Microencapsulation of Copper(II) Sulfate in Ionically Cross-Linked Chitosan by Spray Drying for the Development of Irreversible Moisture Indicators in Paper Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Copper(II) Sulfate-Loaded Chitosan Particles
2.3. Microscopy
2.4. Moisture Determination
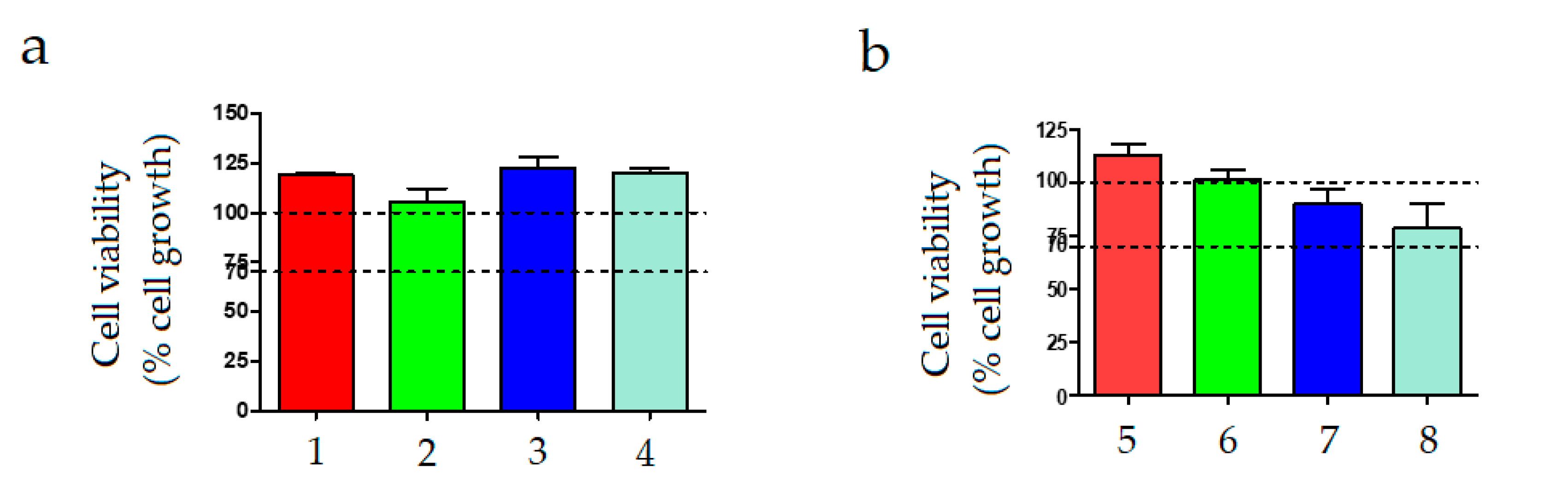
2.5. Cytotoxicity Tests
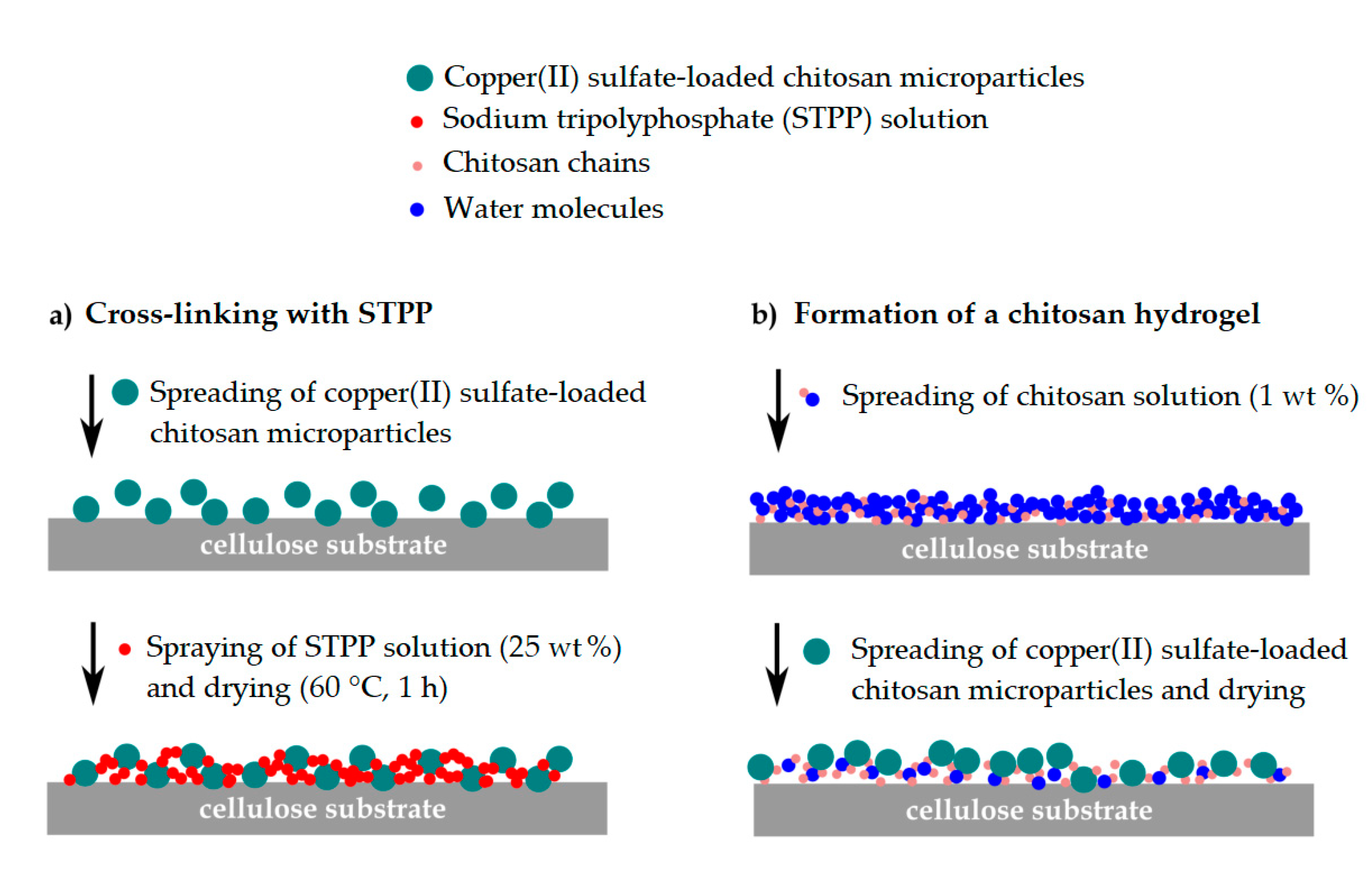
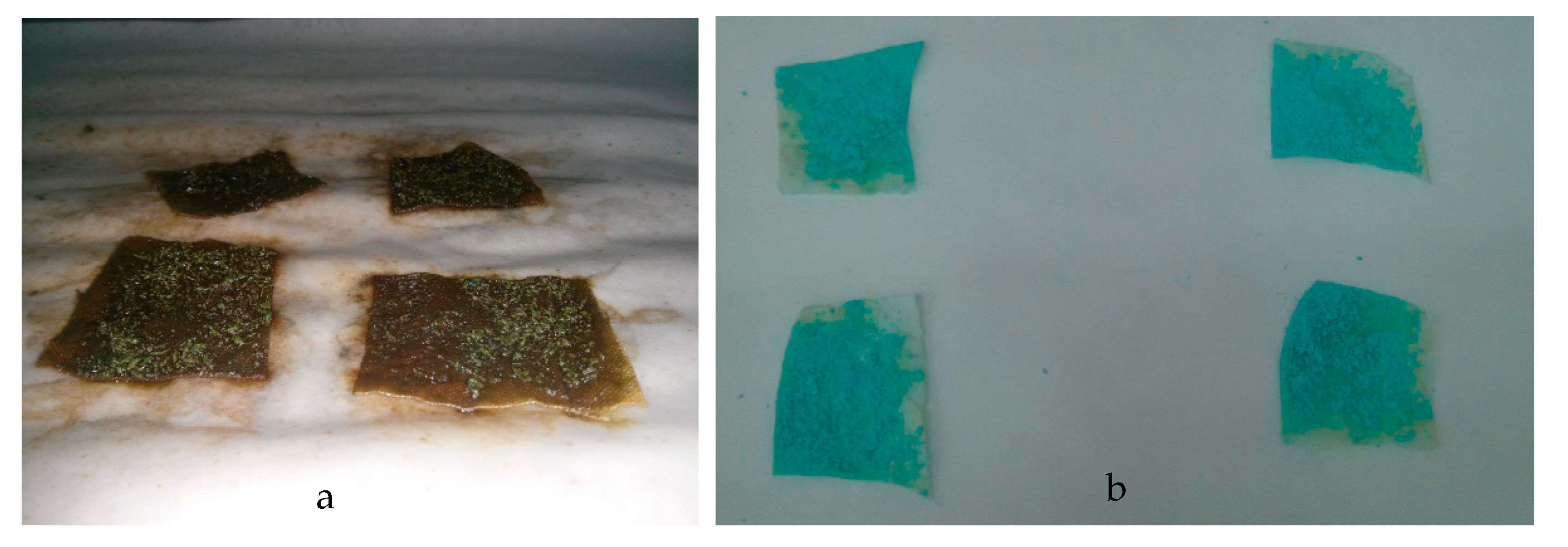
2.6. Fixation of the Copper(II) Sulfate-Loaded Chitosan Particles
2.7. Colorimetric Measurements
3. Results and Discussion
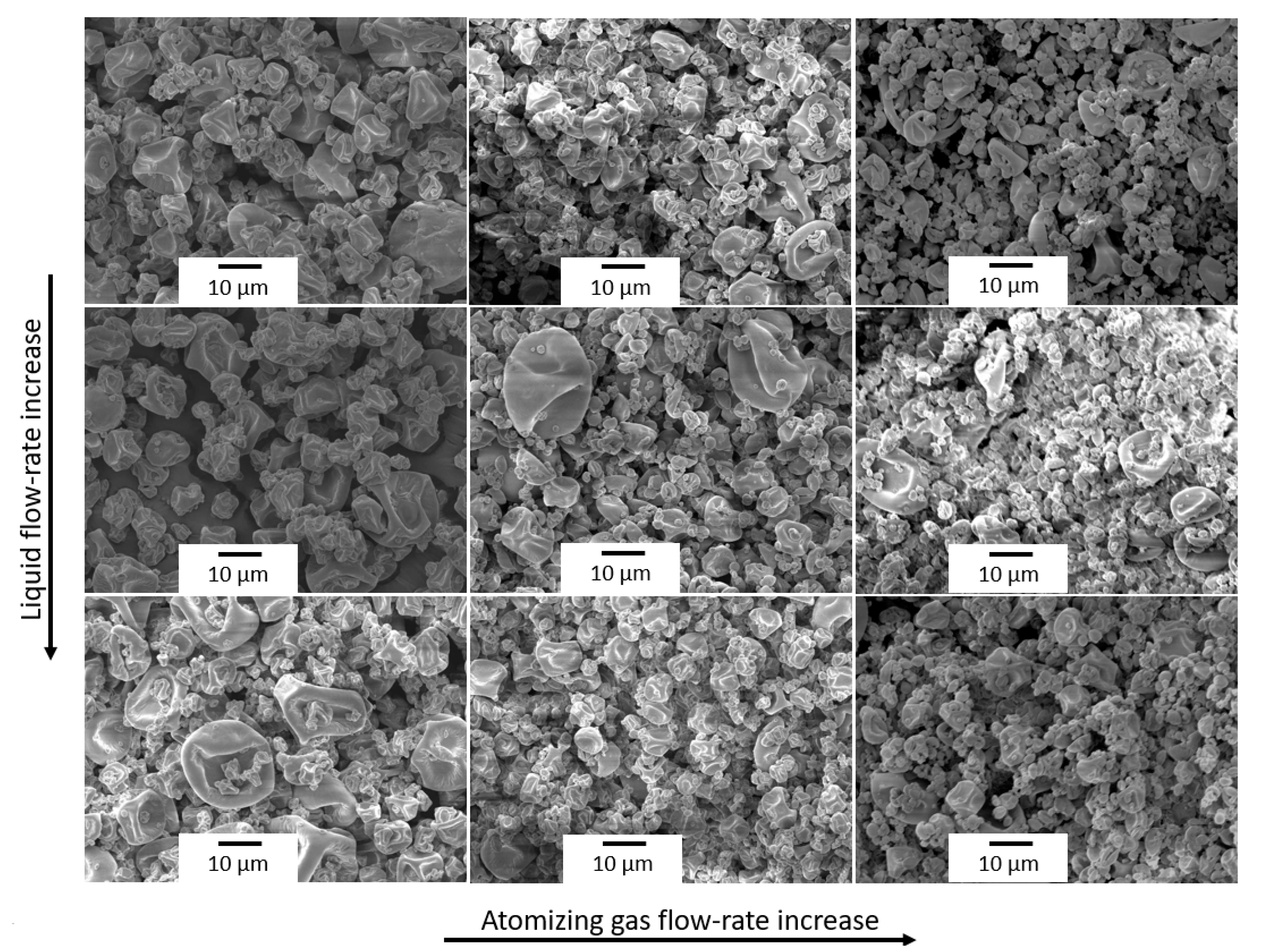
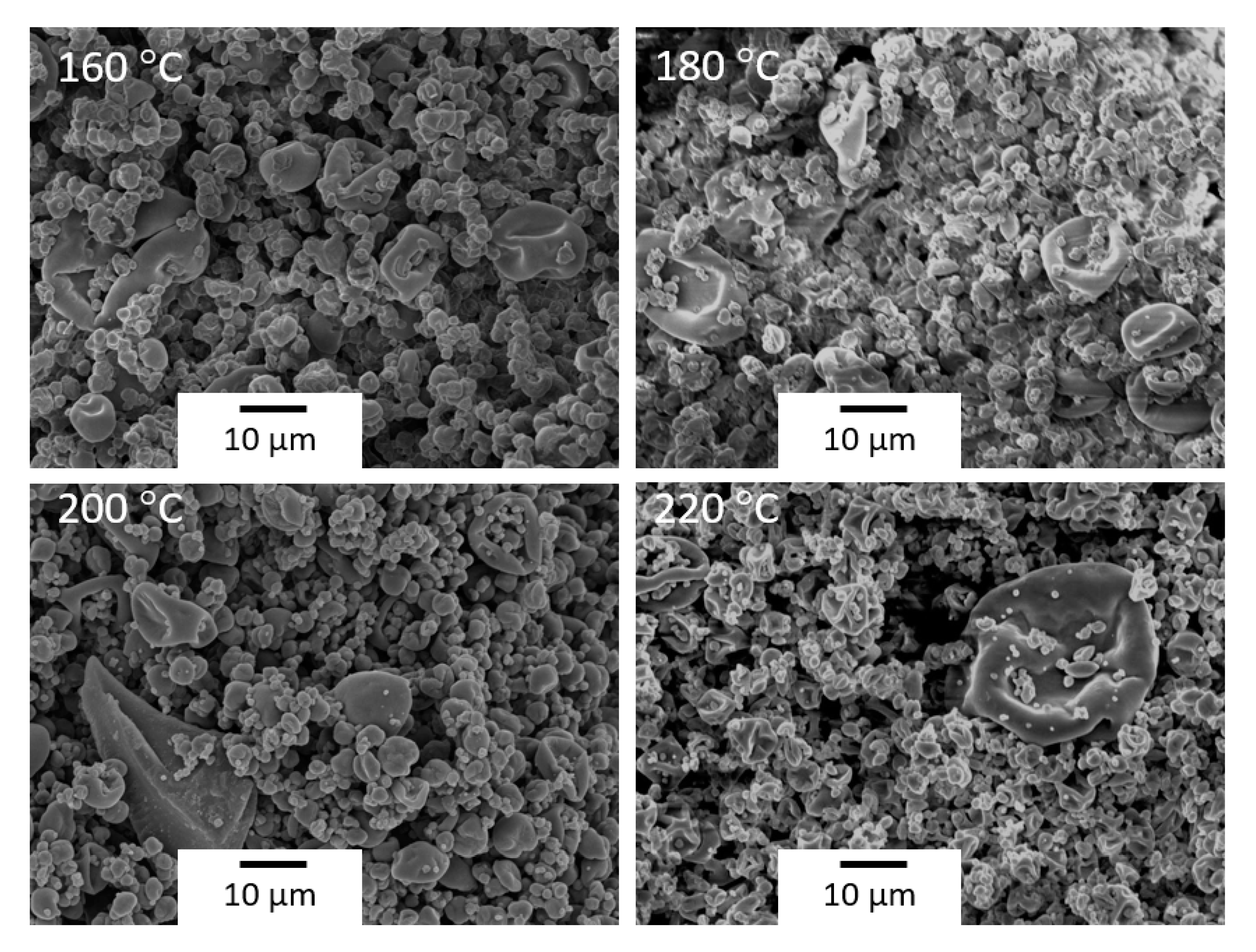
3.1. Optimization of the Spray Drying Process
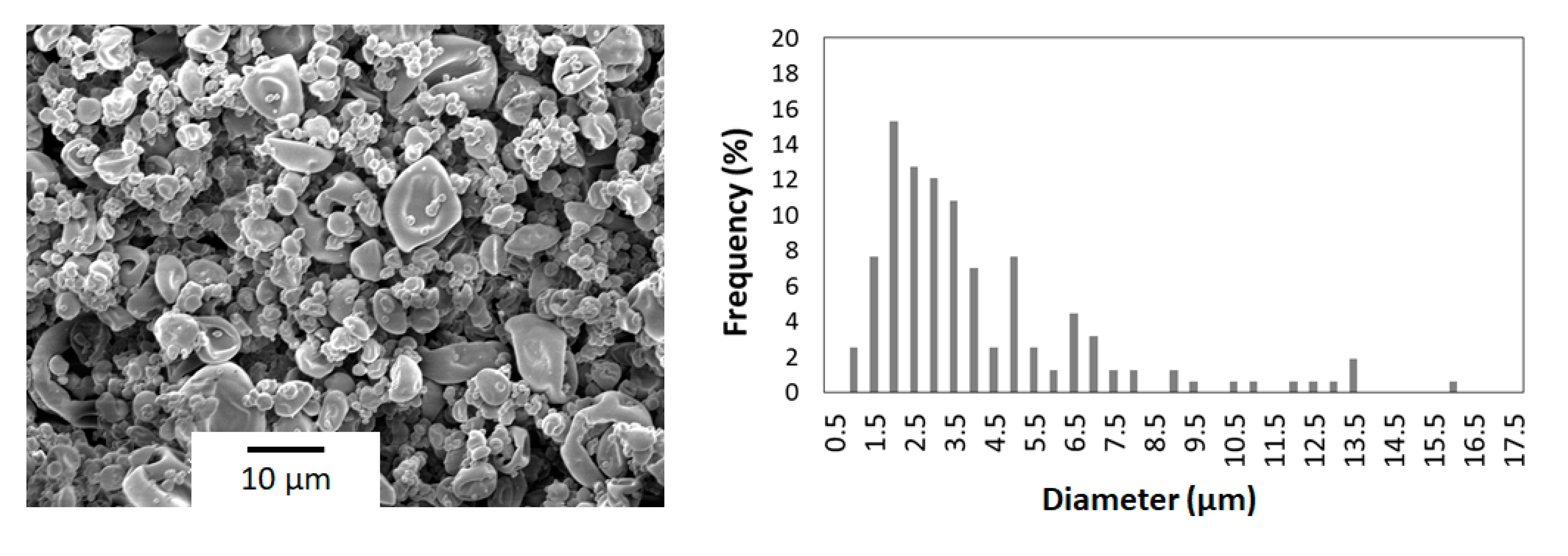
3.2. Morphological Characterization of the Chitosan Microcapsules
3.3. Cytotoxicity of the Chitosan Microcapsules
3.4. Fixation of the Chitosan Microcapsules to the Paper Substrate
3.5. Evaluation of the Paper Substrates as Moisture Indicators
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Rodrigues, E.T.; Han, J.H. Intelligent Packaging, in Encyclopedia of Agricultural and Food Engineering; Heldman, D., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 528–535. [Google Scholar]
- Torres-Giner, S.; Gil, L.; Pascual-Ramírez, L.; Garde-Belza, J.A. Packaging: Food waste reduction. In Encyclopedia of Polymer Applications; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2018; Volume 3, pp. 1990–2009. [Google Scholar]
- Unander, T.; Nilsson, H.-E. Characterization of Printed Moisture Sensors in Packaging Surveillance Applications. IEEE Sens. J. 2009, 9, 922–928. [Google Scholar] [CrossRef]
- Uesaka, T. Dimensional Stability and Environmental Effects on Paper Properties. In Handbook of Physical Testing of Paper; Mark, R.E., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 115–171. [Google Scholar]
- Xu, N.; Chen, H.; Lin, Q.; Li, Z.; Yang, T.; Yuan, Z. Selective and sensitive colorimetric determination of cobalt ions using Ag–Au bimetallic nanoparticles. RSC Adv. 2017, 7, 16295–16301. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, S.; Li, F.; Shi, J. Cellulose-based sensors for metal ions detection. Cellulose 2020, 27, 5477–5507. [Google Scholar] [CrossRef]
- De Godoi, F.C.; Rabelo, R.B.; da Vasconcellos, F.C.; Beppu, M.M. Preparation of Copper Nanoparticles in Chitosan Membranes and their Application as Irreversible Humidity Indicators. Chem. Eng. Trans. 2011, 24, 217–222. [Google Scholar]
- Chandrawat, M.P.S.; Yadav, R.N.; Sharma, S.K. Investigation of blue vitriol (copper sulphate pentahydron) as an admixture on the properties of magnesia cement: An eco-friendly approach. Rasayan. J. Chem. 2008, 1, 914–919. [Google Scholar]
- Bamfield, P. Chromic Phenomena: Technological Applications of Colour Chemistry, 3rd ed.; Royal Society of Chemistry: Cambridge, UK, 2018. [Google Scholar]
- Ferrara, M.; Bengisu, M. Materials That Change Color-Smart Materials Intelligent Design; Springer International Publishing: Heidelberg, Germany, 2014. [Google Scholar]
- Patel, B.B.; Patel, J.K.; Chakraborty, S.; Shukla, D. Revealing facts behind spray dried solid dispersion technology used for solubility enhancement. Saudi Pharm. J. 2013, 23, 352–365. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.T.H.; Wang, S.-L.; Vo, T.P.K.; Nguyen, A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2016, 43, 3527–3537. [Google Scholar] [CrossRef]
- Steenkamp, G.; Keizer, K.; Neomagus, H.; Krieg, H.M. Copper(II) removal from polluted water with alumina/chitosan composite membranes. J. Membr. Sci. 2002, 197, 147–156. [Google Scholar] [CrossRef]
- Deans, J.; Dixon, B. Uptake of Pb2+ and Cu2+ by novel biopolymers. Water Res. 1992, 26, 469–472. [Google Scholar] [CrossRef]
- Findon, A.; McKay, G.; Blair, H.S. Transport studies for the sorption of copper ions by chitosan. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1993, 28, 173–185. [Google Scholar] [CrossRef]
- Pereira, F.S.; De Souza, G.G.; Moraes, P.G.P.; Barroso, R.P.; Lanfredi, S.; Gomes, H.M.; Costa-Filho, A.; González, E.R.P. Study of chitosans interaction with Cu(II) from the corresponding sulfate and chloride salts. Cellulose 2015, 22, 2391–2407. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan- metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Rodriguez, L.B.; Avalos, A.; Chiaia, N.; Nadarajah, A. Effect of Formulation and Process Parameters on Chitosan Microparticles Prepared by an Emulsion Crosslinking Technique. AAPS PharmSciTech 2016, 18, 1084–1094. [Google Scholar] [CrossRef]
- Lim, L.-Y.; Wan, L.S.C.; Thai, P.Y. Chitosan Microspheres Prepared by Emulsification and Ionotropic Gelation. Drug Dev. Ind. Pharm. 1997, 23, 981–985. [Google Scholar] [CrossRef]
- Klein, M.P.; Hackenhaar, C.R.; Lorenzoni, A.S.; Rodrigues, R.C.; Costa, T.M.H.; Ninow, J.L.; Hertz, P.F. Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and β-d-galactosidase immobilization. Carbohydr. Polym. 2016, 137, 184–190. [Google Scholar] [CrossRef]
- Sung, H.; Huang, R.N.; Huang, L.L.H.; Tsai, C.-C. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef]
- Aral, C.; Akbuğa, J. Alternative approach to the preparation of chitosan beads. Int. J. Pharm. 1998, 168, 9–15. [Google Scholar] [CrossRef]
- Desai, K.G.; Park, H.J. Preparation and characterization of drug-loaded chitosan-tripolyphosphate microspheres by spray drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Database of Select Committee on GRAS Substrances (SCOGS) Reviews; US Food and Drug Administration: Silver Spring, MD, USA, 2006. [Google Scholar]
- Paños, I.; Acosta, N.; Heras, Á. New drug delivery systems based on chitosan. Curr. Drug Discov. Technol. 2008, 5, 333–341. [Google Scholar] [CrossRef]
- Sreekumar, S.; Lemke, P.; Moerschbacher, B.M.; Torres-Giner, S.; Lagaron, J.M. Preparation and optimization of submicron chitosan capsules by water-based electrospraying for food and bioactive packaging applications. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.A.; Park, H.J.; Hwang, S.-J.; Park, J.B.; Lee, J.S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002, 249, 165–174. [Google Scholar] [CrossRef]
- Mi, F.-L.; Sung, H.; Shyu, S.-S.; Su, C.-C.; Peng, C.-K. Synthesis and characterization of biodegradable TPP/genipin co-crosslinked chitosan gel beads. Polymer 2003, 44, 6521–6530. [Google Scholar] [CrossRef]
- Shu, X.; Zhu, K.J. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int. J. Pharm. 2000, 201, 51–58. [Google Scholar] [CrossRef]
- Aranaz, I.; Paños, I.; Peniche, C.; Heras, Á.; Acosta, N. Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride. Molecules 2017, 22, 1980. [Google Scholar] [CrossRef]
- Kašpar, O.; Jakubec, M.; Štěpánek, F. Characterization of spray dried chitosan–TPP microparticles formed by two- and three-fluid nozzles. Powder Technol. 2013, 240, 31–40. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Pokharkar, V. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: A technical note. AAPS PharmSciTech 2006, 7, E138–E143. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of ionic and covalent crosslinking agents on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- International Organization for Standardization. 10993–5: 2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- De Carvalho, F.G.; Magalhães, T.C.; Teixeira, N.M.; Gondim, B.L.C.; Carlo, H.L.; Dos Santos, R.L.; De Oliveira, A.R.; Ângelo, M.L.D. Synthesis and characterization of TPP/chitosan nanoparticles: Colloidal mechanism of reaction and antifungal effect on C. albicans biofilm formation. Mater. Sci. Eng. C 2019, 104, 109885. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Helbling, I.M.; Busatto, C.A.; Fioramonti, S.A.; Pesoa, J.I.; Santiago, L.G.; Estenoz, D.A.; Luna, J.A. Preparation of TPP-crosslinked chitosan microparticles by spray drying for the controlled delivery of progesterone intended for estrus synchronization in cattle. Pharm. Res. 2018, 35, 66. [Google Scholar] [CrossRef] [PubMed]
- Learoyd, T.P.; Burrows, J.L.; French, E.; Seville, P.C. Modified release of beclometasone dipropionate from chitosan-based spray-dried respirable powders. Powder Technol. 2008, 187, 231–238. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Y.-H.; Cheng, K.-C.; Song, Y.-L. Investigations of the Influences of Processing Conditions on the Properties of Spray Dried Chitosan-Tripolyphosphate Particles loaded with Theophylline. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2008, 20, 613–619. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Borohydride Reduction of Nickel and Copper Ions in Aqueous and Nonaqueous Media. Controllable Chemistry Leading to Nanoscale Metal and Metal Boride Particles. Langmuir 1994, 10, 4726–4730. [Google Scholar] [CrossRef]
- Leygraf, C.; Chang, T.; Herting, G.; Wallinder, I.O. The origin and evolution of copper patina colour. Corros. Sci. 2019, 157, 337–346. [Google Scholar] [CrossRef]
- Ulutan, S.; Demir, A.P.T.; Balkose, D. Use, Preparation, and Characterization of Copper-Containing Silica Gel. Ind. Eng. Chem. Res. 2020, 59, 9939–9949. [Google Scholar] [CrossRef]



| Sample | Chitosan (g) | STTP (mL) | STTP/Chitosan Ratio (mL/g) |
|---|---|---|---|
| 1 | 0.25 | 1.25 | 5 |
| 2 | 0.25 | 2.50 | 10 |
| 3 | 0.25 | 3.75 | 15 |
| 4 | 0.25 | 5.00 | 20 |
| 5 | 0.50 | 2.50 | 5 |
| 6 | 0.50 | 5.00 | 10 |
| 7 | 0.50 | 7.50 | 15 |
| 8 | 0.50 | 10.00 | 20 |
| Inlet Temperature (°C) | Atomizing Gas Flow-Rate (nL/h) | Liquid Flow-Rate (mL/h) | Aspiration Rate (%) | Yield (%) | Moisture (%) |
|---|---|---|---|---|---|
| 160 | 538 | 290 | 80 | 42.73 ± 2.23 | 6.87 ± 0.32 |
| 363 | 40.45 ± 3.67 | 7.99 ± 0.67 | |||
| 290 | 90 | 30.30 ± 3.05 | 8.43 ± 1.03 | ||
| 363 | 43.64 ± 4.14 | 5.50 ± 0.88 | |||
| 667 | 290 | 80 | 46.52 ± 3.54 | 11.34 ± 0.98 | |
| 363 | 45.61 ± 4.70 | 7.33 ± 0.72 | |||
| 290 | 90 | 46.82 ± 2.46 | 10.02 ± 0.54 | ||
| 363 | 52.57 ± 2.20 | 6.17 ± 0.33 | |||
| 180 | 439 | 213 | 70 | 26.97 ± 2.01 | 11.30 ± 0.27 |
| 290 | 23.09 ± 2.24 | 15.24 ± 1.12 | |||
| 363 | 40.91 ± 1.87 | 10.95 ± 0.98 | |||
| 213 | 80 | 40.00 ± 2.26 | 11.50 ± 1.36 | ||
| 290 | 46.17 ± 3.03 | 10.49 ± 0.74 | |||
| 363 | 26.29 ± 1.23 | 21.24 ± 0.54 | |||
| 213 | 90 | 33.37 ± 1.41 | 10.40 ± 0.75 | ||
| 290 | 36.57 ± 1.20 | 11.49 ± 1.00 | |||
| 363 | 25.37 ± 1.96 | 13.45 ± 1.03 | |||
| 538 | 213 | 70 | 52.80 ± 3.08 | 11.61 ± 0.98 | |
| 290 | 46.63 ± 2.79 | 9.15 ± 0.54 | |||
| 363 | 44.11 ± 3.25 | 9.79 ± 0.76 | |||
| 213 | 80 | 49.60 ± 2.68 | 9.29 ± 1.12 | ||
| 290 | 48.00 ± 3.10 | 9.33 ± 0.97 | |||
| 363 | 45.71 ± 2.41 | 8.15 ± 1.08 | |||
| 213 | 90 | 69.26 ± 4.20 | 7.99 ± 0.44 | ||
| 290 | 38.17 ± 2.98 | 6.81 ± 0.77 | |||
| 363 | 39.31 ± 3.02 | 10.29 ± 0.36 | |||
| 667 | 213 | 70 | 34.06 ± 1.87 | 11.09 ± 0.33 | |
| 290 | 49.83 ± 3.12 | 9.72 ± 0.78 | |||
| 363 | 33.00 ± 2.98 | 12.46 ± 1.54 | |||
| 213 | 80 | 67.20 ± 1.00 | 8.99 ± 0.89 | ||
| 290 | 72.00 ± 3.32 | 8.89 ± 0.21 | |||
| 363 | 62.63 ± 2.49 | 9.74 ± 1.20 | |||
| 213 | 90 | 70.11 ± 3.88 | 14.91 ± 0.65 | ||
| 290 | 66.97 ± 4.57 | 7.83 ± 1.01 | |||
| 363 | 50.74 ± 3.23 | 8.67 ± 0.43 | |||
| 200 | 667 | 213 | 80 | 41.21 ± 2.01 | 6.03 ± 0.26 |
| 290 | 39.24 ± 3.22 | 6.81 ± 0.87 | |||
| 363 | 40.45 ± 2.89 | 7.53 ± 0.22 | |||
| 213 | 90 | 34.39 ± 2.45 | 7.04 ± 1.04 | ||
| 290 | 35.91 ± 1.89 | 4.86 ± 0.83 | |||
| 363 | 34.09 ± 2.40 | 6.67 ± 1.36 | |||
| 220 | 667 | 213 | 80 | 39.24 ± 3.02 | 5.27 ± 0.44 |
| 290 | 33.64 ± 2.41 | 7.46 ± 0.80 | |||
| 363 | 32.42 ± 1.09 | 6.54 ± 0.56 | |||
| 213 | 90 | 39.55 ± 2.08 | 5.83 ± 0.67 | ||
| 290 | 44.24 ± 1.67 | 5.58 ± 0.71 | |||
| 363 | 41.67 ± 3.32 | 5.50 ± 0.58 |
| Test | RH (%) | L | a* | b* |
|---|---|---|---|---|
| 1 | 0 | 54.33 ± 2.32 | 3.08 ± 0.49 | 9.55 ± 0.70 |
| 2 | 15 | 59.98 ± 1.89 | −17.81 ± 1.03 | 9.63 ± 0.82 |
| 3 | 60 | 60.01 ± 2.04 | −18.76 ± 1.16 | 13.39 ± 1.75 |
| 4 | 100 | 61.86 ± 2.04 | −25.43 ± 2.07 | 27.65 ± 1.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Lema, S.; Terol, J.; Fages, E.; Balart, R.; Quiles-Carrillo, L.; Prieto, C.; Torres-Giner, S. Microencapsulation of Copper(II) Sulfate in Ionically Cross-Linked Chitosan by Spray Drying for the Development of Irreversible Moisture Indicators in Paper Packaging. Polymers 2020, 12, 2039. https://doi.org/10.3390/polym12092039
Rojas-Lema S, Terol J, Fages E, Balart R, Quiles-Carrillo L, Prieto C, Torres-Giner S. Microencapsulation of Copper(II) Sulfate in Ionically Cross-Linked Chitosan by Spray Drying for the Development of Irreversible Moisture Indicators in Paper Packaging. Polymers. 2020; 12(9):2039. https://doi.org/10.3390/polym12092039
Chicago/Turabian StyleRojas-Lema, Sandra, Jorge Terol, Eduardo Fages, Rafael Balart, Luis Quiles-Carrillo, Cristina Prieto, and Sergio Torres-Giner. 2020. "Microencapsulation of Copper(II) Sulfate in Ionically Cross-Linked Chitosan by Spray Drying for the Development of Irreversible Moisture Indicators in Paper Packaging" Polymers 12, no. 9: 2039. https://doi.org/10.3390/polym12092039
APA StyleRojas-Lema, S., Terol, J., Fages, E., Balart, R., Quiles-Carrillo, L., Prieto, C., & Torres-Giner, S. (2020). Microencapsulation of Copper(II) Sulfate in Ionically Cross-Linked Chitosan by Spray Drying for the Development of Irreversible Moisture Indicators in Paper Packaging. Polymers, 12(9), 2039. https://doi.org/10.3390/polym12092039









