Enhancing iCVD Modification of Electrospun Membranes for Membrane Distillation Using a 3D Printed Scaffold
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Electrospinning
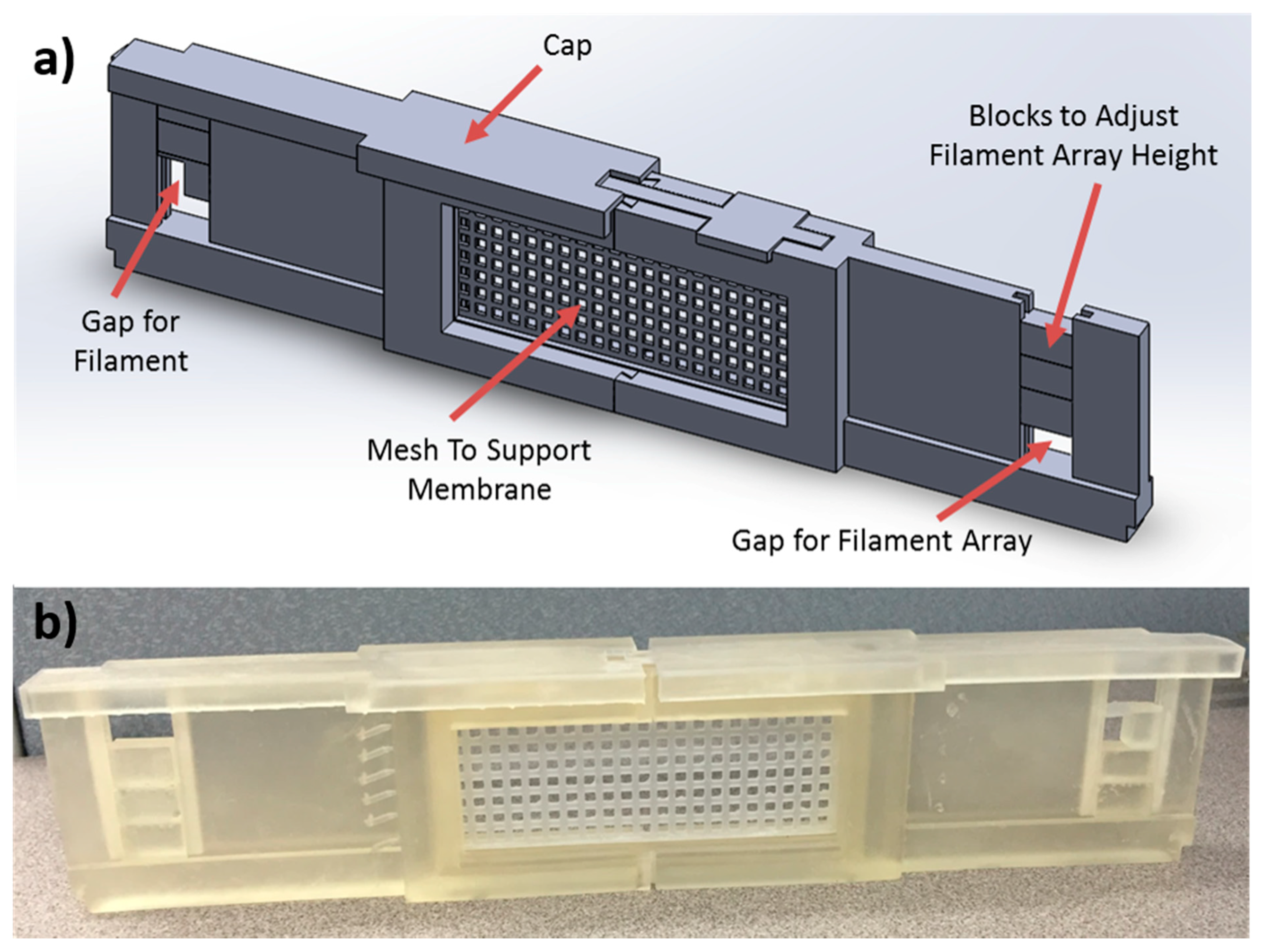
2.3. Scaffold Fabrication
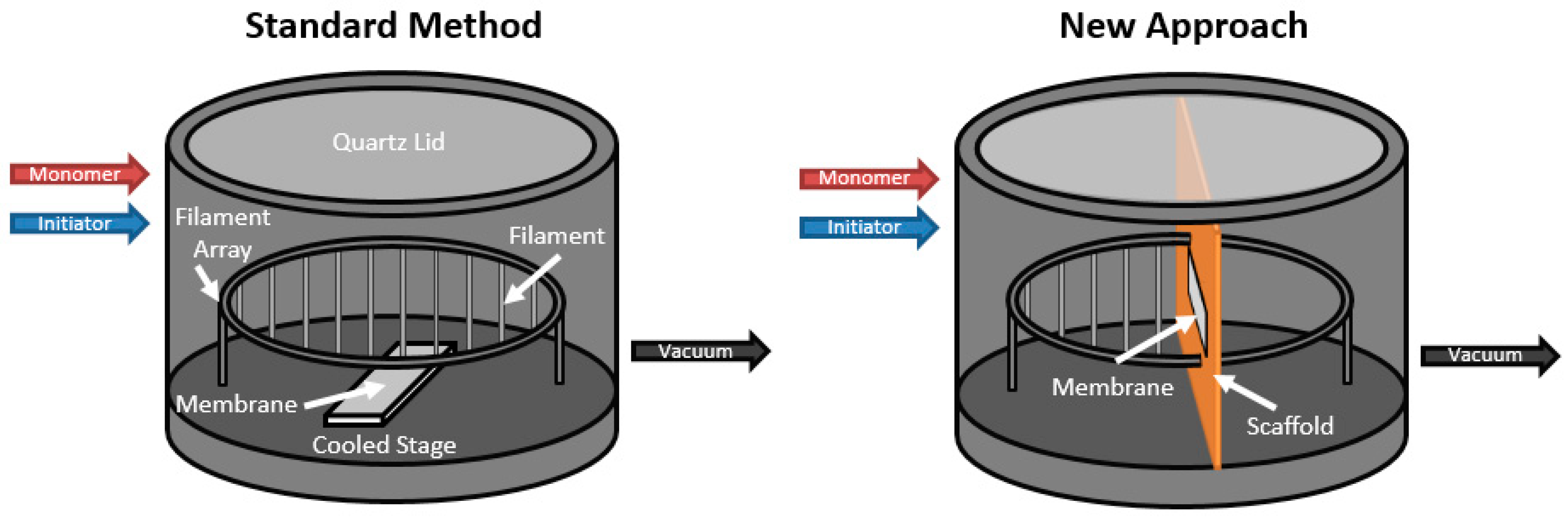
2.4. Initiated Chemical Vapor Deposition
2.5. Membrane Distillation
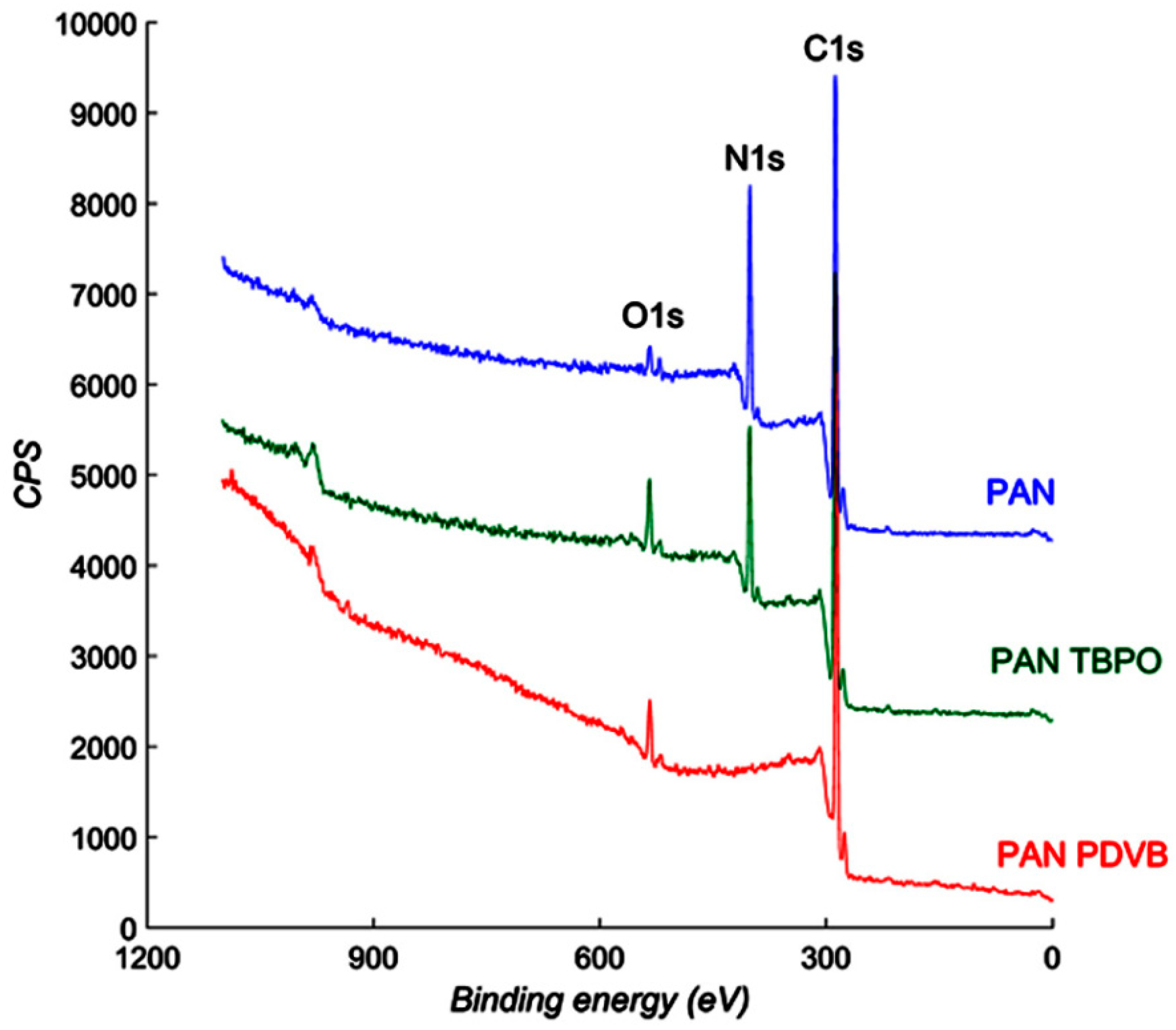
2.6. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
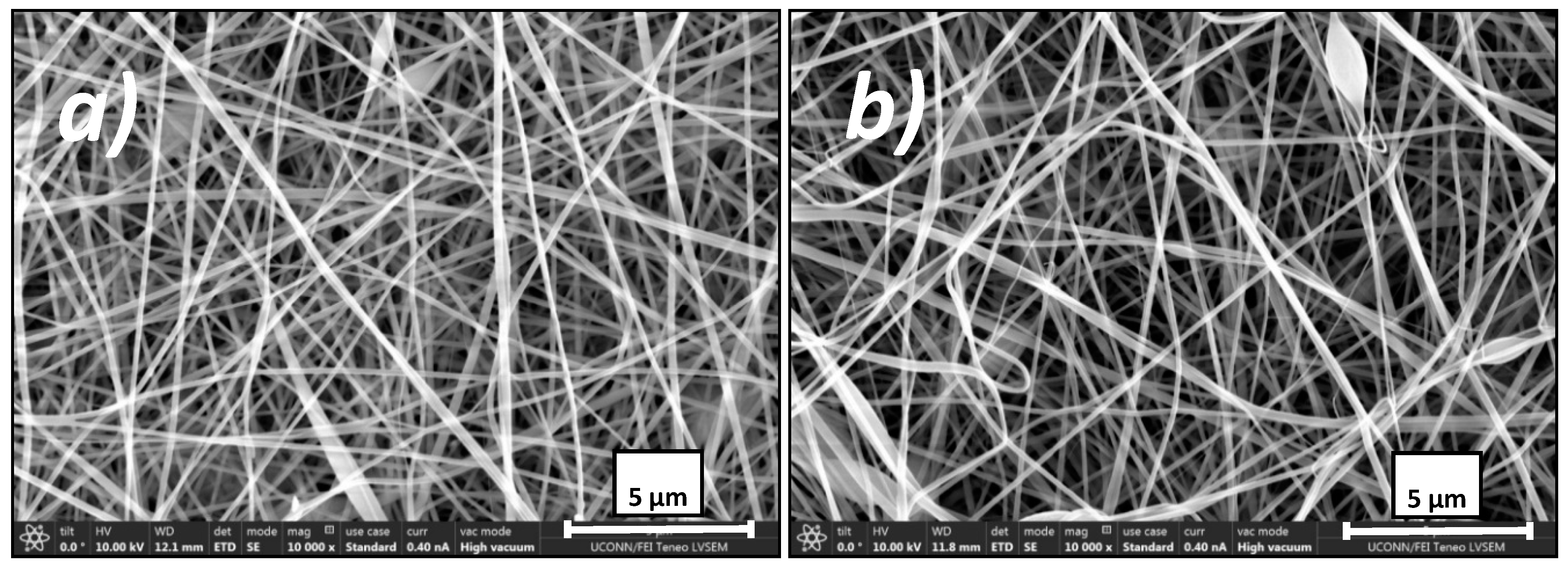
3.1. Membrane Characterization
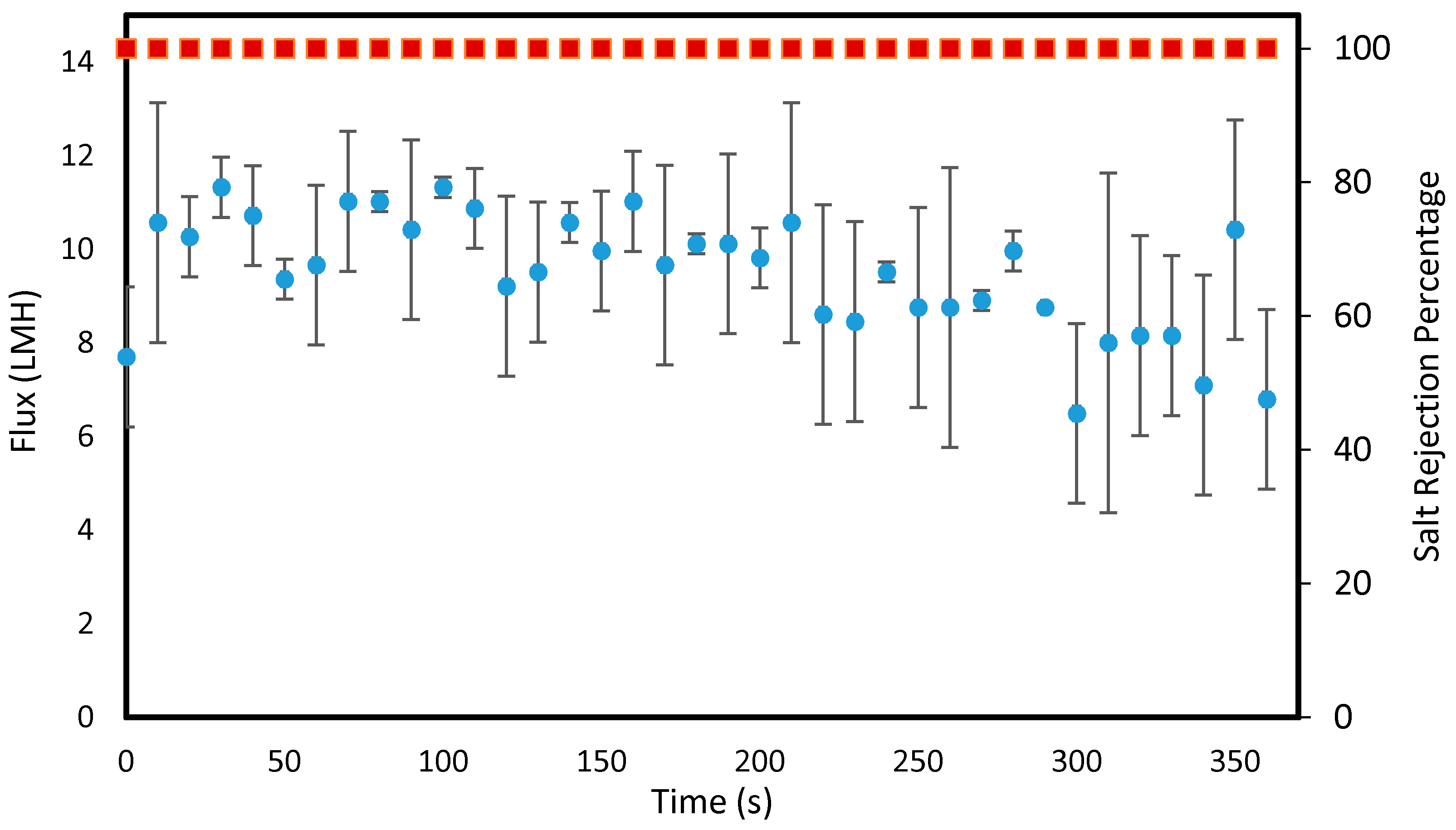
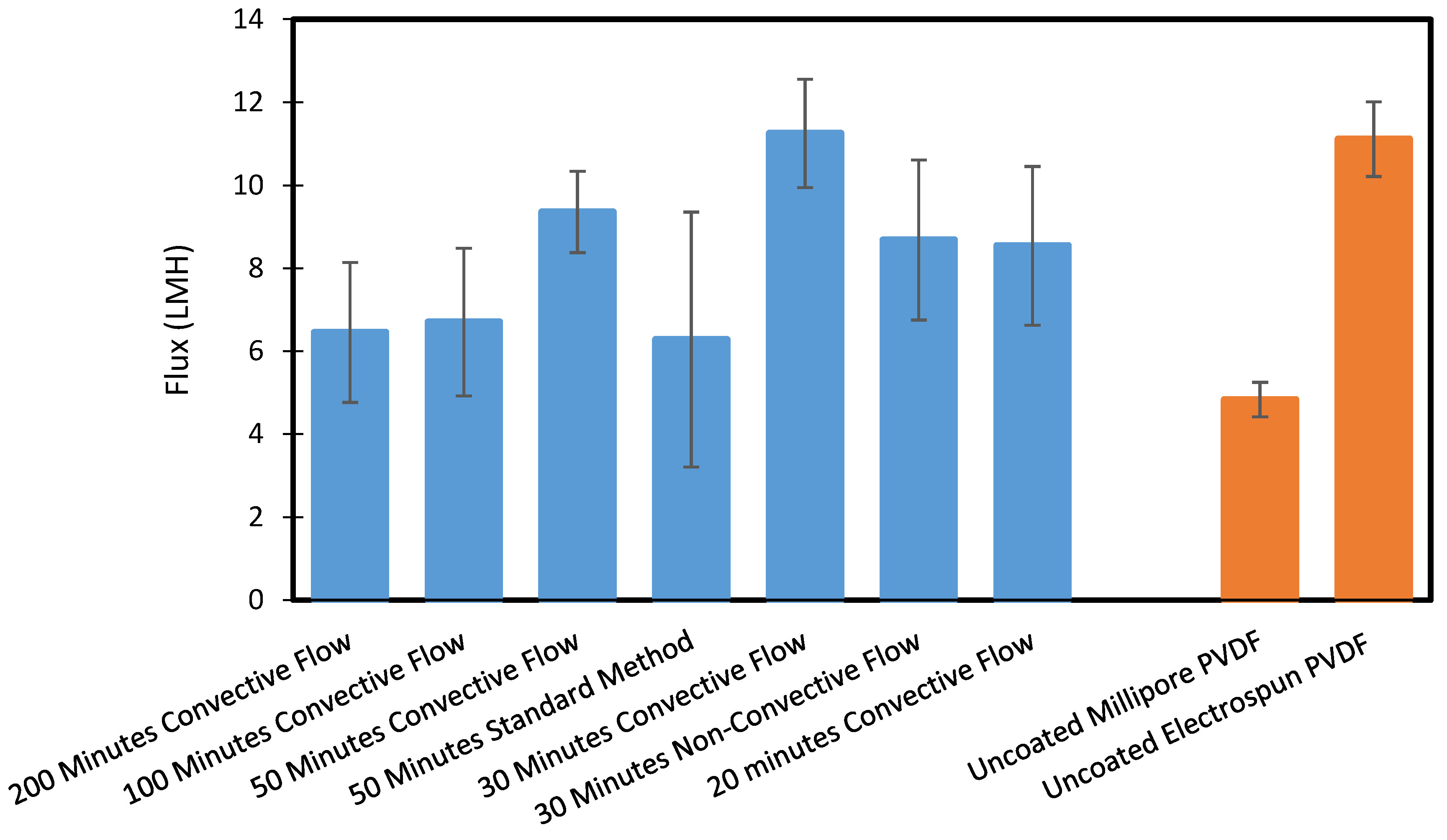
3.2. Membrane Distillation Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camachoo, L.M.; Dumée, L.; Zhang, J.; de Li, J.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Recent progress of membrane distillation using electrospun nanofibrous membrane. J. Memb. Sci. 2014, 453, 435–462. [Google Scholar] [CrossRef]
- Saffarini, R.B.; Summers, E.K.; Arafat, H.A.; Lienhard, J.H. Technical evaluation of stand-alone solar powered membrane distillation systems. Desalination 2012, 286, 332–341. [Google Scholar] [CrossRef]
- Hwang, H.J.; He, K.; Gray, S.; Zhang, J.; Moon, I.S. Direct contact membrane distillation (DCMD): Experimental study on the commercial PTFE membrane and modeling. J. Memb. Sci. 2011, 371, 90–98. [Google Scholar] [CrossRef]
- Huang, L.-T.; Hsu, P.-S.; Kuo, C.-Y.; Chen, S.-C.; Lai, J.-Y. Pore size control of PTFE membranes by stretch operation with asymmetric heating system. Desalination 2008, 233, 64–72. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.-S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Memb. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2014, 356, 56–84. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Dong, Z.-Q.; Ma, X.; Xu, Z.-L.; You, W.-T.; Li, F. Superhydrophobic PVDF–PTFE electrospun nanofibrous membranes for desalination by vacuum membrane distillation. Desalination 2014, 347, 175–183. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Memb. Sci. 2013, 425, 30–39. [Google Scholar] [CrossRef]
- Francis, L.; Maab, H.; AlSaadi, A.; Nunes, S.; Ghaffour, N.; Amy, G.L. Fabrication of electrospun nanofibrous membranes for membrane distillation application. Desalin. Water Treat. 2013, 51, 1337–1343. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nanofibers at different electrospinning times: Desalination by direct contact membrane distillation. J. Memb. Sci. 2013, 433, 167–179. [Google Scholar] [CrossRef]
- Su, C.-I.; Shih, J.-H.; Huang, M.-S.; Wang, C.-M.; Shih, W.-C.; Liu, Y.-S. A Study of Hydrophobic Electrospun Membrane Applied in Seawater Desalination by Membrane Distillation. Fibers Polym. 2012, 13, 698–702. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuurab, T. Application of surface modifying macromolecules for the preparation of membranes for membrane distillation. Desalination 2003, 158, 1–56. [Google Scholar] [CrossRef]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Memb. Sci. 2012, 415, 850–863. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, D.L.; Zhang, S.H.; Jian, X.G. Hydrophobic modification of poly(phthalazinone ether sulfone ketone) hollow fiber membrane for vacuum membrane distillation. J. Memb. Sci. 2008, 310, 20–27. [Google Scholar] [CrossRef]
- Matin, A.; Ozaydin-ince, G.; Khan, Z.; Mohammad, S.; Zaidi, J.; Gleason, K.; Eggenspiler, D. Random copolymer films as potential antifouling coatings for reverse osmosis membranes. Desalin. Water Treat. 2011, 34, 100–105. [Google Scholar] [CrossRef]
- Matin, A.; Khan, Z.; Gleason, K.K.; Khaled, M.; Zaidi, S.M.J.; Khalil, A.; Moni, P.; Yang, R. Surface-modified reverse osmosis membranes applying a copolymer film to reduce adhesion of bacteria as a strategy for biofouling control. Sep. Purif. Technol. 2014, 124, 117–123. [Google Scholar] [CrossRef]
- Ozaydin-Ince, G.; Matin, A.; Khan, Z.; Zaidi, S.M.J.; Gleason, K.K. Surface modification of reverse osmosis desalination membranes by thin-film coatings deposited by initiated chemical vapor deposition. Thin Solid Films 2013, 539, 181–187. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Ozaydin-Ince, G.; Wong, S.Y.; Gleason, K.K. Surface-tethered zwitterionic ultrathin antifouling coatings on reverse osmosis membranes by initiated chemical vapor deposition. Chem. Mater. 2011, 23, 1263–1272. [Google Scholar] [CrossRef]
- Martin, T.P.; Lau, K.K.S.; Chan, K.; Mao, Y.; Gupta, M.; O’Shaughnessy, W.S.; Gleason, K.K. Initiated chemical vapor deposition (iCVD) of polymeric nanocoatings. Surf. Coat. Technol. 2007, 201, 9400–9405. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Gleason, K.K. Initiated and Oxidative Chemical Vapor Deposition of Polymeric Thin Films: iCVD and oCVD. Adv. Funct. Mater. 2008, 18, 979–992. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Gleason, K.K. Initiated Chemical Vapor Deposition (iCVD) of poly(alkyl acrylates): A kinetic model. Macromolecules 2006, 39, 3695–3703. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Gleason, K.K. Initiated chemical vapor deposition (iCVD) of poly(alkyl acrylates): An experimental study. Macromolecules 2006, 39, 3688–3694. [Google Scholar] [CrossRef]
- Gupta, M.; Kapur, V.; Pinkerton, N.M.; Gleason, K.K. Initiated Chemical Vapor Deposition (iCVD) of conformal polymeric nanocoatings for the surface modification of high-aspect-ratio pores. Chem. Mater. 2008, 20, 1646–1651. [Google Scholar] [CrossRef]
- Gupta, M.; Gleason, K.K. Surface modification of high aspect ratio structures with fluoropolymer coatings using chemical vapor deposition. Thin Solid Films 2009, 517, 3547–3550. [Google Scholar] [CrossRef]
- Asatekin, A.; Gleason, K.K. Polymeric nanopore membranes for hydrophobicity-based separations by conformal initiated chemical vapor deposition. Nano Lett. 2011, 11, 677–686. [Google Scholar] [CrossRef]
- Guo, F.; Servi, A.; Liu, A.; Gleason, K.K.; Rutledge, G.C. Desalination by membrane distillation using electrospun polyamide fiber membranes with surface fluorination by chemical vapor deposition. ACS Appl. Mater. Interfaces 2015, 7, 8225–8232. [Google Scholar] [CrossRef]
- Servi, A.T.; Kharraz, J.; Klee, D.; Notarangelo, K.; Eyob, B.; Guillen-Burrieza, E.; Liu, A.; Arafat, H.A.; Gleason, K.K. A systematic study of the impact of hydrophobicity on the wetting of MD membranes. J. Memb. Sci. 2016, 520, 850–859. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Park, J.-H.; Sudarshan, T.S.; ASM International. Chemical Vapor Deposition. 2001. Available online: https://www.scribd.com/doc/75746558/Chemical-Vapor-Deposition-Surface-Engineering-Series-V-2-ASMI (accessed on 26 April 2017).
| Coating Orientation | Coating Time (Minutes) | Contact Angle of Side Directly Exposed to Precursors | Contact Angle of Side Not in Direct Contact with Precursors |
|---|---|---|---|
| Standard: Affixed to Stage | 50 | 122 ± 4.3° | 118 ± 4.0° |
| Scaffold: Allowing Convective Flow | 50 | 135 ± 4.8° | 147 ± 4.3° |
| Scaffold: Allowing Convective Flow | 30 | 149 ± 3.4° | 141 ± 1.5° |
| Scaffold: Prohibiting Convective Flow | 30 | 135 ± 0.9° | 127 ± 0.7° |
| Scaffold: Allowing Convective Flow | 15 | 136 ± 0.6° | 125 ± 0.4° |
| Scaffold: Prohibiting Convective Flow | 15 | 124 ± 0.5° | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beauregard, N.; Al-Furaiji, M.; Dias, G.; Worthington, M.; Suresh, A.; Srivastava, R.; Burkey, D.D.; McCutcheon, J.R. Enhancing iCVD Modification of Electrospun Membranes for Membrane Distillation Using a 3D Printed Scaffold. Polymers 2020, 12, 2074. https://doi.org/10.3390/polym12092074
Beauregard N, Al-Furaiji M, Dias G, Worthington M, Suresh A, Srivastava R, Burkey DD, McCutcheon JR. Enhancing iCVD Modification of Electrospun Membranes for Membrane Distillation Using a 3D Printed Scaffold. Polymers. 2020; 12(9):2074. https://doi.org/10.3390/polym12092074
Chicago/Turabian StyleBeauregard, Nicole, Mustafa Al-Furaiji, Garrett Dias, Matthew Worthington, Aravind Suresh, Ranjan Srivastava, Daniel D. Burkey, and Jeffrey R. McCutcheon. 2020. "Enhancing iCVD Modification of Electrospun Membranes for Membrane Distillation Using a 3D Printed Scaffold" Polymers 12, no. 9: 2074. https://doi.org/10.3390/polym12092074
APA StyleBeauregard, N., Al-Furaiji, M., Dias, G., Worthington, M., Suresh, A., Srivastava, R., Burkey, D. D., & McCutcheon, J. R. (2020). Enhancing iCVD Modification of Electrospun Membranes for Membrane Distillation Using a 3D Printed Scaffold. Polymers, 12(9), 2074. https://doi.org/10.3390/polym12092074







