstructure and topography. Metals 2020, 10, 788. [Google Scholar] [CrossRef]
- Konieczny, B.; Szczesio-Wlodarczyk, A.; Sokolowski, J.; Bociong, K. Challenges of Co-Cr Alloy additive manufacturing methods in dentistry-the current state of knowledge (systematic review). Materials 2020, 13, 3524. [Google Scholar] [CrossRef]
- Svanborg, P.; Hjalmarsson, L. A systematic review on the accuracy of manufacturing techniques for cobalt chromium fixed dental prostheses. Biomater. Investig. Dent. 2020, 7, 31–40. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Adhesion of porcelain to three-dimensionally printed and soft milled cobalt chromium. J. Prosthodont. Res. 2020, 64, 120–127. [Google Scholar] [CrossRef]
- Park, J.M.; Ahn, J.S.; Cha, H.S.; Lee, J.H. Wear Resistance of 3d printing resin material opposing zirconia and metal antagonists. Materials 2018, 11, 1043. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, Y.; Liu, Z.; Wei, B. Effects of repeated firing on the marginal accuracy of Co-Cr copings fabricated by selective laser melting. J. Prothet. Dent. 2015, 113, 135–139. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, N.H.; Kim, S.; Karabucak, B.; Kim, E. Computer-aided Design/Computer-aided manufacturing-guided endodontic surgery: Guided osteotomy and apex localization in a mandibular molar with a thick buccal bone plate. J. Endod. 2018, 44, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Wealleans, J.; Ray, J. Endodontic applications of 3D printing. Int. Endod. J. 2018, 51, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, C.M.; Ray, J.J.; Wealleans, J.A. Targeted endodontic microsurgery: A novel approach to anatomically challenging scenarios using 3-dimensional-printed guides and trephine burs-a report of 3 cases. J. Endod. 2018, 44, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.M.; Monteiro, N.; Tran, V.; Ferracane, J. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef]
- Gok, T.; Capar, I.D.; Akcay, I.; Keles, A. Evaluation of different techniques for filling simulated c-shaped canals of 3-dimensional printed resin teeth. J. Endod. 2017, 43, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Mohmmed, S.A.; Vianna, M.E.; Penny, M.R.; Hilton, S.T.; Mordan, N.J.; Knowles, J.C. Investigations into in situ enterococcus faecalis biofilm removal by passive and active sodium hypochlorite irrigation delivered into the lateral canal of a simulated root canal model. Int. Endod. J. 2018, 51, 649–662. [Google Scholar] [CrossRef]
- Mai, H.-N.; Lee, K.-B.; Lee, D.-H. Fit of interim crowns fabricated using photopolymer-jetting 3D printing. J. Prosthet. Dent. 2017, 118, 208–215. [Google Scholar] [CrossRef]
- Alharbi, N.; Alharbi, S.; Cuijpers, V.; Osman, R.B.; Wismeijer, D. Three-dimensional evaluation of marginal and internal fit of 3D-printed interim restorations fabricated on different finish line designs. J. Prosthodont. Res. 2018, 62, 218–226. [Google Scholar] [CrossRef]
- Yildirim, B. Effect of porcelain firing and cementation on the marginal fit of implant-supported metal-ceramic restorations fabricated by additive or subtractive manufacturing methods. J. Prosthet. Dent. 2020, 124, e1–e476. [Google Scholar] [CrossRef]
- Papadiochou, S.; Pissiotis, A.L. Marginal adaptation and CAD-CAM technology: A systematic review of restorative material and fabrication techniques. J. Prosthet. Dent. 2018, 119, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Prechtel, A.; Stawarczyk, B.; Hickel, R.; Edelhoff, D.; Reymus, M. Fracture load of 3D printed PEEK inlays compared with milled ones, direct resin composite fillings, and sound teeth. Clin. Oral Investig. 2020, 24, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.B.; van der Veen, A.J.; Huiberts, D.; Wismeijer, D.; Alharbi, N. 3D-printing zirconia implants; a dream or a reality? An in-vitro study evaluating the dimensional accuracy, surface topography and mechanical properties of printed zirconia implant and discs. J. Mech. Behav. Biomed. Mater. 2017, 75, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, H.; Liu, Y.; Jiang, X.; Gao, B. Trueness analysis of zirconia crowns fabricated with 3-dimensional printing. J. Prosthet. Dent. 2019, 121, 285–291. [Google Scholar] [CrossRef]
- McLaughlin, J.B.; Ramos, V., Jr.; Dickinson, D.P. Comparison of fit of dentures fabricated by traditional techniques versus cad/cam technology. J. Prosthodont. 2019, 28, 428–435. [Google Scholar] [CrossRef]
- Saponaro, P.C.; Yilmaz, B.; Heshmati, R.H.; McGlumphy, E.A. Clinical performance of CAD-CAM-fabricated complete dentures: A cross-sectional study. J. Prosthet. Dent. 2016, 116, 431–435. [Google Scholar] [CrossRef]
- Kalberer, N.; Mehl, A.; Schimmel, M.; Muller, F.; Srinivasan, M. CAD-CAM milled versus rapidly prototyped (3D-printed) complete dentures: An in vitro evaluation of trueness. J. Prosthet. Dent. 2019, 121, 637–643. [Google Scholar] [CrossRef]
- Schweiger, J.S.; Edelhoff, D.; Güth, J.-F. Systematics and concepts for the digital production of complete dentures-risks and opportunities. Int. J. Comput. Dent. 2018, 21, 41–56. [Google Scholar]
- Schweiger, J.; Guth, J.F.; Edelhoff, D.; Stumbaum, J. Virtual evaluation for CAD-CAM-fabricated complete dentures. J. Prosthet. Dent. 2017, 117, 28–33. [Google Scholar] [CrossRef]
- Lee, S.J.; Betensky, R.A.; Gianneschi, G.E.; Gallucci, G.O. Accuracy of digital versus conventional implant impressions. Clin. Oral Implant. Res. 2015, 26, 715–719. [Google Scholar] [CrossRef]
- Wilk, B.L. Intraoral Digital impressioning for dental implant restorations versus traditional implant impression techniques. Compend. Contin. Educ. Dent. 2015, 36, 529–530. [Google Scholar] [PubMed]
- Clark, W.A.; Duqum, I.; Kowalski, B.J. The digitally replicated denture technique: A case report. J. Esthet. Restor. Dent. 2019, 31, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Flugge, T.V.; Schlager, S.; Nelson, K.; Nahles, S.; Metzger, M.C. Precision of intraoral digital dental impressions with iTero and extraoral digitization with the iTero and a model scanner. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, S.B.; Vonau, S.; Stampf, S.; Att, W. Assessing the feasibility and accuracy of digitizing edentulous jaws. J. Am. Dent. Assoc. 2013, 144, 914–920. [Google Scholar] [CrossRef]
- Presotto, A.G.C.; Barao, V.A.R.; Bhering, C.L.B.; Mesquita, M.F. Dimensional precision of implant-supported frameworks fabricated by 3D printing. J. Prosthet. Dent. 2019, 122, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.J.; Park, J.M.; Kim, T.H.; Ahn, J.S.; Cha, H.S.; Lee, J.H. 3D printing of resin material for denture artificial teeth: Chipping and indirect tensile fracture resistance. Materials 2018, 11, 1798. [Google Scholar] [CrossRef]
- Choi, J.J.E.; Uy, C.E.; Plaksina, P.; Ramani, R.S.; Ganjigatti, R.; Waddell, J.N. Bond strength of denture teeth to heat-cured, cad/cam and 3d printed denture acrylics. J. Prosthodont. 2020, 29, 415–421. [Google Scholar] [CrossRef]
- Saponaro, P.C.; Yilmaz, B.; Johnston, W.; Heshmati, R.H.; McGlumphy, E.A. Evaluation of patient experience and satisfaction with CAD-CAM-fabricated complete dentures: A retrospective survey study. J. Prosthet. Dent. 2016, 116, 524–528. [Google Scholar] [CrossRef]
- Kattadiyil, M.T.; Jekki, R.; Goodacre, C.J.; Baba, N.Z. Comparison of treatment outcomes in digital and conventional complete removable dental prosthesis fabrications in a predoctoral setting. J. Prosthet. Dent. 2015, 114, 818–825. [Google Scholar] [CrossRef]
- AlHelal, A.; Goodacre, B.J.; Kattadiyil, M.T.; Swamidass, R. Errors associated with digital preview of computer-engineered complete dentures and guidelines for reducing them: A technique article. J. Prosthet. Dent. 2018, 119, 17–25. [Google Scholar] [CrossRef]
- Abt, E.; Carr, A.B.; Worthington, H.V. Interventions for replacing missing teeth: Partially absent dentition. Cochrane Database Syst. Rev. 2012, 2, CD003814. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Pei, Z.; Wen, Y. Using Intraoral scanning technology for three-dimensional printing of kennedy class I removable partial denture metal framework: A clinical report. J. Prosthodont. 2019, 28, e473–e476. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Altwaim, B.; Alhassan, M.; Alammari, R. The fit accuracy of removable partial denture metal frameworks using conventional and 3d printed techniques. J. Contemp. Dent. Pract. 2019, 20, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Chen, Y.-J.; Chen, P.-C.; Chen, C.-Y. Fabrication of PDMS passive micromixer by lost-wax casting. Int. J. Precis. Eng. Man. 2015, 16, 2033–2039. [Google Scholar] [CrossRef]
- Negm, E.E.; Aboutaleb, F.A.; Alam-Eldein, A.M. Virtual evaluation of the accuracy of fit and trueness in maxillary poly(etheretherketone) removable partial denture frameworks fabricated by direct and indirect cad/cam techniques. J. Prosthodont. 2019, 28, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, H.; Zhao, Y.; Zhang, X.; Wang, Y.; Lyu, P. Adaptation of removable partial denture frameworks fabricated by selective laser melting. J. Prosthet. Dent. 2019, 122, 316–324. [Google Scholar] [CrossRef]
- Tasaka, A.; Shimizu, T.; Kato, Y.; Okano, H.; Ida, Y.; Higuchi, S.; Yamashita, S. Accuracy of removable partial denture framework fabricated by casting with a 3D printed pattern and selective laser sintering. J. Prosthodont. Res. 2020, 64, 224–230. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, Y.; Gao, B.; Yu, H. A novel digital altered cast impression technique for fabricating a removable partial denture with a distal extension. J. Am. Dent. Assoc. 2020, 151, 297–302. [Google Scholar] [CrossRef]
- Louvrier, A.; Marty, P.; Barrabé, A.; Euvrard, E.; Chatelain, B.; Weber, E.; Meyer, C. How useful is 3D printing in maxillofacial surgery? J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 206–212. [Google Scholar] [CrossRef]
- Hidalgo, D.A. Fibula free flap: A new method of mandible reconstruction. Plast. Reconstr. Surg. 1989, 84, 71–79. [Google Scholar] [CrossRef]
- Fang, F.; Chung, K.C. An evolutionary perspective on the history of flap reconstruction in the upper extremity. Hand Clin. 2014, 30, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.-C.; Demirkan, F.; Chen, H.-C.; Chen, I.-H. Double free flaps in reconstruction of extensive composite mandibular defects in head and neck cancer. Plast. Reconstr. Surg. 1999, 103, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Smithers, F.A.; Cheng, K.; Jayaram, R.; Mukherjee, P.; Clark, J.R. Maxillofacial reconstruction using in-house virtual surgical planning. ANZ J. Surg. 2018, 88, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Momoh, A.O.; Yu, P.; Skoracki, R.J.; Liu, S.; Feng, L.; Hanasono, M.M. A prospective cohort study of fibula free flap donor-site morbidity in 157 consecutive patients. Plast. Reconstr. Surg. 2011, 128, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.A.; Lin, A.Y. A new classification of three-dimensional printing technologies: Systematic review of three-dimensional printing for patient-specific craniomaxillofacial surgery. Plast. Reconstr. Surg. 2017, 139, 1211–1220. [Google Scholar] [CrossRef]
- Lin, A.Y.; Yarholar, L.M. Plastic surgery innovation with 3D printing for craniomaxillofacial operations. Mo. Med. 2020, 117, 136–142. [Google Scholar]
- Largo, R.D.; Garvey, P.B. Updates in head and neck reconstruction. Plast. Reconstr. Surg. 2018, 141, 271e–285e. [Google Scholar] [CrossRef]
- Ganry, L.; Quilichini, J.; Bandini, C.; Leyder, P.; Hersant, B.; Meningaud, J. Three-dimensional surgical modelling with an open-source software protocol: Study of precision and reproducibility in mandibular reconstruction with the fibula free flap. Int. J. Oral Maxillofac. Surg. 2017, 46, 946–957. [Google Scholar] [CrossRef]
- Kadowaki, M.; Kubo, T.; Fujikawa, M.; Tashima, H.; Nagayama, H.; Ishihara, O.; Yamada, R.; Otake, I.; Hosokawa, K. A two-tiered structure device based on stereolithography for residual mandible repositioning in mandibular reconstruction with fibular flap. Microsurgery 2017, 37, 509–515. [Google Scholar] [CrossRef]
- Emodi, O.; Shilo, D.; Israel, Y.; Rachmiel, A. Three-dimensional planning and printing of guides and templates for reconstruction of the mandibular ramus and condyle using autogenous costochondral grafts. Br. J. Oral Maxillofac. Surg. 2017, 55, 102–104. [Google Scholar] [CrossRef]
- Bosc, R.; Hersant, B.; Carloni, R.; Niddam, J.; Bouhassira, J.; De Kermadec, H.; Bequignon, E.; Wojcik, T.; Julieron, M.; Meningaud, J.-P. Mandibular reconstruction after cancer: An in-house approach to manufacturing cutting guides. Int. J. Oral Maxillofac. Surg. 2017, 46, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mascha, F.; Winter, K.; Pietzka, S.; Heufelder, M.; Schramm, A.; Wilde, F. Accuracy of computer-assisted mandibular reconstructions using patient-specific implants in combination with CAD/CAM fabricated transfer keys. J. Craniomaxillofac. Surg. 2017, 45, 1884–1897. [Google Scholar] [CrossRef]
- Jacek, B.; Maciej, P.; Tomasz, P.; Agata, B.; Wiesław, K.; Radosław, W.; Filip, G. 3D printed models in mandibular reconstruction with bony free flaps. J. Mater. Sci. Mater. Med. 2018, 29, 23. [Google Scholar] [CrossRef]
- Resnick, C. Precise osteotomies for mandibular distraction in infants with Robin sequence using virtual surgical planning. Int. J. Oral Maxillofac. Surg. 2018, 47, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Rachmiel, A.; Shilo, D.; Blanc, O.; Emodi, O. Reconstruction of complex mandibular defects using integrated dental custom-made titanium implants. Br. J. Oral Maxillofac. Surg. 2017, 55, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, M. Additively manufactured sub-periosteal jaw implants. Int. J. Oral Maxillofac. Surg. 2017, 46, 938–940. [Google Scholar] [CrossRef]
- Shaheen, E.; Sun, Y.; Jacobs, R.; Politis, C. Three-dimensional printed final occlusal splint for orthognathic surgery: Design and validation. Int. J. Oral Maxillofac. Surg. 2017, 46, 67–71. [Google Scholar] [CrossRef]
- Li, B.; Shen, S.; Jiang, W.; Li, J.; Jiang, T.; Xia, J.; Shen, S.G.; Wang, X. A new approach of splint-less orthognathic surgery using a personalized orthognathic surgical guide system: A preliminary study. Int. J. Oral Maxillofac. Surg. 2017, 46, 1298–1305. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, X.; Wang, L.; Zhang, Y.; Chen, K.; Wu, G. The application of 3D printing technology for simultaneous orthognathic surgery and mandibular contour osteoplasty in the treatment of craniofacial deformities. Aesthetic Plast. Surg. 2017, 41, 1413–1424. [Google Scholar] [CrossRef]
- Ackland, D.C.; Robinson, D.; Redhead, M.; Lee, P.V.S.; Moskaljuk, A.; Dimitroulis, G. A personalized 3D-printed prosthetic joint replacement for the human temporomandibular joint: From implant design to implantation. J. Mech. Behav. Biomed. Mater. 2017, 69, 404–411. [Google Scholar] [CrossRef]
- Wang, L.; Tian, D.; Sun, X.; Xiao, Y.; Chen, L.; Wu, G. The precise repositioning instrument for Genioplasty and a three-dimensional printing technique for treatment of complex facial asymmetry. Aesthetic Plast. Surg. 2017, 41, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Yeung, E.; Osher, J.; Huppa, C. Novel treatment planning of hemimandibular hyperplasia by the use of three-dimensional computer-aided-design and computer-aided-manufacturing technologies. J. Craniofac. Surg. 2017, 28, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.P.; Moin, D.A.; Wismeijer, D.; van Merkesteyn, J.R. Replacing heavily damaged teeth by third molar autotransplantation with the use of cone-beam computed tomography and rapid prototyping. J. Oral Maxillofac. Surg. 2017, 75, 1809–1816. [Google Scholar] [CrossRef]
- Tarsitano, A.; Ciocca, L.; Scotti, R.; Marchetti, C. Morphological results of customized microvascular mandibular reconstruction: A comparative study. J. Craniomaxillofac. Surg. 2016, 44, 697–702. [Google Scholar] [CrossRef]
- Lei, L.; Yu, Y.; Ke, T.; Sun, W.; Chen, L. The application of three-dimensional printing model and platelet-rich fibrin technology in guided tissue regeneration surgery for severe bone defects. J. Oral Implantol. 2019, 45, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P.; Magner, A.W.; Fletcher, P. The effect of the distance from the contact point to the crest of bone on the presence or absence of the interproximal dental papilla. J. Periodontol. 1992, 63, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, D.; Huang, Y.; Wu, L.; Tang, X. CAD/CAM techniques help in the rebuilding of ideal marginal gingiva contours of anterior maxillary teeth: A case report. J. Am. Dent. Assoc. 2017, 148, 834–839. [Google Scholar] [CrossRef]
- Wang, P.; Tang, C.; Tang, Y.; Wu, Y. Immediate implant placement and complete mouth rehabilitation with CAD-CAM titanium frameworks and cemented crowns for a patient with severe periodontal disease: A clinical report. J. Prosthet. Dent. 2018, 119, 511–515. [Google Scholar] [CrossRef]
- Oh, S.; Kim, S.; Lo, H.S.; Choi, J.-Y.; Kim, H.-J.; Ryu, G.-J.; Kim, S.-Y.; Choi, K.-K.; Kim, D.-S.; Jang, J.-H. Virtual simulation of autotransplantation using 3-dimensional printing prototyping model and computer-assisted design program. J. Endod. 2018, 44, 1883–1888. [Google Scholar] [CrossRef]
- Van-der-Meer, W.J.; Jansma, J.; Delli, K.; Livas, C. Computer-aided planning and surgical guiding system fabrication in premolar autotransplantation: A 12-month follow up. Dent. Traumatol. 2016, 32, 336–340. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.; Flanagan, C.; Park, C.; Pagni, G.; Hollister, S.; Giannobile, W.V. 3D-printed bioresorbable scaffold for periodontal repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.L.; Kadioglu, O.; Currier, G.F.; Kierl, J.P.; Li, J. Accuracy of digital light processing printing of 3-dimensional dental models. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.B.; Currier, G.F.; Kadioglu, O.; Kierl, J.P. Accuracy of 3-dimensional printed dental models reconstructed from digital intraoral impressions. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.S.; Hoye, L.N.; Elnagar, M.H.; Atsawasuwan, P.; Galang-Boquiren, M.T.; Caplin, J.; Viana, G.C.; Obrez, C.; Kusnoto, C. Accuracy of dental monitoring 3D digital dental models using photograph and video mode. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhang, Z.; Li, X.; Wang, Y.; Wang, P.; Li, J. One-Stage treatment for maxillofacial asymmetry with orthognathic and contouring surgery using virtual surgical planning and 3D-printed surgical templates. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Hwang, C.H.; Lee, K.H.; Kang, B.C. Maxillofacial 3-dimensional image analysis for the diagnosis of facial asymmetry. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Mitsugi, M.; Kanno, T.; Nomachi, A.; Wajima, T.; Tatemoto, Y. Three-dimensional virtual operations can facilitate complicated surgical planning for the treatment of patients with jaw deformities associated with facial asymmetry: A case report. Int. J. Oral Sci. 2013, 5, 176–182. [Google Scholar] [CrossRef][Green Version]
- Zinser, M.J.; Mischkowski, R.A.; Dreiseidler, T.; Thamm, O.C.; Rothamel, D.; Zoller, J.E. Computer-assisted orthognathic surgery: Waferless maxillary positioning, versatility, and accuracy of an image-guided visualisation display. Br. J. Oral Maxillofac. Surg. 2013, 51, 827–833. [Google Scholar] [CrossRef]
- Camardella, L.T.; Vilella, O.V.; van Hezel, M.M.; Breuning, K.H. Accuracy of stereolithographically printed digital models compared to plaster models. J. Orofac. Orthop. 2017, 78, 394–402. [Google Scholar] [CrossRef]
- Gonzalez Guzman, J.F.; Teramoto Ohara, A. Evaluation of three-dimensional printed virtual setups. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 288–295. [Google Scholar] [CrossRef]
- Wan Hassan, W.N.; Yusoff, Y.; Mardi, N.A. Comparison of reconstructed rapid prototyping models produced by 3-dimensional printing and conventional stone models with different degrees of crowding. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kook, M.S.; Kim, H.M.; Oh, H.K.; Lee, K.M. Clear Aligner Use Following Surgery-First Mandibular Prognathism Correction. J. Craniofac. Surg. 2019, 30, e544–e547. [Google Scholar] [CrossRef] [PubMed]
- Jindal, P.; Juneja, M.; Siena, F.L.; Bajaj, D.; Breedon, P. Mechanical and geometric properties of thermoformed and 3D printed clear dental aligners. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Jheon, A.H.; Oberoi, S.; Solem, R.C.; Kapila, S. Moving towards precision orthodontics: An evolving paradigm shift in the planning and delivery of customized orthodontic therapy. Orthod. Craniofac. Res. 2017, 20, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yin, G.; Liao, X.; Yin, X.; Ye, N. A novel customized ceramic bracket for esthetic orthodontics: In vitro study. Prog. Orthod. 2019, 20, 39. [Google Scholar] [CrossRef]
- Tavares, A.; Braga, E.; Araujo, T.M. Digital models: How can dental arch form be verified chairside? Dental Press J. Orthod. 2017, 22, 68–73. [Google Scholar] [CrossRef]
- Pawar, B.A. Maintenance of space by innovative three-dimensional-printed band and loop space maintainer. J. Indian Soc. Pedod. Prev. Dent. 2019, 37, 205–208. [Google Scholar] [CrossRef]
- Sanchez-Monescillo, A.; Duarte, S. PROA concept: Prosthetic restoration with orthodontic appliance. Quintessence Int. 2020, 51, 304–308. [Google Scholar]
- Graf, S.; Vasudavan, S.; Wilmes, B. CAD-CAM design and 3-dimensional printing of mini-implant retained orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 877–882. [Google Scholar] [CrossRef]
- Cassetta, M.; Ivani, M. The accuracy of computer-guided piezocision: A prospective clinical pilot study. Int. J. Oral Maxillofac. Surg. 2017, 46, 756–765. [Google Scholar] [CrossRef]
- Wilcko, W.M.; Wilcko, T.; Bouquot, J.E.; Ferguson, D.J. Rapid orthodontics with alveolar reshaping: Two case reports of decrowding. Int. J. Periodontics Restor. Dent. 2001, 21, 9–19. [Google Scholar]
- Dibart, S.; Sebaoun, J.D.; Surmenian, J. Piezocision: A minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Compend. Contin. Educ. Dent. 2009, 30, 342–344. [Google Scholar] [PubMed]
- Dibart, S.; Surmenian, J.; Sebaoun, J.D.; Montesani, L. Rapid treatment of Class II malocclusion with piezocision: Two case reports. Int. J. Periodontics Restor. Dent. 2010, 30, 487–493. [Google Scholar]
- Hou, H.Y.; Li, C.H.; Chen, M.C.; Lin, P.Y.; Liu, W.C.; Tsai, C.Y.W.; Huang, R.-Y. A novel 3D-printed computer-assisted piezocision guide for surgically facilitated orthodontics. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Charavet, C.; Lecloux, G.; Jackers, N.; Albert, A.; Lambert, F. Piezocision-assisted orthodontic treatment using CAD/CAM customized orthodontic appliances: A randomized controlled trial in adults. Eur. J. Orthod. 2019, 41, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.; Dean, K.; Appachi, S.; Drake, A.F. Craniofacial Interventions in Children. Otolaryngol. Clin. N. Am. 2019, 52, 903–922. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, H.; Kuang, W.; Yuan, W. Presurgical nasoalveolar molding with 3D printing for a patient with unilateral cleft lip, alveolus, and palate. Am. J. Orthod. Dentofacial Orthop. 2019, 156, 412–419. [Google Scholar] [CrossRef]
- Krey, K.F.; Ratzmann, A.; Metelmann, P.H.; Hartmann, M.; Ruge, S.; Kordass, B. Fully digital workflow for presurgical orthodontic plate in cleft lip and palate patients. Int. J. Comput. Dent. 2018, 21, 251–259. [Google Scholar]
- Bollman, M.; Malbrue, R.; Li, C.; Yao, H.; Guo, S.; Yao, S. Improvement of osseointegration by recruiting stem cells to titanium implants fabricated with 3D printing. Ann. N. Y. Acad. Sci. 2020, 1463, 37–44. [Google Scholar] [CrossRef]
- Alghamdi, H.S. Methods to improve osseointegration of dental implants in low quality (Type-IV) bone: An overview. J. Funct. Biomater. 2018, 9, 7. [Google Scholar] [CrossRef]
- Altay, M.A.; Sindel, A.; Ozalp, O.; Yildirimyan, N.; Kader, D.; Bilge, U.; Baur, D.A. Does the Intake of selective serotonin reuptake inhibitors negatively affect dental implant osseointegration? A retrospective study. J. Oral Implantol. 2018, 44, 260–265. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Analysis of risk factors for cluster behavior of dental implant failures. Clin. Implant Dent. Relat. Res. 2017, 19, 632–642. [Google Scholar] [CrossRef]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors affecting the survival rate of dental implants: A retrospective study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, M.D.; Taschieri, S.; Canciani, E.; Addis, A.; Musto, F.; Weinstein, R.; Dellavia, C. Osseointegration of titanium implants with different rough surfaces: A histologic and histomorphometric study in an adult minipig model. Implant Dent. 2017, 26, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Dalal, N.; Ammoun, R.; Abdulmajeed, A.A.; Deeb, G.R.; Bencharit, S. Intaglio surface dimension and guide tube deviations of implant surgical guides influenced by printing layer thickness and angulation setting. J. Prosthodont. 2020, 29, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Nesic, D.; Schaefer, B.M.; Sun, Y.; Saulacic, N.; Sailer, I. 3D Printing approach in dentistry: The future for personalized oral soft tissue regeneration. J. Clin. Med. 2020, 9, 2238. [Google Scholar] [CrossRef]
- Kalman, L. 3D printing of a novel dental implant abutment. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 299–303. [Google Scholar] [CrossRef]
- Tedesco, J.; Lee, B.E.J.; Lin, A.Y.W.; Binkley, D.M.; Delaney, K.H.; Kwiecien, J.M.; Granfield, K. Osseointegration of a 3D printed stemmed titanium dental implant: A pilot study. Int. J. Dent. 2017, 2017, 5920714. [Google Scholar] [CrossRef]
- Mangano, C.; Bianchi, A.; Mangano, F.G.; Dana, J.; Colombo, M.; Solop, I.; Admakin, O. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: A case series. 3D Print. Med. 2020, 6, 1. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.; Yang, C.; Spintzyk, S.; Scheideler, L.; Li, P.; Li, D.; Geis-Gerstorfer, J.; Rupp, F. Carbon fiber reinforced PEEK composites based on 3D-printing technology for orthopedic and dental applications. J. Clin. Med. 2019, 8, 240. [Google Scholar] [CrossRef]
- Sikder, P.; Ferreira, J.A.; Fakhrabadi, E.A.; Kantorski, K.Z.; Liberatore, M.W.; Bottino, M.C.; Bhaduri, S.B. Bioactive amorphous magnesium phosphate-polyetheretherketone composite filaments for 3D printing. Dent. Mater. 2020, 36, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Hong, M.-H.; Lee, H.; Lee, C.-H.; Hong, M.; Lee, J.; Lee, D.H. Reliability of metal 3D printing with respect to the marginal fit of fixed dental prostheses: A systematic review and meta-analysis. Materials 2020, 13, 4781. [Google Scholar] [CrossRef] [PubMed]
- Chang Tu, C.; Tsai, P.I.; Chen, S.Y.; Kuo, M.Y.; Sun, J.S.; Chang, J.Z. 3D laser-printed porous Ti6Al4V dental implants for compromised bone support. J. Formos. Med. Assoc. 2020, 119, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Odkhuu, M.; Cho, S.; Li, J.; Park, B.Y.; Kim, J.W. 3D-printed titanium implant with pre-mounted dental implants for mandible reconstruction: A case report. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 28. [Google Scholar] [CrossRef] [PubMed]
- Reymus, M.; Fotiadou, C.; Hickel, R.; Diegritz, C. 3D-printed model for hands-on training in dental traumatology. Int. Endod. J. 2018, 51, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Höhne, C.; Schmitter, M. 3D printed teeth for the preclinical education of dental students. J. Dent. Educ. 2019, 83, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Höhne, C.; Schwarzbauer, R.; Schmitter, M. 3D printed teeth with enamel and dentin layer for educating dental students in crown preparation. J. Dent. Educ. 2019, 83, 1457–1463. [Google Scholar] [CrossRef]
- Nicot, R.; Druelle, C.; Schlund, M.; Roland-Billecart, T.; Gwénaël, R.; Ferri, J.; Gosset, D. Use of 3D printed models in student education of craniofacial traumas. Dent. Traumatol. 2019, 35, 296–299. [Google Scholar] [CrossRef]
- Fu, X.; Qiao, J.; Gui, L.; Girod, S.; Niu, F.; Liu, J.; Jin, Q.; Zhang, H.; Xu, S.; Mao, X.; et al. An effective simulator for intraoral facial skeletal contour surgeries. Ann. Plast. Surg. 2019, 82, 99–103. [Google Scholar] [CrossRef]
- Marty, M.; Broutin, A.; Vergnes, J.N.; Vaysse, F. Comparison of student’s perceptions between 3D printed models versus series models in paediatric dentistry hands-on session. Eur. J. Dent. Educ. 2019, 23, 68–72. [Google Scholar] [CrossRef]
- Kessler, A.; Hickel, R.; Reymus, M. 3D printing in dentistry—State of the art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.; Marti Marti, B.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Abduo, J.; Elseyoufi, M. Accuracy of intraoral scanners: A systematic review of influencing factors. Eur. J. Prosthodont. Restor. Dent. 2018, 26, 101–121. [Google Scholar] [PubMed]
- Favaretto, M.; Shaw, D.; De Clercq, E.; Joda, T.; Elger, B.S. Big data and digitalization in dentistry: A systematic review of the ethical issues. Int. J. Environ. Res. Public Health 2020, 17, 2495. [Google Scholar] [CrossRef]
| Type of Printing Technique | Advantages | Disadvantages |
|---|---|---|
| SLA |
|
|
| SLS |
|
|
| DLP |
|
|
| FDM |
|
|
| AM Technology Type | Compatible Dental Materials | Approximate Accuracy * |
|---|---|---|
| Inkjet Printing (IJP) | Low viscosity cell slurries or polymer hydrogels | 35 to 40 µm |
| Polyjet Printing (PJP) | Photopolymers | 20 to 85 µm |
| Multi-Jet-Printing (MJP) | Ceramic, Metal or Plastic | 25 to 35 µm |
| FDM | Acrylonitrile butadiene styrene (ABS), Polyesters, Polypropylene or Polycarbonate | 35 to 40 µm |
| SLA | Ceramics, Acrylate photopolymers or Plastic | 50 to 55 µm |
| SLS | Ceramic, Metal, Thermoplastics or Plastic | 45 to 50 µm |
| Direct Metal Laser Sintering (DMLS) or Selective Laser Melting (SLM) | Cobalt, Titanium, Aluminum, Steel Bronze or Nickel | 20 to 35 µm |
| Colour-Jet Printing (JCP) | Gypsum | 23 to 30 µm |
| Electron Beam Melting (EBM) | Metal, such as titanium | 40 to 50 µm |
| Laminated Object Manufacturing (LOM) | Metal or Plastic | 60 to 70 µm |
| Surgery/Application | Prosthesis/Treatment Using 3D Printing | Ref. |
|---|---|---|
| Mandibular reconstruction |
| [100] |
| [99] | |
| [101] | |
| [102] | |
| [103] | |
| [104] | |
| Mandibular distraction Osteogenesis (MDO) |
| [105] |
| Implants |
| [106] |
| [107] | |
| Orthognathic Surgery |
| [108] |
| [109] | |
| [110] | |
| Temporomandibular Joint reconstruction (TMJ) |
| [111] |
| Facial Asymmetry |
| [112] |
| [113] | |
| Auto transplantation |
| [114] |
| S. No. | Materials Used for 3D Printing of Dental Implants | Studies in the Literature |
|---|---|---|
| 1 | Plastic (MED690 VeroDentPlus) | Kalman, 2018 [158] |
| 2 | Stainless Steel (Duraform 316L) | Kalman, 2018 [158] |
| 3 | Zirconia | Osman et al., 2017 [64] |
| 4 | Titanium | Tedesco et al., 2017 [159] |
| 5 | Acrylic Resin | Mangano et al., 2020 [160] |
| 6 | PEEK | Xingting et al., 2019 [161] |
| 7 | Amorphous Magnesium Phosphate (AMP) blended with PEEK | Sikder et al., 2020 [162] |
| 8 | Cobalt-Chromium (Co-Cr) Alloy | Bae et al., 2020 [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. https://doi.org/10.3390/polym13010157
Pillai S, Upadhyay A, Khayambashi P, Farooq I, Sabri H, Tarar M, Lee KT, Harb I, Zhou S, Wang Y, et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers. 2021; 13(1):157. https://doi.org/10.3390/polym13010157
Chicago/Turabian StylePillai, Sangeeth, Akshaya Upadhyay, Parisa Khayambashi, Imran Farooq, Hisham Sabri, Maryam Tarar, Kyungjun T. Lee, Ingrid Harb, Stephanie Zhou, Yifei Wang, and et al. 2021. "Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics" Polymers 13, no. 1: 157. https://doi.org/10.3390/polym13010157
APA StylePillai, S., Upadhyay, A., Khayambashi, P., Farooq, I., Sabri, H., Tarar, M., Lee, K. T., Harb, I., Zhou, S., Wang, Y., & Tran, S. D. (2021). Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers, 13(1), 157. https://doi.org/10.3390/polym13010157









