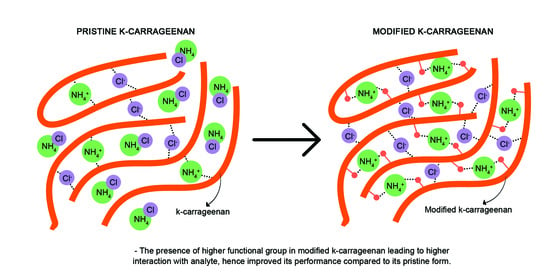
Effect of Active Site Modification towards Performance Enhancement in Biopolymer κ-Carrageenan Derivatives
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of Succinyl κ-Carrageenan
3. Characterization
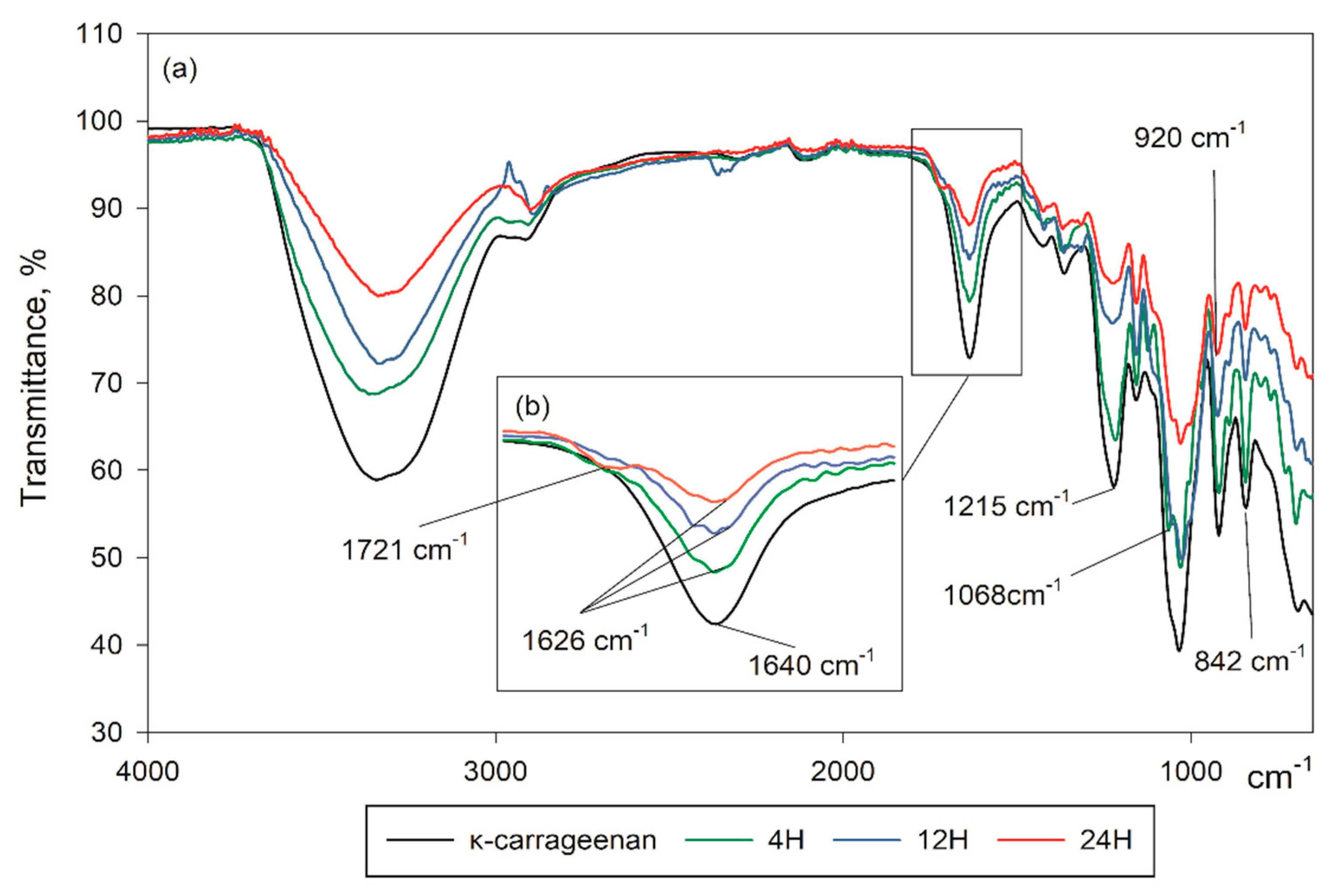
3.1. Fourier Transform Infra-Red Analysis (FTIR)
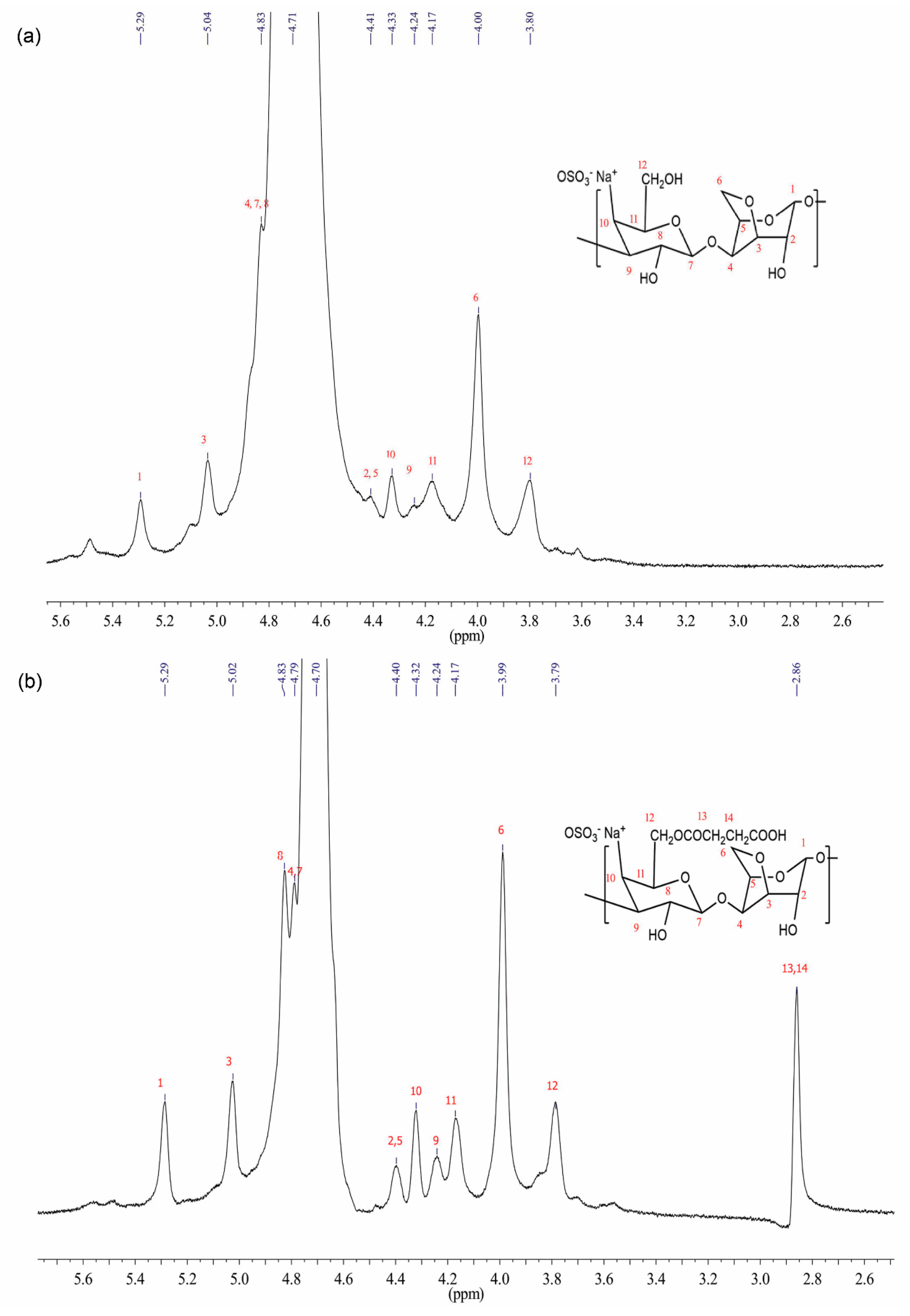
3.2. Nuclear Magnetic Resonance Analysis (NMR)
3.3. X-Ray Diffraction Analysis (XRD) and Elemental Analysis
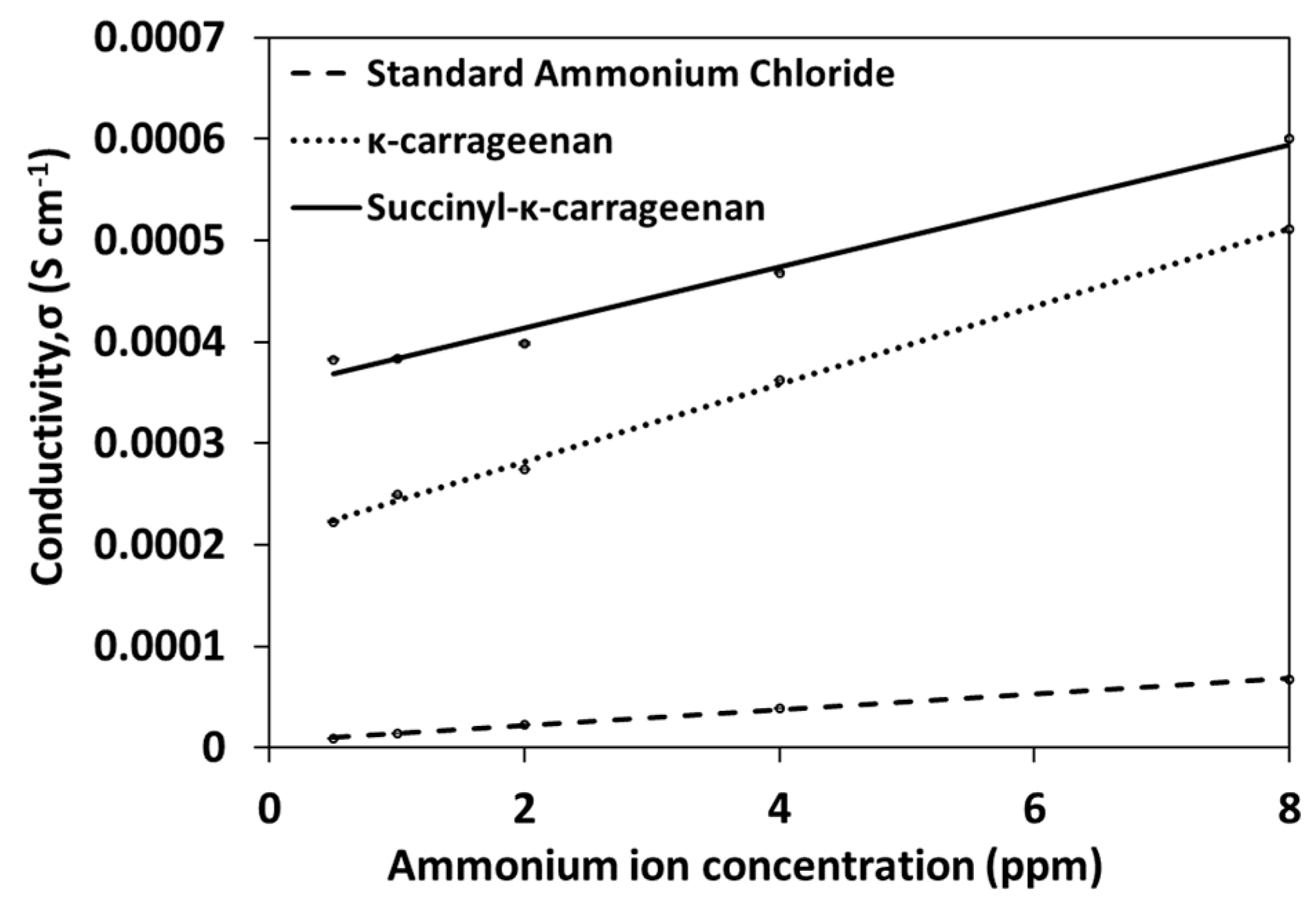
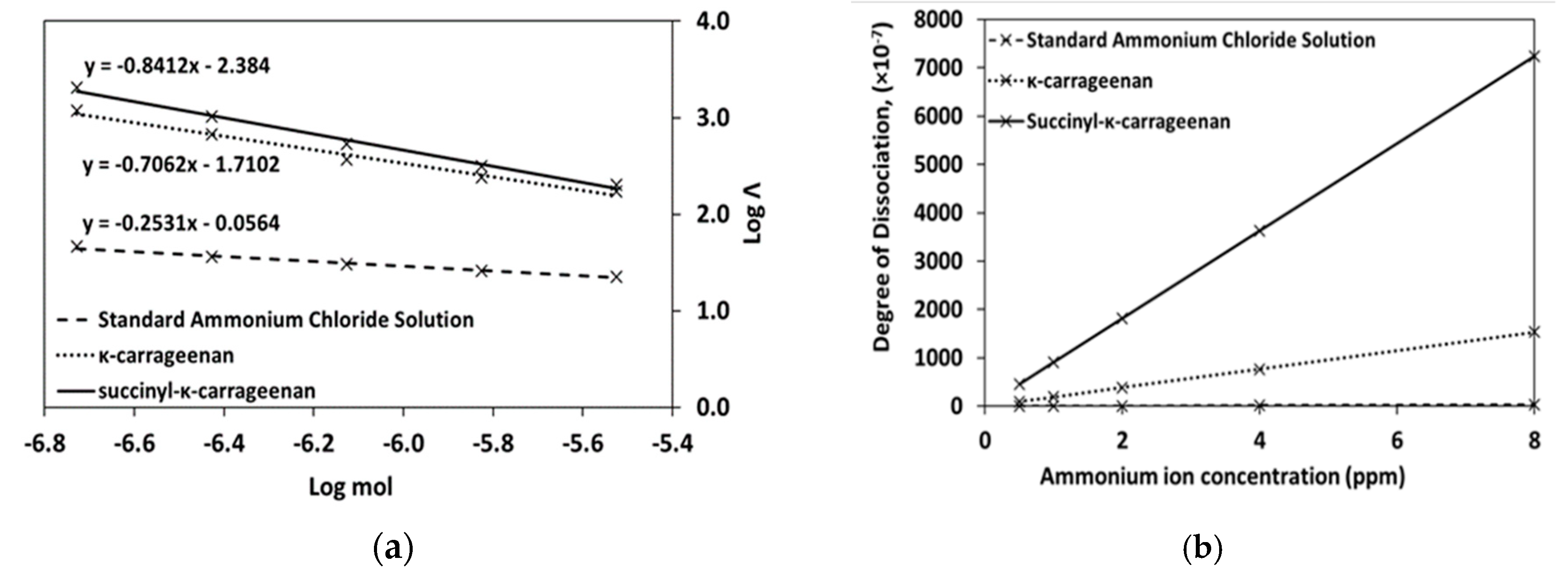
3.4. Conductivity Analysis
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azeman, N.H.; Arsad, N.; Bakar, A.A.A. Polysaccharides as the Sensing Material for Metal Ion Detection-Based Optical Sensor Applications. Sensors 2020, 20, 3924. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Bai, Z.; Zhu, Y. Fast removal of Co(ii) from aqueous solution using porous carboxymethyl chitosan beads and its adsorption mechanism. RSC Adv. 2018, 8, 13370–13387. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Omer, A.M.; Ouyang, X.K. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int. J. Biol. Macromol. 2018, 106, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liew, Y.; Loh, K.S.; Ahmad, A.; Lim, K.L.; Wan, W.R. Synthesis and characterization of modified κ -carrageenan for enhanced proton conductivity as polymer electrolyte membrane. PLoS ONE 2017, 12, e0185313. [Google Scholar]
- Wang, J.; Niu, S.; Zhao, B.; Luo, T.; Liu, D.; Zhang, J. Catalytic synthesis of sulfated polysaccharides. II: Comparative studies of solution conformation and antioxidant activities. Carbohydr. Polym. 2014, 107, 221–231. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ramli, N.; Ahmad, A.; Rahman, M.Y.A. Chemical interaction and conductivity of carboxymethyl κ -carrageenan based green polymer electrolyte. Solid State Ion. 2012, 224, 51–57. [Google Scholar] [CrossRef]
- Wang, J.; Bao, A.; Wang, Q.; Guo, H.; Zhang, Y.; Liang, J.; Kong, W.; Yao, J.; Zhang, J. Sulfation can enhance antitumor activities of Artemisia sphaerocephala polysaccharide in vitro and vivo. Int. J. Biol. Macromol. 2017, 107, 502–511. [Google Scholar] [CrossRef]
- Huang, J.; Deng, Y.; Ren, J.; Chen, G.; Wang, G.; Wang, F.; Wu, X. Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr. Polym. 2018, 186, 54–63. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Veeramachineni, K.; Owh, C. Biodegradable Polysaccharides for Controlled Drug Delivery. ChemPlusChem 2016, 81, 504–514. [Google Scholar] [CrossRef]
- Latifi, M.; Ahmad, A.; Kaddami, H.; Hassan, N.H.; Dieden, R.; Habibi, Y. Chemical Modification and Processing of Chitin for Sustainable Production of Biobased Electrolytes. Polymers 2020, 12, 207. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.; Yuan, L.; Lu, Y. Synthesis and aggregation behavior of N-succinyl-o-carboxymethylchitosan in aqueous solutions. Colloid Polym. Sci. 2007, 285, 1535–1541. [Google Scholar] [CrossRef]
- Rudhziah, S.; Rani, M.S.A.; Ahmad, A.; Mohamed, N.S.; Kaddami, H. Potential of blend of kappa-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind. Crop. Prod. 2015, 72, 133–141. [Google Scholar] [CrossRef]
- Bezerra, R.D.; Silva, M.M.; Morais, A.I.; Osajima, J.A.; Santos, M.R.; Airoldi, C. Phosphated Cellulose as an Efficient Biomaterial for Aqueous Drug Ranitidine Removal. Materials 2014, 7, 7907–7924. [Google Scholar] [CrossRef] [Green Version]
- Elbarbary, A.M.; Ghobashy, M.M. Phosphorylation of chitosan/HEMA interpenetrating polymer network prepared by γ-radiation for metal ions removal from aqueous solutions. Carbohydr. Polym. 2017, 162, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Abu-Saied, M.A.; Wycisk, R.; Abbassy, M.M.; El-Naim, G.A.; El-Demerdash, F.; Youssef, M.E.; Bassuony, H.; Pintauro, P.N. Sulfated chitosan/PVA absorbent membrane for removal of copper and nickel ions from aqueous solutions—Fabrication and sorption studies. Carbohydr. Polym. 2017, 165, 149–158. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, D.; Yao, S. Review on biomedical and bioengineering applications of cellulose sulfate. Carbohydr. Polym. 2015, 132, 311–322. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Y.; He, W. Research progress on chemical modification of alginate. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Ling, Y.P.; Heng, L.Y. Reflectance based sensor for carrageenan utilizing methylene blue embedded acrylic microspheres. Sens. Actuators B Chem. 2014, 192, 247–252. [Google Scholar] [CrossRef]
- Abdullah, S.; Azeman, N.H.; Mobarak, N.N.; Zan, M.S.D.; Bakar, A.A.A. Sensitivity enhancement of localized SPR sensor towards Pb (II) ion detection using natural bio-polymer based carrageenan. Optic 2018, 168, 784–793. [Google Scholar] [CrossRef]
- Xin, P.-P.; Huang, Y.-B.; Hse, C.-Y.; Cheng, H.N.; Huang, C.; Pan, H. Modification of cellulose with succinic anhydride in TBAA/DMSO mixed solvent under catalyst-free conditions. Materials 2017, 10, 526. [Google Scholar] [CrossRef]
- Rahman, N.A.; Hanifah, S.A.; Mobarak, N.N.; Su, S.; Ahmad, A.; Shyuan, L.K.; Khoon, L.T. Synthesis and characterizations of o- nitrochitosan based biopolymer electrolyte for electrochemical devices. PLoS ONE 2019, 14, e0212066. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Du, Y.; Yang, J.; Zhang, B.; Sun, L. Effect of Degree of Substitution and Molecular Weight of Carboxymethyl Chitosan Nanoparticles on Doxorubicin Delivery. J. Appl. Polym. Sci. 2005, 100, 4689–4696. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Abdullah, P. Synthesis and Characterization of Several Lauryl Chitosan Derivatives. Malays. J. Anal. Sci. 2010, 14, 88–99. [Google Scholar]
- Wade, L.G. Organic Chemistry, 8th ed.; Pearson: Harlow, UK, 2013; p. 1039. [Google Scholar]
- Hanibah, H.; Ahmad, A.; Hassan, N.H. A new approach in determining limiting molar conductivity value for liquid electrolyte. Electrochim. Acta 2014, 147, 758–764. [Google Scholar] [CrossRef]
- Tranquilan-Aranilla, C.; Nagasawa, N.; Bayquen, A.; Dela Rosa, A. Synthesis and characterization of carboxymethyl derivatives of kappa-carrageenan. Carbohydr. Polym. 2012, 87, 1810–1816. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, X.; Tian, X. Synthesis and N.M.R. structural analysis of O -succinyl derivative of low-molecular-weight k -carrageenan. Carbohydr. Polym. 2005, 61, 399–406. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J. Introduction to Spectroscopy, 3rd ed.; Brooks/Cole Thomson Learning: Albert Complex, Singapore, 2001; pp. 50–72. [Google Scholar]
- Kolender, A.A.; Matulewicz, M.C. Desulfation of sulfated galactans with chlorotrimethylsilane. Characterization of β-carrageenan by 1H N.M.R. spectroscopy. Carbohydr. Res. 2004, 339, 1619–1629. [Google Scholar] [CrossRef]
- Forget, A.; Christensen, J.; Lüdeke, S.; Kohler, E.; Tobias, S.; Matloubi, M. Polysaccharide hydrogels with tunable stiffness and provasculogenic properties via α -helix to β -sheet switch in secondary structure. Proc. Natl. Acad. Sci. USA 2013, 110, 12887–12892. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Mo, J.; Li, J.; Liu, J.; Yan, H.; Lyu, J.; Jiang, B.; Chu, L.; Li, M. Comprehensively-modified polymer electrolyte membranes with multifunctional P.M.I.A. for highly-stable all-solid-state lithium-ion batteries. J. Energy Chem. 2020, 48, 334–343. [Google Scholar] [CrossRef]
- Hanibah, H.; Ahmad, A.; Hassan, N.H. Studies on the molar conductivity behavior of PMMA-co-M.A. electrolyte solution at different polymer concentrations. In AIP Conference Proceedings; American Institute of Physics: Melville, NY, USA, 2014; Volume 1614, pp. 295–301. [Google Scholar]
- Lu, F.; Zhang, C.; Lu, B.; Yu, K.; Liu, J.; Kang, H.; Liu, R.; Lan, G. Cellobiose as a model compound for cellulose to study the interactions in cellulose/lithium chloride/N-methyl-2-pyrrolidone systems. Cellulose 2017, 24, 1621–1629. [Google Scholar] [CrossRef]



| Functional Groups | Biopolymer Polysaccharides | Applications | References |
|---|---|---|---|
| Carboxymethyl | Chitosan, chitin, cellulose, κ-carrageenan | Polymer electrolyte, ion removal, drug delivery | [1,2,3,9,10,11] |
| Phosphate | κ-carrageenan, chitosan, cellulose | Polymer electrolyte, medical, ion removal, drug removal | [4,12,13,14] |
| Sulfate | Artemisia sphaerocephala polysaccharide, chitosan, cellulose, alginate | Biological and medical application, ion removal | [5,15,16,17] |
| Sample | Carbon, (%) | Sulfur, (%) | Oxygen, (%) | Ratio, (C/S) | DS |
|---|---|---|---|---|---|
| κ-Carrageenan | 27.54 | 61.6 | 5.52 | 4.99 | - |
| Succinyl κ-carrageenan (4 h) | 26.94 | 62.16 | 5.17 | 5.21 | 0.06 |
| Succinyl κ-carrageenan (12 h) | 32.50 | 57.86 | 3.10 | 10.48 | 1.37 |
| Succinyl κ-carrageenan (24 h) | 33.17 | 57.88 | 2.74 | 12.10 | 1.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Bakar, M.H.; Azeman, N.H.; Mobarak, N.N.; Mokhtar, M.H.H.; A Bakar, A.A. Effect of Active Site Modification towards Performance Enhancement in Biopolymer κ-Carrageenan Derivatives. Polymers 2020, 12, 2040. https://doi.org/10.3390/polym12092040
Abu Bakar MH, Azeman NH, Mobarak NN, Mokhtar MHH, A Bakar AA. Effect of Active Site Modification towards Performance Enhancement in Biopolymer κ-Carrageenan Derivatives. Polymers. 2020; 12(9):2040. https://doi.org/10.3390/polym12092040
Chicago/Turabian StyleAbu Bakar, Mohd Hafiz, Nur Hidayah Azeman, Nadhratun Naiim Mobarak, Mohd Hadri Hafiz Mokhtar, and Ahmad Ashrif A Bakar. 2020. "Effect of Active Site Modification towards Performance Enhancement in Biopolymer κ-Carrageenan Derivatives" Polymers 12, no. 9: 2040. https://doi.org/10.3390/polym12092040
APA StyleAbu Bakar, M. H., Azeman, N. H., Mobarak, N. N., Mokhtar, M. H. H., & A Bakar, A. A. (2020). Effect of Active Site Modification towards Performance Enhancement in Biopolymer κ-Carrageenan Derivatives. Polymers, 12(9), 2040. https://doi.org/10.3390/polym12092040