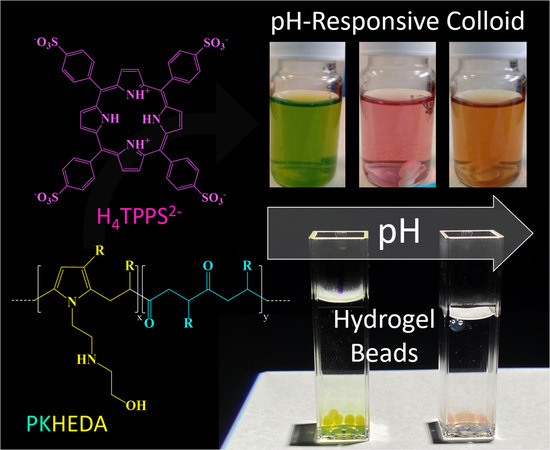
pH-Responsive Polyketone/5,10,15,20-Tetrakis-(Sulfonatophenyl)Porphyrin Supramolecular Submicron Colloidal Structures
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
2.3. Surface Activity Study of Functional Polyketones
2.4. Solubility Studies of Functional Polyketones
2.5. Study of PKHEDA/TPPS Colloids
2.6. Ultrafiltration
2.7. Colloidal Stability
2.8. Calcium Alginate Sensor Beads
3. Result and Discussion
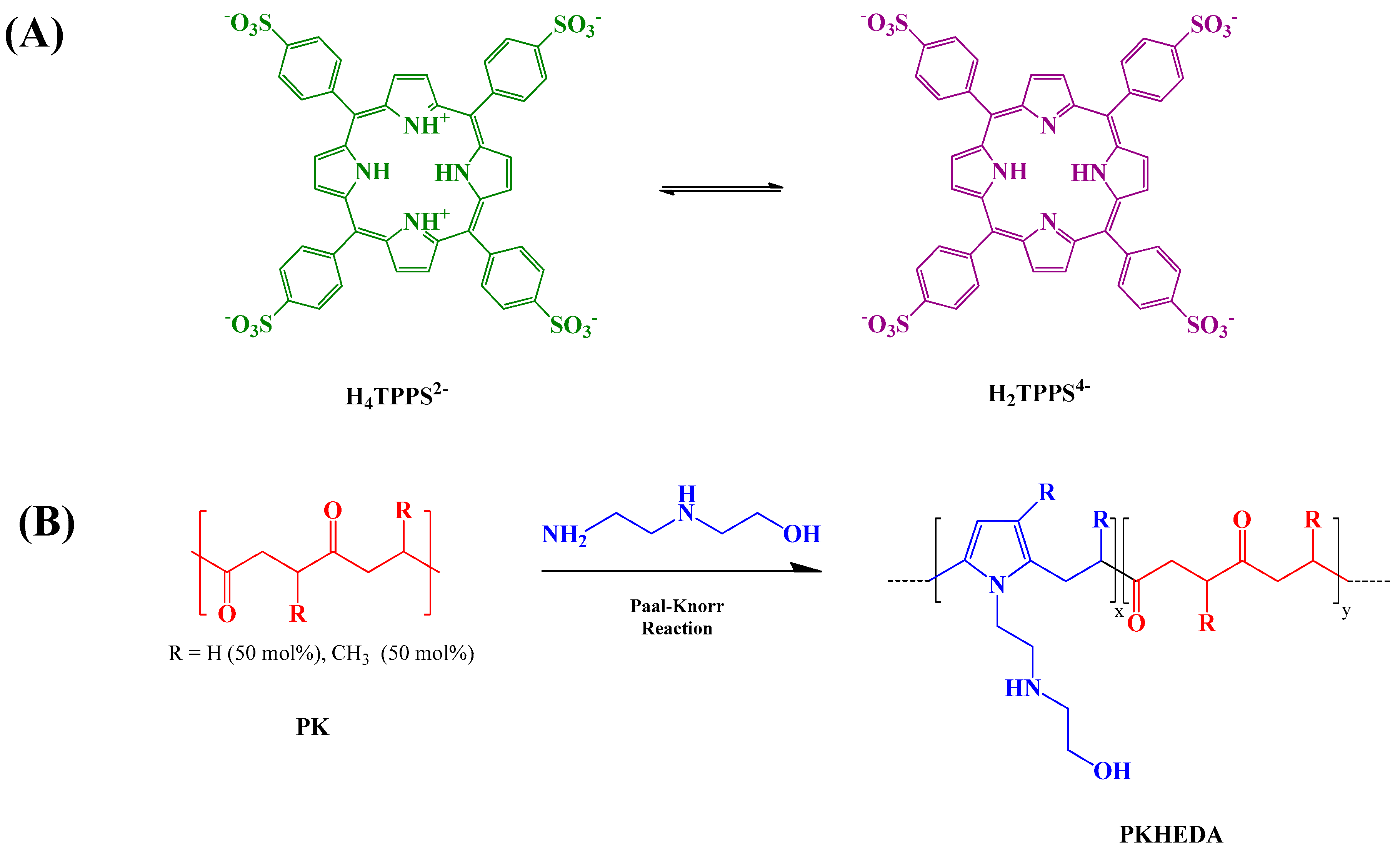
3.1. Polyketone Modification
3.2. Surface Tension and Self-Aggregation Process of PKHEDA
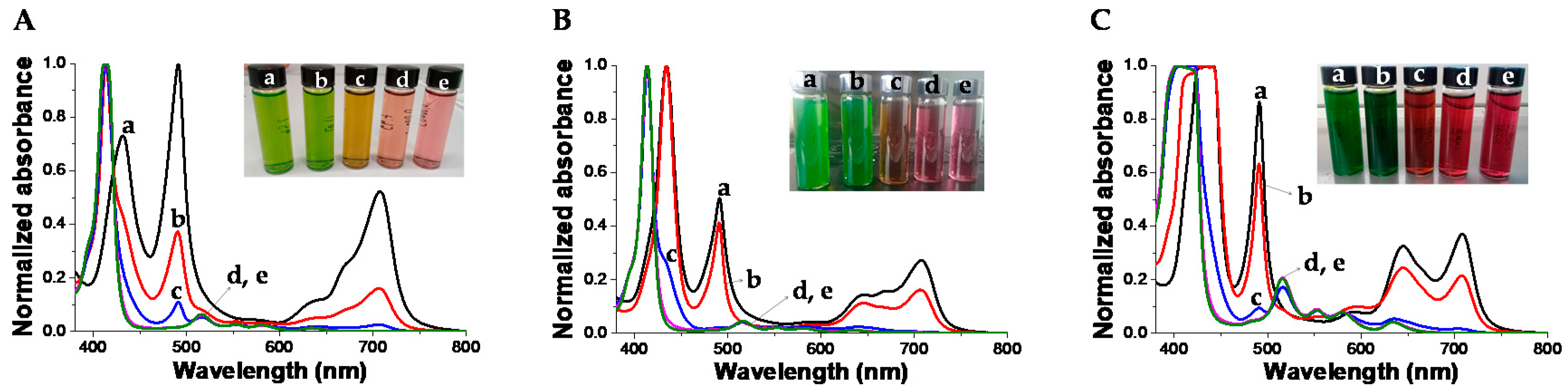
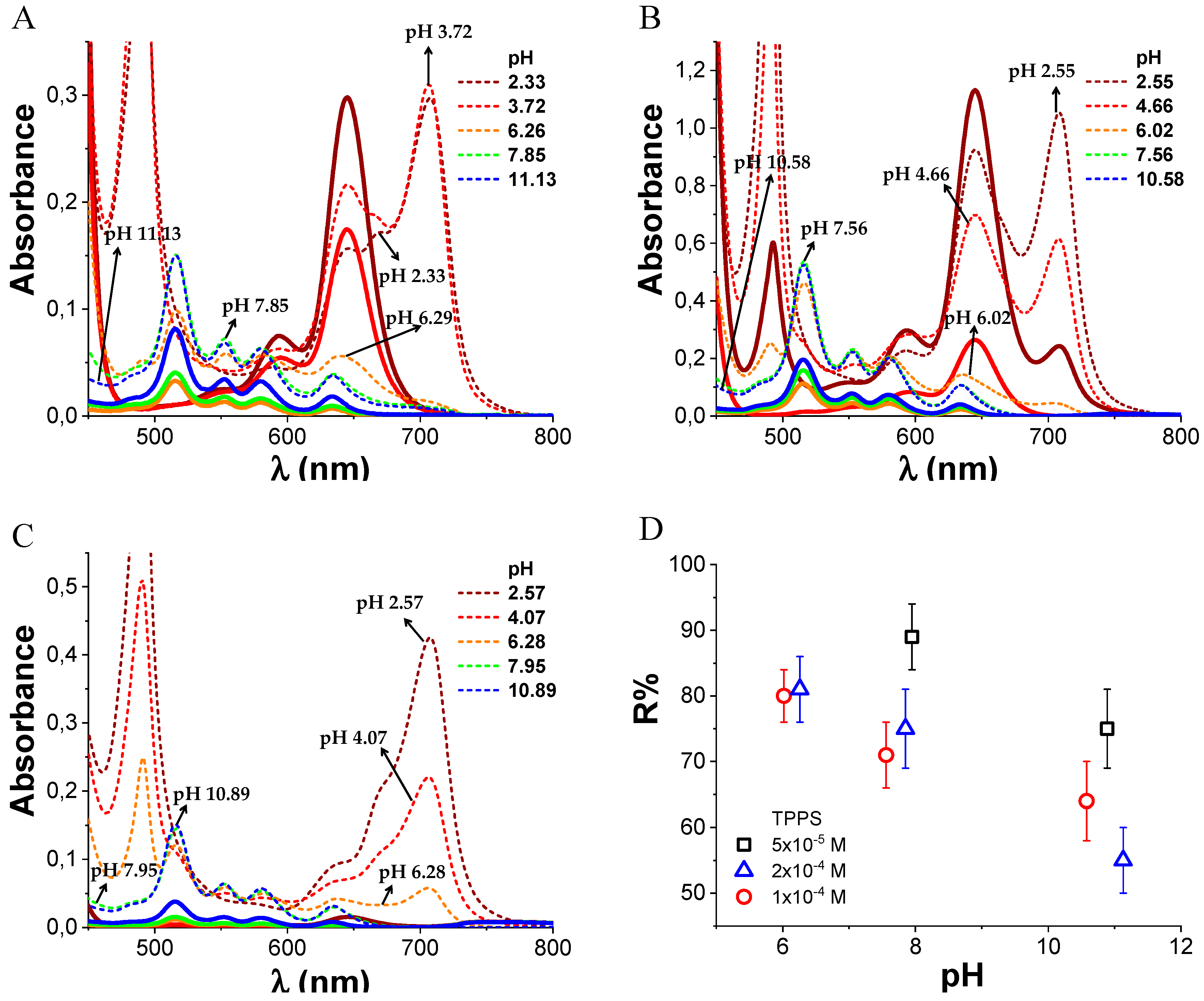
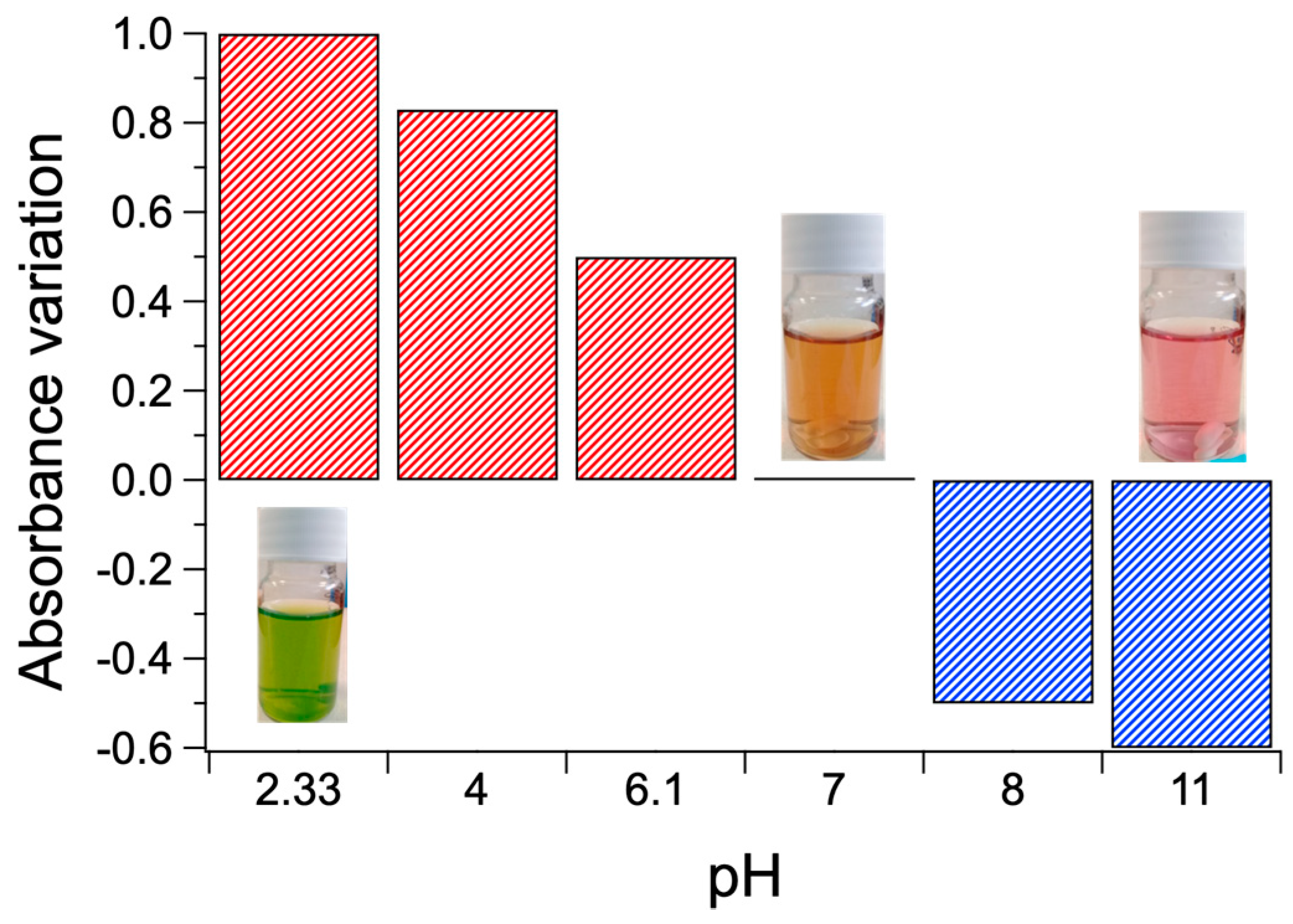
3.3. PKHEDA/TPPS Colloids
3.4. Ultrafiltration Studies
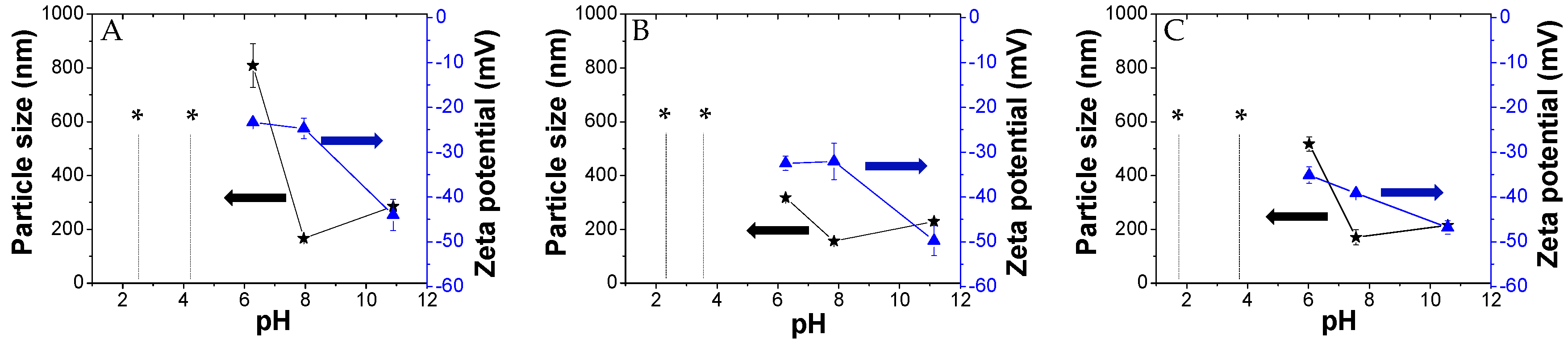
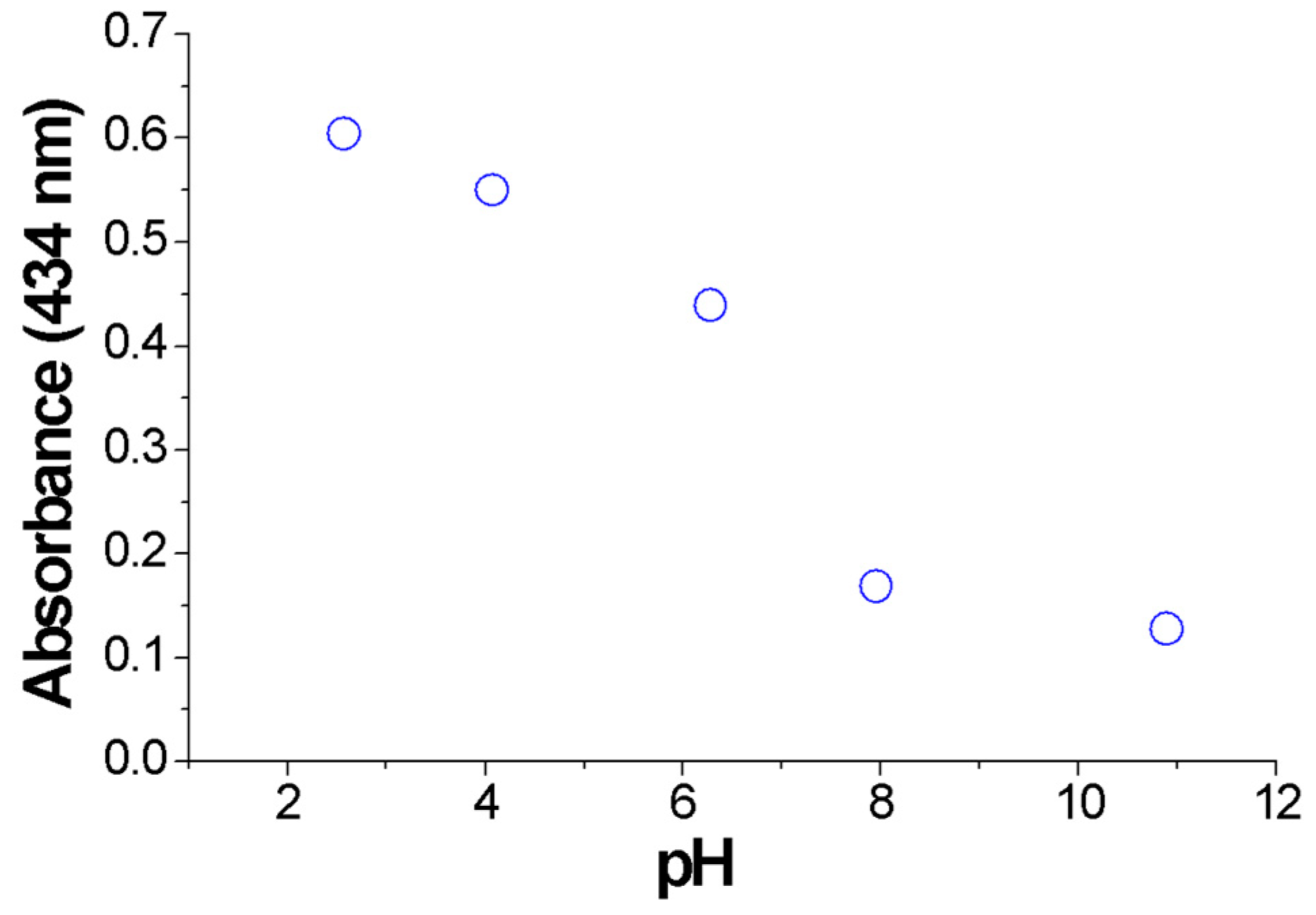
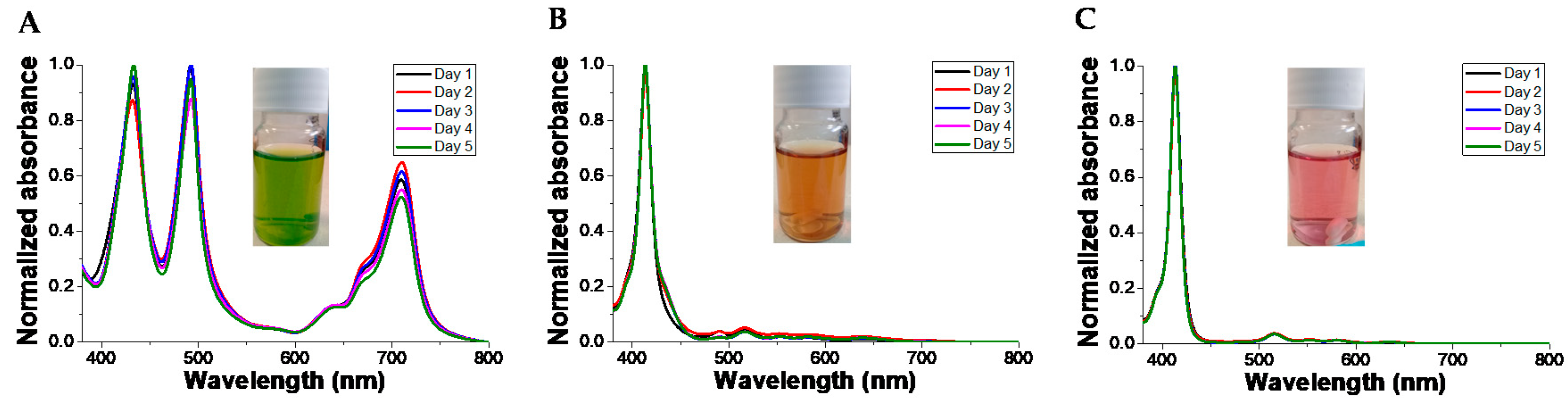
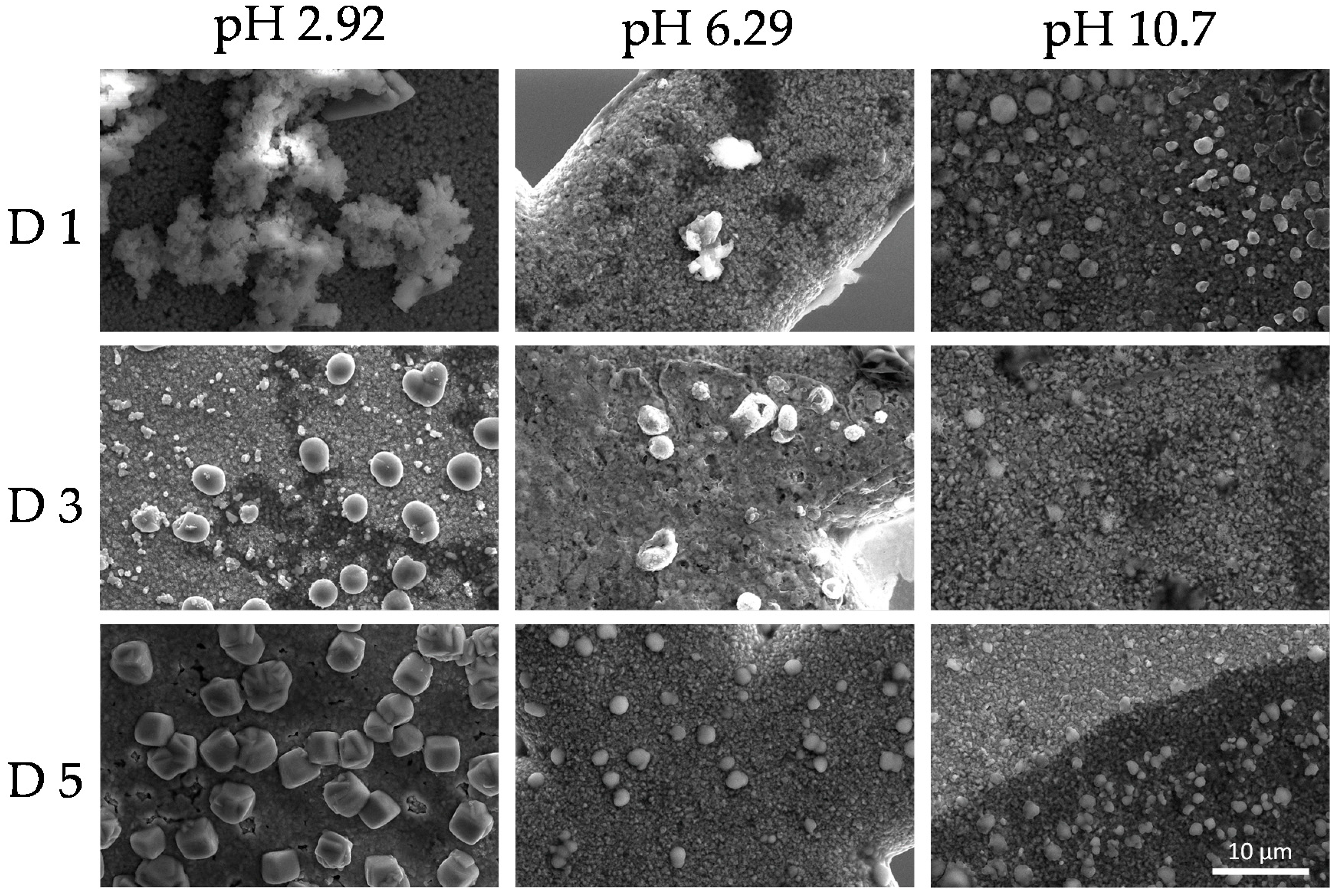
3.5. Colloid Stability
3.6. Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lehn, J.M. Cryptates: Inclusion complexes of macropolycyclic receptor molecules. Pure Appl. Chem. 1978, 50, 871–892. [Google Scholar] [CrossRef]
- Albrecht, M. Supramolecular chemistry—General principles and selected examples from anion recognition and metallosupramolecular chemistry. Naturwissenschaften 2007, 94, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Boekhoven, J.; Stupp, S.I. 25th anniversary article: Supramolecular materials for regenerative medicine. Adv. Mater. 2014, 26, 1642–1659. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tang, B.; Liu, Y.; Gao, Q.; Wang, Z.; Zhang, X. The fabrication of a supra-amphiphile for dissipative self-assembly. Chem. Sci. 2016, 7, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Gröhn, F.; Klein, K.; Koynov, K. A novel type of vesicles based on ionic and π–π interactions. Macromol. Rapid Commun. 2010, 31, 75–80. [Google Scholar] [CrossRef]
- Li, Q.; Luo, L.; Yan, X.; Zhou, W.; Wang, F. Ionic self-assembled fluorescent microfibres with electrochemical properties. Supramol. Chem. 2014, 26, 358–362. [Google Scholar] [CrossRef]
- Serpe, M.J.; Craig, S.L. Physical organic chemistry of supramolecular polymers. Langmuir 2007, 23, 1626–1634. [Google Scholar] [CrossRef]
- Lehn, J.-M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763. [Google Scholar] [CrossRef]
- Ruthard, C.; Maskos, M.; Yildiz, H.; Gröhn, F. Association of a cylindrical polyelectrolyte brush with tetravalent counterions. Macromol. Rapid Commun. 2011, 32, 523–527. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, Y.S. Organic nanophotonics: From controllable assembly of functional molecules to low-dimensional materials with desired photonic properties. Chem. Soc. Rev. 2014, 43, 4325–4340. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, R.; Shi, L. Cooperative macromolecular self-assembly toward polymeric assemblies with multiple and bioactive functions. Account. Chem. Res. 2014, 47, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Shibu, E.S.; Sonoda, A.; Tao, Z.; Feng, Q.; Furube, A.; Masuo, S.; Wang, L.; Tamai, N.; Ishikawa, M.; Biju, V. Energy materials: Supramolecular nanoparticles for solar energy harvesting. Nano Rev. 2013, 4, 21079. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T.; Murata, H.; Fukuzumi, S.; Kamat, P.V. Porphyrin-based molecular architectures for light energy conversion. Mol. Cryst. Liq. Cryst. 2007, 471, 39–51. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Wang, R.; Xu, H.; Chi, B. Supramolecular assemblies of histidinylated β-cyclodextrin for enhanced oligopeptide delivery into osteoclast precursors. J. Biomater. Sci. Polym. Ed. 2016, 27, 490–504. [Google Scholar] [CrossRef]
- Frühbeißer, S.; Gröhn, F. Catalytic activity of macroion–porphyrin nanoassemblies. J. Am. Chem. Soc. 2012, 134, 14267–14270. [Google Scholar] [CrossRef]
- Ruthard, C.; Schmidt, M.; Gröhn, F. Porphyrin–polymer networks, worms, and nanorods: pH-triggerable hierarchical self-assembly. Macromol. Rapid Commun. 2011, 32, 706–711. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Y.; Zheng, L.; Dai, C. Construction of supramolecular self-assembled microfibers with fluorescent properties through a modified Ionic Self-Assembly (ISA) strategy. Chem. A Eur. J. 2013, 19, 1076–1081. [Google Scholar] [CrossRef]
- Faul, C.F.J.; Antonietti, M. Ionic self-assembly: Facile synthesis of supramolecular materials. Adv. Mater. 2003, 15, 673–683. [Google Scholar] [CrossRef]
- Velichkova, R.S.; Christova, D.C. Amphiphilic polymers from macromonomers and telechelics. Prog. Polym. Sci. 1995, 20, 819–887. [Google Scholar] [CrossRef]
- Jun, H.; Le Kim, T.H.; Han, S.W.; Seo, M.; Kim, J.W.; Nam, Y.S. Polyglycerol-poly(ε-caprolactone) block copolymer as a new semi-solid polymeric emulsifier to stabilize O/W nanoemulsions. Colloid Polym. Sci. 2015, 293, 2949–2956. [Google Scholar] [CrossRef]
- Kronberg, B.; Holmberg, K.; Lindman, B. Types of surfactants, their synthesis, and applications. In Surface Chemistry of Surfactants and Polymers; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–47. [Google Scholar] [CrossRef]
- Laschewsky, A. Molecular concepts, self-organisation and properties of polysoaps. In Polysoaps/Stabilizers/Nitrogen-15 NMR; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–86. [Google Scholar] [CrossRef]
- Tadros, T. Polymeric surfactants in disperse systems. Adv. Colloid Interface Sci. 2009, 147–148, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Khristov, K.; Czarnecki, J. Emulsion films stabilized by natural and polymeric surfactants. Curr. Opin. Colloid Interface Sci. 2010, 15, 324–329. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Cheng, D.; Wang, Y.; Shuai, X. A pH-sensitive micelle for codelivery of siRNA and doxorubicin to hepatoma cells. Polymer 2014, 55, 3217–3226. [Google Scholar] [CrossRef]
- Yi, Y.; Lin, G.; Chen, S.; Liu, J.; Zhang, H.; Mi, P. Polyester micelles for drug delivery and cancer theranostics: Current achievements, progresses and future perspectives. Mater. Sci. Eng. C 2018, 83, 218–232. [Google Scholar] [CrossRef]
- Solov’eva, A.B.; Aksenova, N.A.; Glagolev, N.N.; Melik-Nubarov, N.S.; Ivanov, A.V.; Volkov, V.I.; Chernyak, A.V. Amphiphilic polymers in photodynamic therapy. Russ. J. Phys. Chem. B 2012, 6, 433–440. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Amphiphilic copolymers based on PEG-acrylate as surface active water viscosifiers: Towards new potential systems for enhanced oil recovery. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef]
- Barakat, Y.; Gendy, T.S.; Basily, I.K.; Mohamad, A.I. Polymeric surfactants for enhanced oil recovery. Part II—The hlb-cmc relationship of ethoxylated alkylphenol-formaldehyde polymeric surfactants. Br. Polym. J. 1989, 21, 451–457. [Google Scholar] [CrossRef]
- Migliore, N.; Picchioni, F.; Raffa, P. The effect of macromolecular structure on the rheology and surface properties of amphiphilic random polystyrene-r-poly(meth)acrylate copolymers prepared by RDRP. Soft Matter 2020, 16, 2836–2846. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Raffa, P.; Picchioni, F.; Broekhuis, A.A. Acrylamide homopolymers and acrylamide–N-isopropylacrylamide block copolymers by atomic transfer radical polymerization in water. Macromolecules 2012, 45, 4040–4045. [Google Scholar] [CrossRef][Green Version]
- Raffa, P.; Brandenburg, P.; Wever, D.A.Z.; Broekhuis, A.A.; Picchioni, F. Polystyrene–poly(sodium methacrylate) amphiphilic block copolymers by ATRP: Effect of structure, pH, and ionic strength on rheology of aqueous solutions. Macromolecules 2013, 46, 7106–7111. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition−fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Chong, Y.K.; Le, T.P.T.; Moad, G.; Rizzardo, E.; Thang, S.H. A more versatile route to block copolymers and other polymers of complex architecture by living radical polymerization: The RAFT process. Macromolecules 1999, 32, 2071–2074. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Yeow, J.; Chapman, R.; Gormley, A.J.; Boyer, C. Up in the air: Oxygen tolerance in controlled/living radical polymerisation. Chem. Soc. Rev. 2018, 47, 4357–4387. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Catalán-Toledo, J.; Muñoz-Suescun, F.; Oyarzun-Ampuero, F.; Raffa, P.; Polgar, L.M.; Picchioni, F.; Moreno-Villoslada, I. Totally organic redox-active pH-sensitive nanoparticles stabilized by amphiphilic aromatic polyketones. J. Phys. Chem. B 2018, 122, 1747–1755. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Broekhuis, A.A.; Picchioni, F. Reversible polymer networks containing covalent and hydrogen bonding interactions. Eur. Polym. J. 2014, 50, 127–134. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Fortunato, G.; Pucci, A.; Raffa, P.; Polgar, L.; Broekhuis, A.A.; Pourhossein, P.; Lima, G.M.R.; Beljaars, M.; Picchioni, F. Thermally reversible rubber-toughened thermoset networks via Diels–Alder chemistry. Eur. Polym. J. 2016, 74, 229–240. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Lima, G.M.R.; Raffa, P.; Fortunato, G.; Pucci, A.; Flores, M.E.; Moreno-Villoslada, I.; Broekhuis, A.A.; Picchioni, F. Intrinsic self-healing thermoset through covalent and hydrogen bonding interactions. Eur. Polym. J. 2016, 81, 186–197. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Pucci, A.; Araya-Hermosilla, E.; Pescarmona, P.P.; Raffa, P.; Polgar, L.M.; Moreno-Villoslada, I.; Flores, M.; Fortunato, G.; Broekhuis, A.A.; et al. An easy synthetic way to exfoliate and stabilize MWCNTs in a thermoplastic pyrrole-containing matrix assisted by hydrogen bonds. RSC Adv. 2016, 6, 85829–85837. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Pucci, A.; Raffa, P.; Santosa, D.; Pescarmona, P.P.; Gengler, Y.N.R.; Rudolf, P.; Moreno-Villoslada, I.; Picchioni, F. Electrically-responsive reversible polyketone/MWCNT network through diels-alder chemistry. Polymers 2018, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Migliore, N.; Polgar, M.L.; Araya-Hermosilla, R.; Picchioni, F.; Raffa, P.; Pucci, A. Effect of the polyketone aromatic pendent groups on the electrical conductivity of the derived MWCNTs-based nanocomposites. Polymers 2018, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Raffa, P.; Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymeric surfactants: Synthesis, properties, and links to applications. Chem. Rev. 2015, 115, 8504–8563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Aqueous polymer emulsions by chemical modifications of thermosetting alternating polyketones. J. Appl. Polym. Sci. 2007, 106, 3237–3247. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Stuart, M.C.A.; Picchioni, F. Polymeric amines by chemical modifications of alternating aliphatic polyketones. J. Appl. Polym. Sci. 2008, 107, 262–271. [Google Scholar] [CrossRef]
- Hamarneh, A.I.; Heeres, H.J.; Broekhuis, A.A.; Sjollema, K.A.; Zhang, Y.; Picchioni, F. Use of soy proteins in polyketone-based wood adhesives. Int. J. Adhes. Adhes. 2010, 30, 626–635. [Google Scholar] [CrossRef]
- Toncelli, C.; Schoonhoven, M.-J.; Broekhuis, A.A.; Picchioni, F. Paal-Knorr kinetics in waterborne polyketone-based formulations as modulating cross-linking tool in electrodeposition coatings. Mater. Des. 2016, 108, 718–724. [Google Scholar] [CrossRef]
- Drain, C.M.; Hupp, J.T.; Suslick, K.S.; Wasielewski, M.R.; Chen, X. A perspective on four new porphyrin-based functional materials and devices. J. Porphyr. Phthalocyanines 2002, 06, 243–258. [Google Scholar] [CrossRef]
- Gouterman, M. Study of the effects of substitution on the absorption spectra of porphin. J. Chem. Phys. 1959, 30, 1139–1161. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Prigorchenko, E.; Ustrnul, L.; Borovkov, V.; Aav, R. Heterocomponent ternary supramolecular complexes of porphyrins: A review. J. Porphyr. Phthalocyanines 2019, 23, 1308–1325. [Google Scholar] [CrossRef]
- Schwab, A.D.; Smith, D.E.; Bond-Watts, B.; Johnston, D.E.; Hone, J.; Johnson, A.T.; de Paula, J.C.; Smith, W.F. Photoconductivity of self-assembled porphyrin nanorods. Nano Lett. 2004, 4, 1261–1265. [Google Scholar] [CrossRef]
- Komagoe, K.; Tamagake, K.; Katsu, T. The influence of aggregation of porphyrins on the efficiency of photogeneration of hydrogen peroxide in aqueous solution. Chem. Pharm. Bull. 2006, 54, 1004–1009. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lapes, M.; Petera, J.; Jirsa, M. Photodynamic therapy of cutaneous metastases of breast cancer after local application of meso-tetra-(para-sulphophenyl)-porphin (TPPS4). J. Photochem. Photobiol. B 1996, 36, 205–207. [Google Scholar] [CrossRef]
- Binder, S.; Kolarova, H.; Tomankova, K.; Bajgar, R.; Daskova, A.; Mosinger, J. Phototoxic effect of TPPS4 and MgTPPS4 on DNA fragmentation of HeLa cells. Toxicol. Vitr. 2011, 25, 1169–1172. [Google Scholar] [CrossRef]
- Canaparo, R.; Varchi, G.; Ballestri, M.; Foglietta, F.; Sotgiu, G.; Guerrini, A.; Francovich, A.; Civera, P.; Frairia, R.; Serpe, L. Polymeric nanoparticles enhance the sonodynamic activity of meso-tetrakis (4-sulfonatophenyl) porphyrin in an in vitro neuroblastoma model. Int. J. Nanomed. 2013, 8, 4247–4263. [Google Scholar] [CrossRef]
- Grinstaff, M.W.; Hill, M.G.; Labinger, J.A.; Gray, H.B. Mechanism of catalytic oxygenation of alkanes by halogenated iron porphyrins. Science 1994, 264, 1311. [Google Scholar] [CrossRef]
- Tian, J.; Liu, S.; Liu, Z.; Yang, J.; Zhu, J.; Qiao, M.; Hu, X. Fluorescence quenching and spectrophotometric methods for the determination of daunorubicin with meso-tera (4-sulphophenyl) porphyrin as probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 120, 7–13. [Google Scholar] [CrossRef]
- Caselli, M. Porphyrin-based electrostatically self-assembled multilayers as fluorescent probes for mercury(ii) ions: A study of the adsorption kinetics of metal ions on ultrathin films for sensing applications. RSC Adv. 2015, 5, 1350–1358. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Liu, M.; Zhang, Y.; Wu, C.; Zhang, Y. Porphyrin-functionalized porous polysulfone membrane towards an optical sensor membrane for sorption and detection of cadmium(II). J. Hazard. Mater. 2016, 301, 233–241. [Google Scholar] [CrossRef]
- Ishida, Y.; Shimada, T.; Masui, D.; Tachibana, H.; Inoue, H.; Takagi, S. Efficient excited energy transfer reaction in clay/porphyrin complex toward an artificial light-harvesting system. J. Am. Chem. Soc. 2011, 133, 14280–14286. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, L.P.F.; Borissevitch, I.E. On the dynamics of the TPPS4 aggregation in aqueous solutions: Successive formation of H and J aggregates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 63, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Egawa, Y.; Hayashida, R.; Anzai, J.-i. pH-Induced Interconversion between J-Aggregates and H-Aggregates of 5,10,15,20-Tetrakis(4-sulfonatophenyl)porphyrin in Polyelectrolyte Multilayer Films. Langmuir 2007, 23, 13146–13150. [Google Scholar] [CrossRef] [PubMed]
- Koti, A.S.R.; Taneja, J.; Periasamy, N. Control of coherence length and aggregate size in the J-aggregate of porphyrin. Chem. Phys. Lett. 2003, 375, 171–176. [Google Scholar] [CrossRef]
- Toncelli, C.; Pino-Pinto, J.P.; Sano, N.; Picchioni, F.; Broekhuis, A.A.; Nishide, H.; Moreno-Villoslada, I. Controlling the aggregation of 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin by the use of polycations derived from polyketones bearing charged aromatic groups. Dye. Pigment. 2013, 98, 51–63. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Murakami, T.; Nishide, H. Comment on “J- and H-aggregates of 5,10,15,20-tetrakis-(4−sulfonatophenyl)-porphyrin and interconversion in PEG-b-P4VP micelles”. Biomacromolecules 2009, 10, 3341–3342. [Google Scholar] [CrossRef]
- Kubát, P.; Lang, K.; Janda, P.; Anzenbacher, P. Interaction of porphyrins with a dendrimer template: Self-aggregation controlled by pH. Langmuir 2005, 21, 9714–9720. [Google Scholar] [CrossRef]
- Paulo, P.M.R.; Costa, S.M.B. Non-covalent dendrimer–porphyrin interactions: The intermediacy of H-aggregates? Photochem. Photobiol. Sci. 2003, 2, 597–604. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, R.; Li, J.; Li, Y.; An, Y.; Shi, L. J- and H-aggregates of 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin and interconversion in PEG-b-P4VP micelles. Biomacromolecules 2008, 9, 2601–2608. [Google Scholar] [CrossRef]
- Andrade, S.M.; Teixeira, R.; Costa, S.M.B.; Sobral, A.J.F.N. Self-aggregation of free base porphyrins in aqueous solution and in DMPC vesicles. Biophys. Chem. 2008, 133, 1–10. [Google Scholar] [CrossRef]
- Lee, S.J.; Hupp, J.T.; Nguyen, S.T. Growth of narrowly dispersed porphyrin nanowires and their hierarchical assembly into macroscopic columns. J. Am. Chem. Soc. 2008, 130, 9632–9633. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; An, Y.; Chen, X.; Xiong, D.a.; Li, Y.; Huang, N.; Shi, L. Chiral polymeric micelles from electrostatic assembly between achiral porphyrins and block copolymers. Macromol. Rapid Commun. 2008, 29, 214–218. [Google Scholar] [CrossRef]
- Zhao, L.; Qu, R.; Li, A.; Ma, R.; Shi, L. Cooperative self-assembly of porphyrins with polymers possessing bioactive functions. Chem. Commun. 2016, 52, 13543–13555. [Google Scholar] [CrossRef] [PubMed]
- Ruthard, C.; Maskos, M.; Kolb, U.; Gröhn, F. Polystyrene sulfonate–porphyrin assemblies: Influence of polyelectrolyte and porphyrin structure. J. Phys. Chem. B 2011, 115, 5716–5729. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Romeo, A.; Angelini, N.; Micali, N.; Longo, A.; Mazzaglia, A.; Scolaro, L.M. Structural features of meso-tetrakis(4-carboxyphenyl)porphyrin interacting with amino-terminated poly(propylene oxide). Macromolecules 2006, 39, 5489–5496. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, L.; Qu, R.; Huang, F.; Gao, H.; An, Y.; Shi, L. Complex micelles with the bioactive function of reversible oxygen transfer. Nano Res. 2015, 8, 491–501. [Google Scholar] [CrossRef]
- Wang, M.; Yan, F.; Zhao, L.; Zhang, Y.; Sorci, M. Preparation and characterization of a pH-responsive membrane carrier for meso-tetraphenylsulfonato porphyrin. RSC Adv. 2017, 7, 1687–1696. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Li, S.; Ye, H.; An, H.; Zhang, Y. pH-responsive ethylene vinyl alcohol copolymer membrane based on porphyrin supramolecular self-assembly. RSC Adv. 2016, 6, 10704–10712. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Vlascici, D.; Birdeanu, M.; Fagadar-Cosma, G. Novel fluorescent pH sensor based on 5-(4-carboxy-phenyl)-10,15,20-tris(phenyl)-porphyrin. Arab. J. Chem. 2019, 12, 1587–1594. [Google Scholar] [CrossRef]
- Thyagarajan, S.; Leiding, T.; Årsköld, S.P.; Cheprakov, A.V.; Vinogradov, S.A. Highly non-planar dendritic porphyrin for pH sensing: Observation of porphyrin monocation. Inorg. Chem. 2010, 49, 9909–9920. [Google Scholar] [CrossRef]
- Igarashi, S.; Kuwae, K.; Yotsuyanagi, T. Optical pH sensor of electrostatically immobilized porphyrin on the surface of sulfonated-polystyrene. Anal. Sci. 1994, 10, 821–822. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Roscam Abbing, M.; Catalán-Toledo, J.; Oyarzun-Ampuero, F.; Pucci, A.; Raffa, P.; Picchioni, F.; Moreno-Villoslada, I. Synthesis of tuneable amphiphilic-modified polyketone polymers, their complexes with 5,10,15,20-tetrakis-(4-sulfonatophenyl)porphyrin, and their role in the photooxidation of 1,3,5-triphenylformazan confined in polymeric nanoparticles. Polymer 2019, 167, 215–223. [Google Scholar] [CrossRef]
- Mul, W.P.; Dirkzwager, H.; Broekhuis, A.A.; Heeres, H.J.; van der Linden, A.J.; Guy Orpen, A. Highly active, recyclable catalyst for the manufacture of viscous, low molecular weight, CO–ethene–propene-based polyketone, base component for a new class of resins. Inorg. Chim. Acta 2002, 327, 147–159. [Google Scholar] [CrossRef]
- Aydinoglu, S.; Biver, T.; Ceccarini, A.; Secco, F.; Venturini, M. Gold(III) extraction and recovery and gold(III)/copper(II) separation using micelles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 324–328. [Google Scholar] [CrossRef][Green Version]
- Dai, S.; Ravi, P.; Tam, K.C. pH-Responsive polymers: Synthesis, properties and applications. Soft Matter 2008, 4, 435–449. [Google Scholar] [CrossRef]
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef]
- Ghorbani Gorji, S.; Ghorbani Gorji, E.; Mohammadifar, M.A. Effect of pH on turbidity, size, viscosity and the shape of sodium caseinate aggregates with light scattering and rheometry. J. Food Sci. Technol. 2015, 52, 1820–1824. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Chai, Z.; Ma, R.; Zhao, L.; Zhang, Z.; An, Y.; Shi, L. Enhancement of the photostability and photoactivity of metallo-meso-5,10,15,20-tetrakis-(4-sulfonatophenyl)porphyrins by polymeric micelles. J. Colloid Interface Sci. 2012, 388, 80–85. [Google Scholar] [CrossRef]
- Li, A.; Zhao, L.; Hao, J.; Ma, R.; An, Y.; Shi, L. Aggregation behavior of the template-removed 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin chiral array directed by poly(ethylene glycol)-block-poly(l-lysine). Langmuir 2014, 30, 4797–4805. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-aggregates of porphyrin−surfactant complexes: Time-resolved fluorescence and other spectroscopic studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Xu, W.; Guo, H.; Akins, D.L. Aggregation of tetrakis(p-sulfonatophenyl)porphyrin within modified mesoporous MCM-41. J. Phys. Chem. B 2001, 105, 1543–1546. [Google Scholar] [CrossRef]
- Ma, H.-L.; Jin, W.-J. Studies on the effects of metal ions and counter anions on the aggregate behaviors of meso-tetrakis(p-sulfonatophenyl)porphyrin by absorption and fluorescence spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Tardajos, M.; Pino-Pinto, J.P.; Díaz-Soto, C.; Flores, M.E.; Gallardo, A.; Elvira, C.; Reinecke, H.; Nishide, H.; Moreno-Villoslada, I. Confinement of 5,10,15,20-tetrakis-(4-sulfonatophenyl)-porphyrin in novel poly(vinylpyrrolidone)s modified with aromatic amines. Dye. Pigment. 2013, 99, 759–770. [Google Scholar] [CrossRef]
- Feng, X.; Chen, L.; Dong, Y.; Jiang, D. Porphyrin-based two-dimensional covalent organic frameworks: Synchronized synthetic control of macroscopic structures and pore parameters. Chem. Commun. 2011, 47, 1979–1981. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mukherjee, K.; Shoukat, R.; Dong, H. A review on pH sensitive materials for sensors and detection methods. Microsyst. Technol. 2017, 23, 4391–4404. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araya-Hermosilla, E.; Moreno-Villoslada, I.; Araya-Hermosilla, R.; Flores, M.E.; Raffa, P.; Biver, T.; Pucci, A.; Picchioni, F.; Mattoli, V. pH-Responsive Polyketone/5,10,15,20-Tetrakis-(Sulfonatophenyl)Porphyrin Supramolecular Submicron Colloidal Structures. Polymers 2020, 12, 2017. https://doi.org/10.3390/polym12092017
Araya-Hermosilla E, Moreno-Villoslada I, Araya-Hermosilla R, Flores ME, Raffa P, Biver T, Pucci A, Picchioni F, Mattoli V. pH-Responsive Polyketone/5,10,15,20-Tetrakis-(Sulfonatophenyl)Porphyrin Supramolecular Submicron Colloidal Structures. Polymers. 2020; 12(9):2017. https://doi.org/10.3390/polym12092017
Chicago/Turabian StyleAraya-Hermosilla, Esteban, Ignacio Moreno-Villoslada, Rodrigo Araya-Hermosilla, Mario E. Flores, Patrizio Raffa, Tarita Biver, Andrea Pucci, Francesco Picchioni, and Virgilio Mattoli. 2020. "pH-Responsive Polyketone/5,10,15,20-Tetrakis-(Sulfonatophenyl)Porphyrin Supramolecular Submicron Colloidal Structures" Polymers 12, no. 9: 2017. https://doi.org/10.3390/polym12092017
APA StyleAraya-Hermosilla, E., Moreno-Villoslada, I., Araya-Hermosilla, R., Flores, M. E., Raffa, P., Biver, T., Pucci, A., Picchioni, F., & Mattoli, V. (2020). pH-Responsive Polyketone/5,10,15,20-Tetrakis-(Sulfonatophenyl)Porphyrin Supramolecular Submicron Colloidal Structures. Polymers, 12(9), 2017. https://doi.org/10.3390/polym12092017