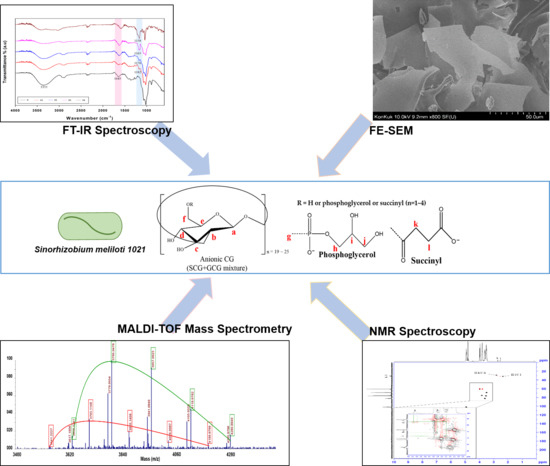
Structural Characterization of Glycerophosphorylated and Succinylated Cyclic β-(1→2)-d-Glucan Produced by Sinorhizobium mliloti 1021
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Condition
2.2. Preparation of Neutral and Anionic Cyclic β-(1→2)-d-Glucan
2.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. Matrix Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry
2.5. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.6. Field Emission Scanning Electron Microscopy (FE-SEM)
3. Results
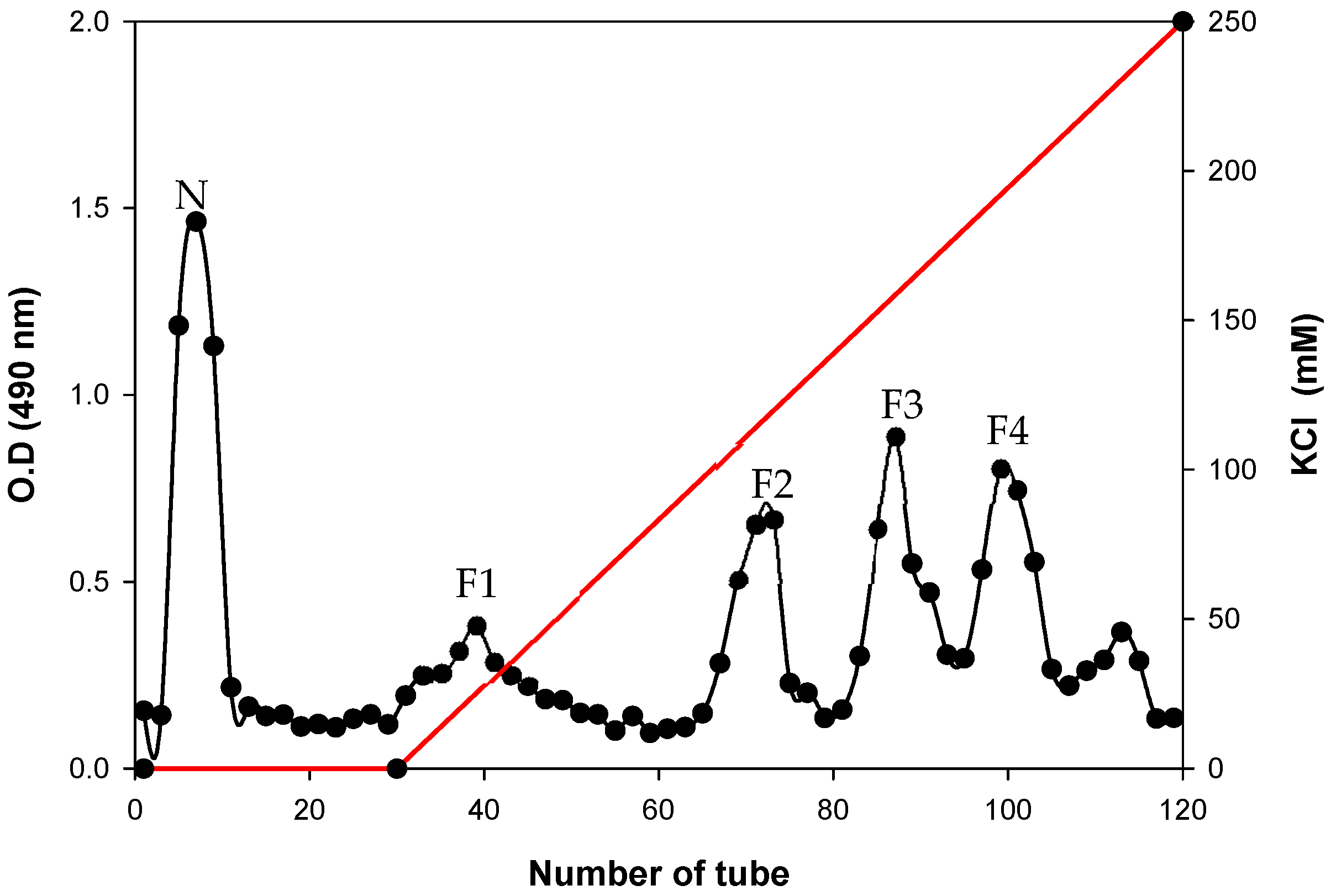
3.1. DEAE-Sephadex Chromatography of CG
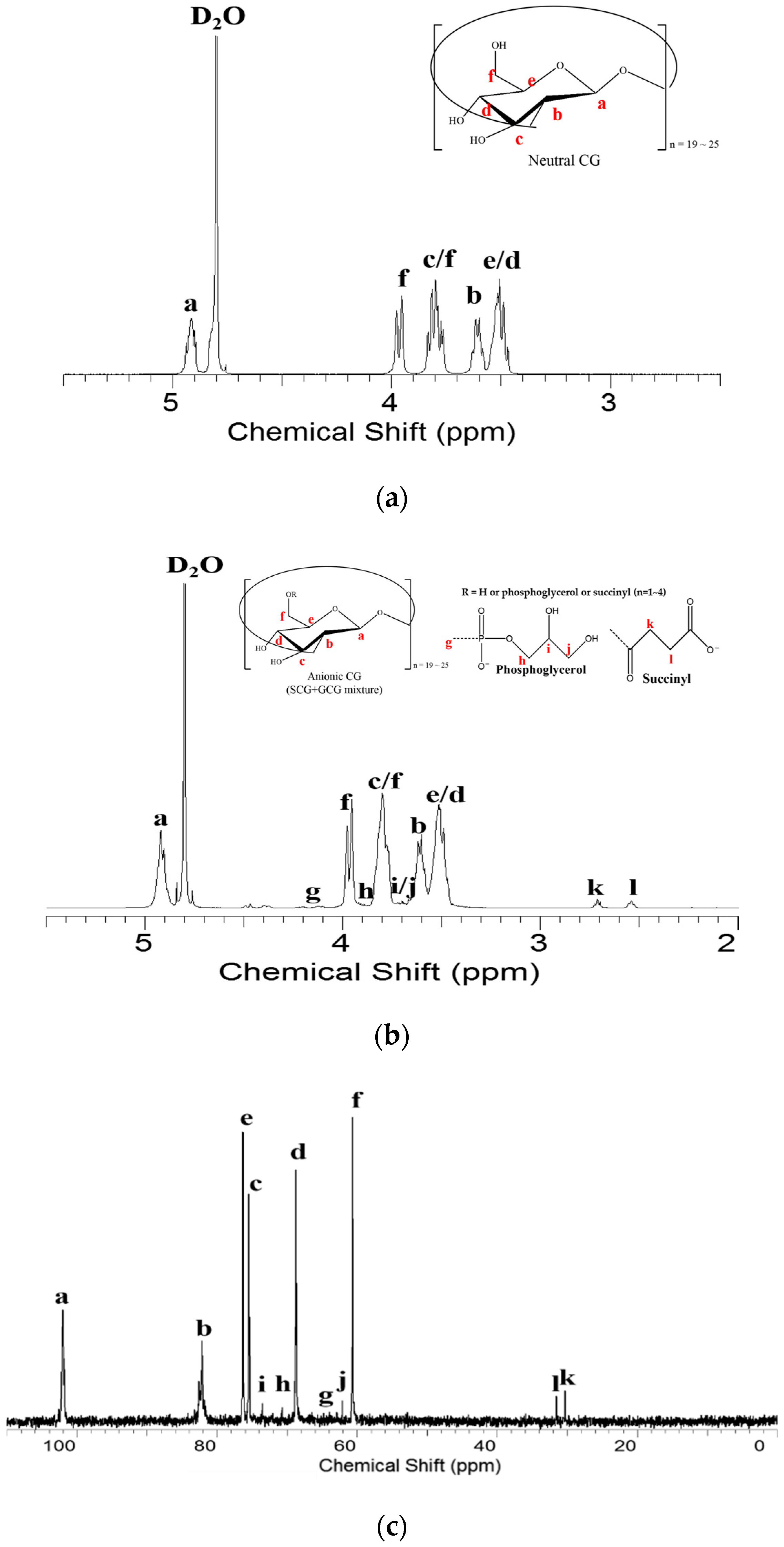
3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
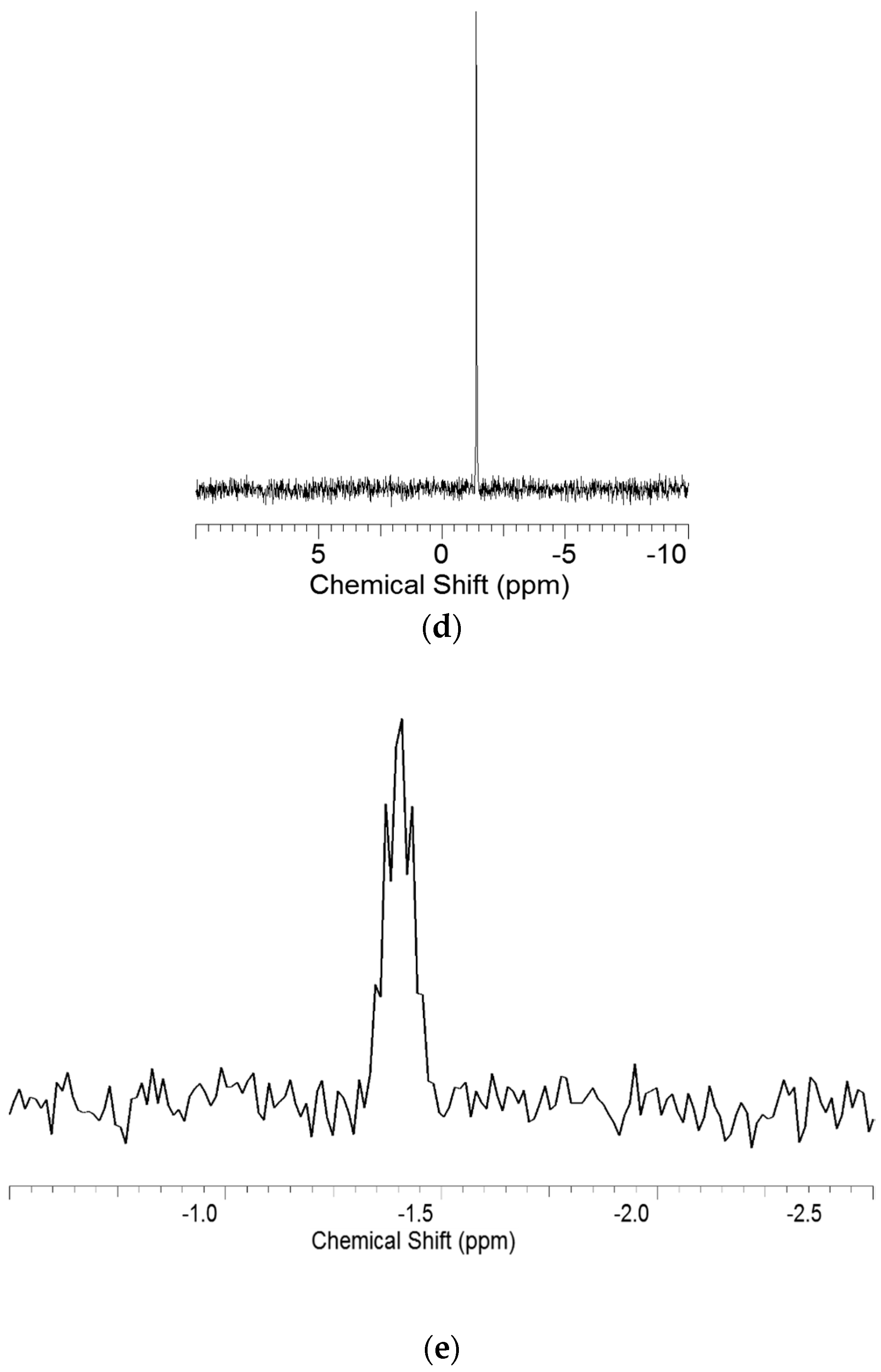
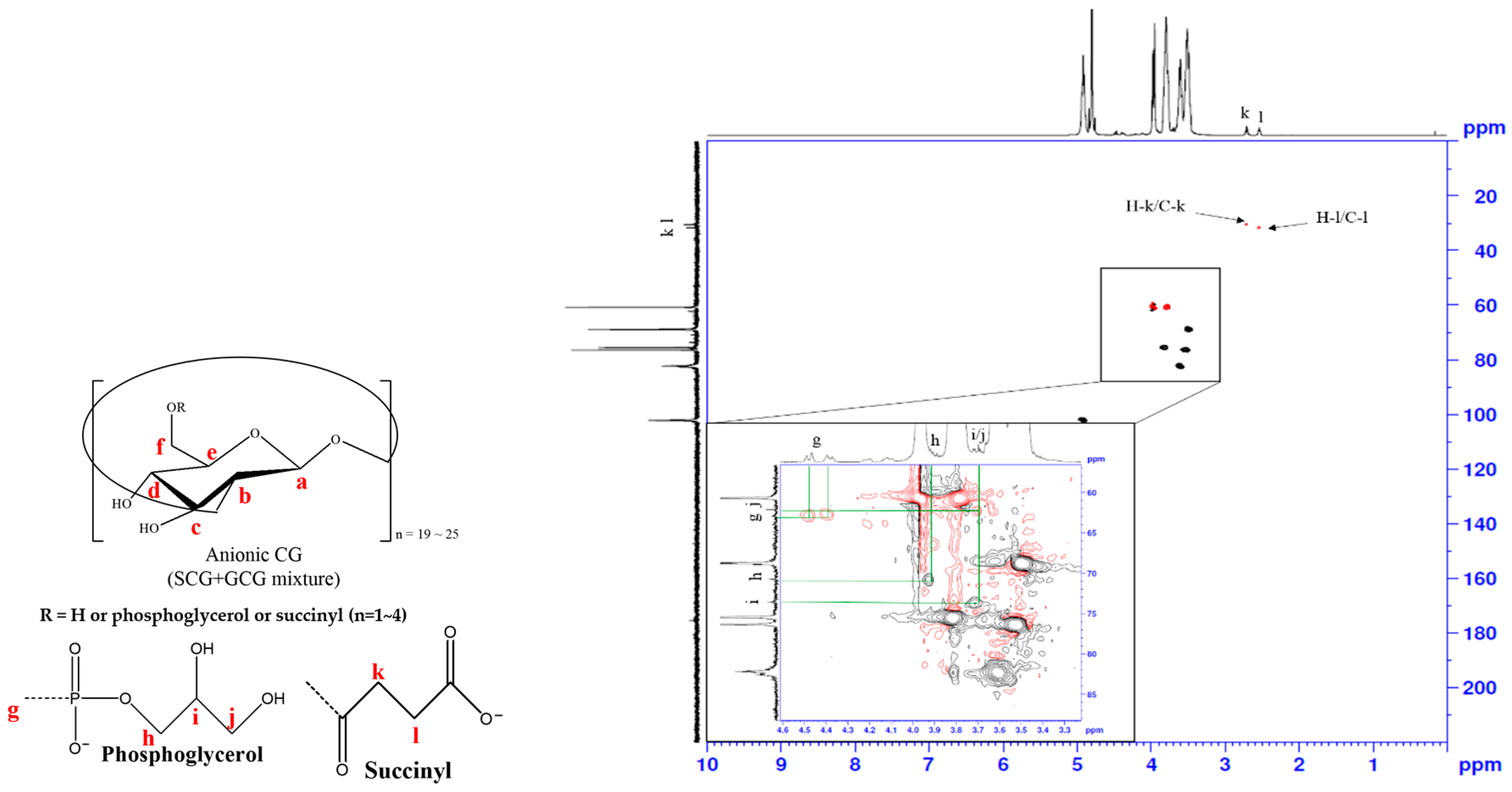
3.3. Heteronuclear Single Quantum Coherence Spectroscopy (HSQC) Spectra Analysis
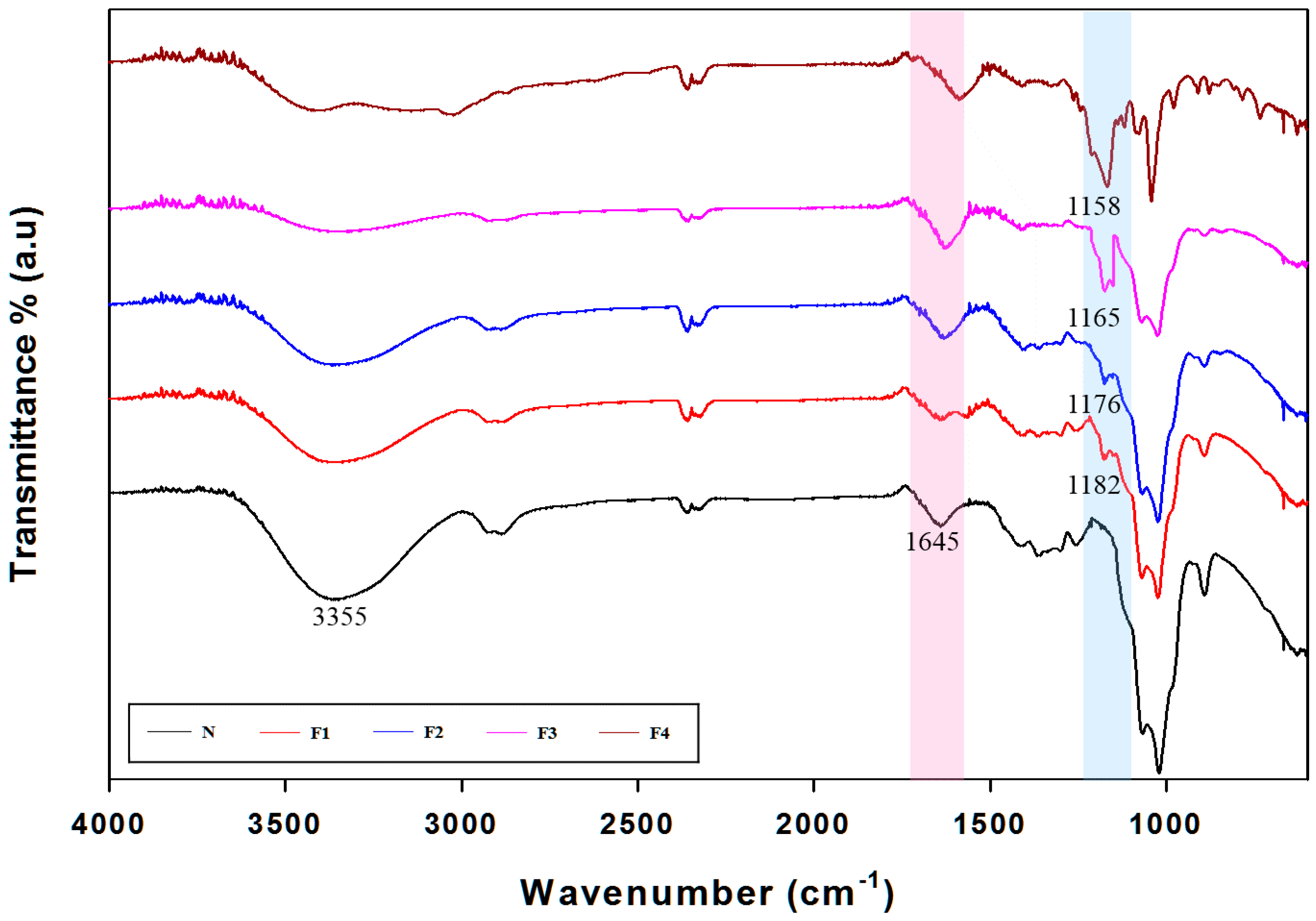
3.4. Attenuated Total Reflection (ATR)-Fourier Transform Infrared (FTIR) Spectra Analysis
3.5. Matrix Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometric Analysis
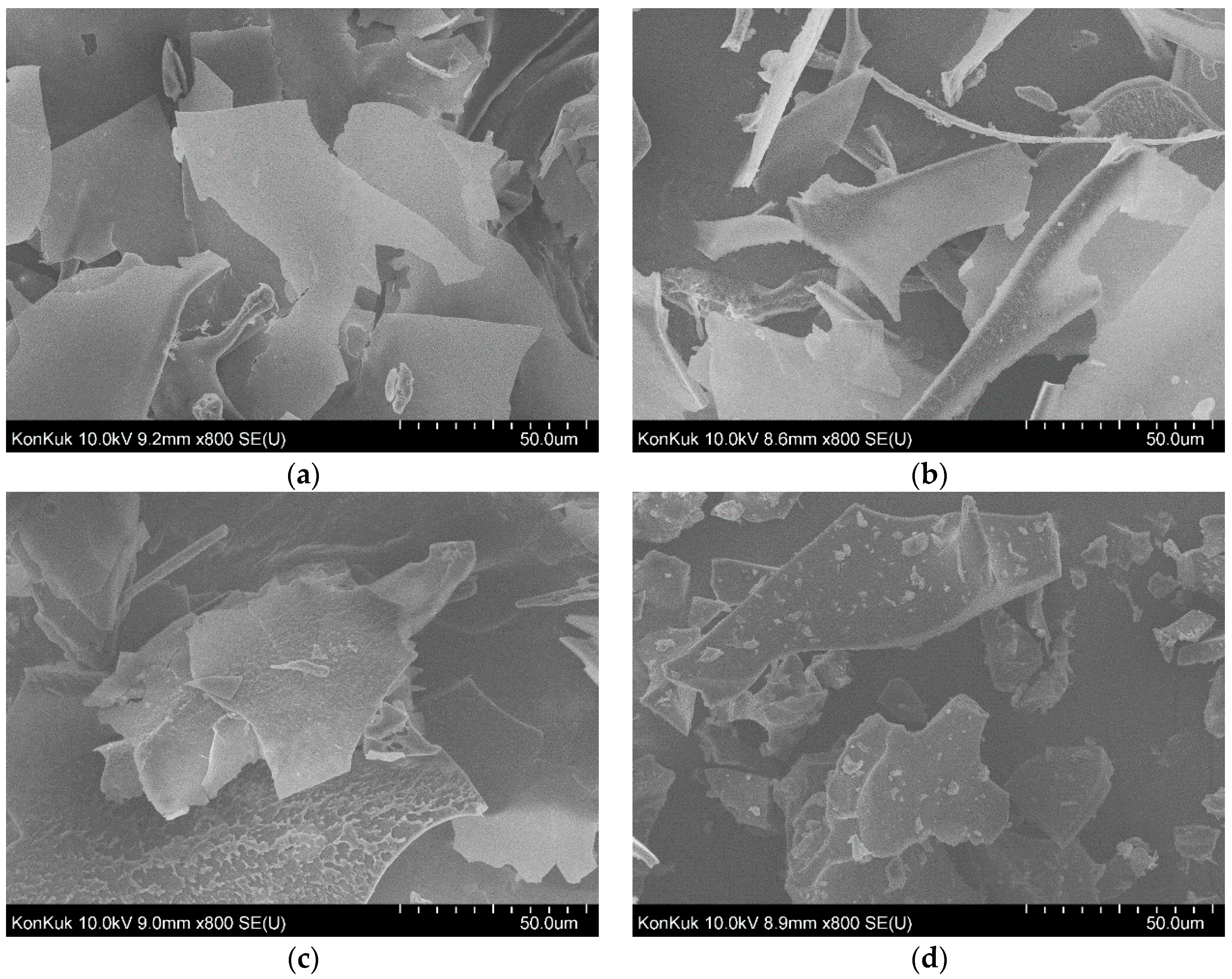
3.6. Field Emission Scannintg Electron Microscopy (FE-SEM) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dylan, T.; Ielpi, L.; Stanfield, S.; Kashyap, L.; Douglas, C.; Yanofsky, M.; Nester, E.; Helinski, D.R.; Ditta, G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA 1986, 83, 4403–4407. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Maiti, T.K. Structure of Extracellular Polysaccharides (EPS) produced by rhizobia and their functions in legume–bacteria symbiosis—A review. Achiev. Life Sci. 2016, 10, 136–143. [Google Scholar] [CrossRef]
- Miller, K.; Kennedy, E.; Reinhold, V. Osmotic adaptation by gram-negative bacteria: Possible role for periplasmic oligosaccharides. Science 1986, 231, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, M.W.; Miller, K.J. Cyclic beta-glucans of members of the family Rhizobiaceae. Microbiol. Rev. 1994, 58, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, M.W.; Hadley, J.A.; Miller, K.J. A novel cyclic beta-1,2-glucan mutant of Rhizobium meliloti. J. Bacteriol. 1995, 177, 6346–6351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seo, D.-H.; Lee, S.-H.; Park, H.-L.; Lee, D.-H.; Ji, E.-J.; Shin, D.-H.; Jung, S.-H. Structural analysis of total anionic cyclosophoraoses synthesized by Rhizobium meliloti 2011. Bull. Korean Chem. Soc. 2002, 23, 899–903. [Google Scholar]
- Kwon, C.; Choi, Y.-H.; Kim, N.; Yoo, J.S.; Yang, C.-H.; Kim, H.-W.; Jung, S. Complex forming ability of a family of isolated cyclosophoraoses with ergosterol and its monte carlo docking computational analysis. J. Incl. Phenom. Macrocycl. Chem. 2000, 36, 55–64. [Google Scholar] [CrossRef]
- Koizumi, K.; Okada, Y.; Horiyama, S.; Utamura, T.; Higashiura, T.; Ikeda, M. Preparation of cyclosophoraose-A and its complex-forming ability. J. Incl. Phenom. Macrocycl. Chem. 1984, 2, 891–899. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwon, C.-H.; Choi, Y.-J.; Seo, D.-H.; Kim, H.-W.; Jung, S.-H. Inclusion complexation of a family of cyclsohoraoses with indomethacin. J. Microbiol. Biotechnol. 2001, 11, 463–468. [Google Scholar]
- Cho, E.; Jung, S. Biotinylated cyclooligosaccharides for paclitaxel solubilization. Molecules 2018, 23, 90. [Google Scholar] [CrossRef]
- Kim, Y.; Shinde, V.V.; Jeong, D.; Choi, Y.; Jung, S. Solubility enhancement of atrazine by complexation with cyclosophoraose isolated from Rhizobium leguminosarum biovar trifolii TA-1. Polymers 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.O.; Mahaguna, V.; Sriwongjanya, M. Characterization of an inclusion complex of cholesterol and hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Biopharm. 1998, 46, 355–360. [Google Scholar] [CrossRef]
- Erden, N.; Çelebi, N. A study of the inclusion complex of naproxen with β-cyclodextrin. Int. J. Pharm. 1988, 48, 83–89. [Google Scholar] [CrossRef]
- Wen, X.; Tan, F.; Jing, Z.; Liu, Z. Preparation and study the 1:2 inclusion complex of carvedilol with β-cyclodextrin. J. Pharm. Biomed. Anal. 2004, 34, 517–523. [Google Scholar] [CrossRef]
- Miller, K.J.; Reinhold, V.N.; Weissborn, A.C.; Kennedy, E.P. Cyclic glucans produced by Agrobacterium tumefaciens are substituted with sn-1-phosphoglycerol residues. Biochim. Biophys. Acta (BBA) Biomembr. 1987, 901, 112–118. [Google Scholar] [CrossRef]
- Roset, M.S.; Ciocchini, A.E.; Ugalde, R.A.; De Iannino, N.I. The brucella abortus cyclic β-1,2-glucan virulence factor is substituted with o-ester-linked succinyl residues. J. Bacteriol. 2006, 188, 5003–5013. [Google Scholar] [CrossRef]
- Miller, K.J.; Gore, R.S.; Benesi, A.J. Phosphoglycerol substituents present on the cyclic beta-1,2-glucans of Rhizobium meliloti 1021 are derived from phosphatidylglycerol. J. Bacteriol. 1988, 170, 4569–4575. [Google Scholar] [CrossRef]
- Hisamatsu, M.; Yamada, T.; Higashiura, T.; Ikeda, M. The production of acidic, O-acylated cyclosophorans [cyclic (1→2)-β-d-glucans] by Agrobacterium and Rhizobium species. Carbohydr. Res. 1987, 163, 115–122. [Google Scholar] [CrossRef]
- Kawaharada, Y.; Kiyota, H.; Eda, S.; Minamisawa, K.; Mitsui, H. Structural characterization of neutral and anionic glucans from Mesorhizobium loti. Carbohydr. Res. 2008, 343, 2422–2427. [Google Scholar] [CrossRef]
- Kennedy, E. Membrane-derived oligosaccharides (periplasmic beta-D-glucans) of Escherichia coli. In Escherichia Coli and Salmonella. Cellular and Molecular Biology, 2nd ed.; Neidhardt, F.C., Curtiss, R., III, Ingraham, J.L., Lin, E.C.C., Low, K.B., Maganasik, B., Reznikoff, W.S., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; American Society for Microbiology: Washington, DC, USA, 1996; pp. 1064–1074. [Google Scholar]
- Breedveld, M.W.; Zevenhuizen, L.P.; Zehnder, A.J. Excessive excretion of cyclic beta-(1,2)-glucan by Rhizobium trifolii TA-1. Appl. Environ. Microbiol. 1990, 56, 2080–2086. [Google Scholar] [CrossRef]
- Batley, M.; Redmond, J.; Djordjevic, S.; Rolfe, B. Characterisation of glycerophosphorylated cyclic β-1,2-glucans from a fast-growing Rhizobium species. Biochim. Biophys. Acta (BBA) Biomembr. 1987, 901, 119–126. [Google Scholar] [CrossRef]
- Dell, A.; York, W.S.; McNeil, M.; Darvill, A.G.; Albersheim, P. The cyclic structure of β-d-(1→2)-linked d-glucans secreted by Rhizobia and Agrobacteria. Carbohydr. Res. 1983, 117, 185–200. [Google Scholar] [CrossRef]
- Garozzo, D.; Spina, E.; Sturiale, L.; Montaudo, G.; Rizzo, R.; Daolio, S. Quantitative determination of β (1–2) cyclic glucans by matrix-assisted laser desorption mass spectrometry. Rapid Commun. Mass Spectrom. 1994, 8, 358–360. [Google Scholar] [CrossRef]
- Kicuntod, J.; Khuntawee, W.; Wolschann, P.; Pongsawasdi, P.; Chavasiri, W.; Kungwan, N.; Rungrotmongkol, T. Inclusion complexation of pinostrobin with various cyclodextrin derivatives. J. Mol. Graph. Model. 2016, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Jullian, C.; Moyano, L.; Yáñez, C.; Olea-Azar, C. Complexation of quercetin with three kinds of cyclodextrins: An antioxidant study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 230–234. [Google Scholar] [CrossRef] [PubMed]


| Fraction | Measured Mass | Composition | Phosphoglycerol | Succinic Acid |
| F1 | 3541.2227 | Glc21+SU+K | 0 | 1 |
| 3617.1806 | Glc21+PG+3Na-2H | 1 | 0 | |
| 3633.1241 | Glc21+PG+K+2Na-2H | 1 | 0 | |
| 3703.1146 | Glc22+Su+K | 0 | 1 | |
| 3779.0554 | Glc22 +PG+3Na-2H | 1 | 0 | |
| 3795.0478 | Glc22 +PG+K+2Na-2H | 1 | 0 | |
| 3865.1409 | Glc23 +SU+K | 0 | 1 | |
| 3941.0840 | Glc23+PG+3Na-2H | 1 | 0 | |
| 3957.0023 | Glc23+PG+K+2Na-2H | 1 | 0 | |
| 4026.9987 | Glc24+SU+K | 0 | 1 | |
| 4103.0329 | Glc24+PG+3Na-2H | 1 | 0 | |
| 4118.9782 | Glc24+PG+K+2Na-2H | 1 | 0 | |
| 4189.9799 | Glc25+SU+K | 0 | 1 | |
| 4265.9796 | Glc25+PG+3Na-2H | 1 | 0 | |
| 4280.8649 | Glc25+PG+K+2Na-2H | 1 | 0 | |
| Fraction | Measured Mass | Composition | Phosphoglycerol | Succinic Acid |
| F2 | 3468.9384 | Glc19+2PG+4Na-3H | 2 | 0 |
| 3485.9207 | Glc19+2PG+K+3Na-3H | 2 | 0 | |
| 3503.0003 | Glc20+2SU+K+Na-H | 0 | 2 | |
| 3633.9621 | Glc20+2PG+4Na-3H | 2 | 0 | |
| 3648.7888 | Glc20+2PG+ K+3Na-3H | 2 | 0 | |
| 3664.5169 | Glc21+2SU+K+Na-H | 0 | 2 | |
| 3795.2472 | Glc20+2PG+4Na-3H | 2 | 0 | |
| 3809.8366 | Glc20+2PG+ K+3Na-3H | 2 | 0 | |
| 3827.6578 | Glc21+2SU+K+Na-H | 0 | 2 | |
| 3957.6616 | Glc21+2PG+4Na-3H | 2 | 0 | |
| 3972.6173 | Glc21+2PG+K+3Na-3H | 2 | 0 | |
| 3989.0377 | Glc22+2SU+K+Na-H | 0 | 2 | |
| 4119.8103 | Glc22+2PG+4Na-3H | 2 | 0 | |
| 4134.5388 | Glc22+2PG+K+3Na-3H | 2 | 0 | |
| 4150.5249 | Glc23+2SU+K+Na-H | 0 | 2 | |
| 4280.8828 | Glc23 +2PG+4Na-3H | 2 | 0 | |
| 4296.9708 | Glc23+2PG+K+3Na-3H | 2 | 0 | |
| 4313.0491 | Glc24+2SU+K+Na-H | 0 | 2 | |
| Fraction | Measured Mass | Composition | Phosphoglycerol | Succinic Acid |
| F3 | 3649.0279 | Glc19+3PG+5Na-4H | 3 | 0 |
| 3663.0904 | Glc19+3PG+K+4Na-4H | 3 | 0 | |
| 3679.7335 | Glc20+3SU+3K+Na-3H | 0 | 3 | |
| 3803.0274 | Glc20+3PG+5Na-4H | 3 | 0 | |
| 3825.6591 | Glc20+3PG+K+4Na-4H | 3 | 0 | |
| 3840.5305 | Glc21+3SU+3K+Na-3H | 0 | 3 | |
| 3971.3438 | Glc21+3PG+5Na-4H | 3 | 0 | |
| 3987.2496 | Glc21+3PG+K+4Na-4H | 3 | 0 | |
| 4002.2742 | Glc22+3SU+3K+Na-3H | 0 | 3 | |
| 4132.1205 | Glc22+3PG+5Na-4H | 3 | 0 | |
| 4148.4018 | Glc22+3PG+K+4Na-4H | 3 | 0 | |
| 4166.3030 | Glc23+3SU+3K+Na-3H | 0 | 3 | |
| 4294.0445 | Glc23+3PG+5Na-4H | 3 | 0 | |
| 4313.1442 | Glc23+3PG+K+4Na-4H | 3 | 0 | |
| 4329.1122 | Glc24+3SU+3K+Na-3H | 0 | 3 | |
| 4458.1510 | Glc24+3PG+5Na-4H | 3 | 0 | |
| 4474.1148 | Glc24+3PG+K+4Na-4H | 3 | 0 | |
| 4490.0541 | Glc25+3SU+3K+Na-3H | 0 | 3 | |
| Fraction | Measured Mass | Composition | Phosphoglycerol | Succinic Acid |
| F4 | 3872.7687 | Glc20+4PG+Na | 4 | 0 |
| 3888.3342 | Glc20+4SU+K | 0 | 4 | |
| 4034.6601 | Glc21+4PG+Na | 4 | 0 | |
| 4050.2674 | Glc21+4SU+K | 0 | 4 | |
| 4196.1505 | Glc22+4PG+Na | 4 | 0 | |
| 4213.0479 | Glc22+4SU+K | 0 | 4 | |
| 4358.5967 | Glc23+4PG+Na | 4 | 0 | |
| 4373.6066 | Glc23+4SU+K | 0 | 4 | |
| 4519.6441 | Glc24+4PG+Na | 4 | 0 | |
| 4535.7733 | Glc24+4SU+K | 0 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, S.; Kim, Y.; Jung, S. Structural Characterization of Glycerophosphorylated and Succinylated Cyclic β-(1→2)-d-Glucan Produced by Sinorhizobium mliloti 1021. Polymers 2020, 12, 2073. https://doi.org/10.3390/polym12092073
Lee H, Kim S, Kim Y, Jung S. Structural Characterization of Glycerophosphorylated and Succinylated Cyclic β-(1→2)-d-Glucan Produced by Sinorhizobium mliloti 1021. Polymers. 2020; 12(9):2073. https://doi.org/10.3390/polym12092073
Chicago/Turabian StyleLee, Hyojeong, Seonmok Kim, Yohan Kim, and Seunho Jung. 2020. "Structural Characterization of Glycerophosphorylated and Succinylated Cyclic β-(1→2)-d-Glucan Produced by Sinorhizobium mliloti 1021" Polymers 12, no. 9: 2073. https://doi.org/10.3390/polym12092073
APA StyleLee, H., Kim, S., Kim, Y., & Jung, S. (2020). Structural Characterization of Glycerophosphorylated and Succinylated Cyclic β-(1→2)-d-Glucan Produced by Sinorhizobium mliloti 1021. Polymers, 12(9), 2073. https://doi.org/10.3390/polym12092073