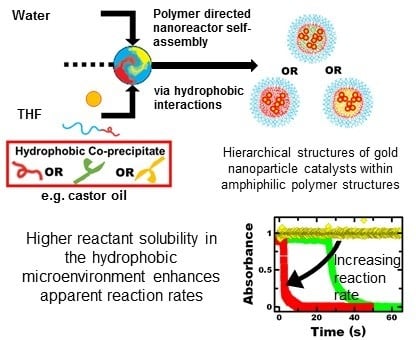
Accelerated Reaction Rates within Self-Assembled Polymer Nanoreactors with Tunable Hydrophobic Microenvironments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoreactor Assembly
2.3. Nanoreactor Characterization
2.4. Kinetic Analysis
2.5. Partition Coefficient Determination
2.6. Langmuir–Hinshelwood Kinetics
2.7. Sequential Reactions
3. Results
3.1. Nanoreactor Formulation and Characterization
3.2. Analysis of Apparent Reaction Kinetics
3.3. Analysis of Intrinsic Reaction Kinetics via Partition Coefficient Analysis and Langmuir–Hinshelwood Kinetics
3.4. Comparison of Catalytic Performance
3.5. Nanoreactor Reuse
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- La Sorella, G.; Strukul, G.; Scarso, A. Recent Advances in Catalysis in Micellar Media. Green Chem. 2015, 17, 644–683. [Google Scholar] [CrossRef]
- Lu, J.; Dimroth, J.; Weck, M. Compartmentalization of Incompatible Catalytic Transformations for Tandem Catalysis. J. Am. Chem. Soc. 2015, 137, 12984–12989. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. “On Water”: Unique Reactivity of Organic Compounds in Aqueous Suspension. Angew. Chemie Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Abela, A.R. Micellar Catalysis of Suzuki-Miyaura Cross-Couplings with Heteroaromatics in Water. Org. Lett. 2008, 10, 5329–5332. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Petersen, T.B.; Abela, A.R. Room-Temperature Suzuki-Miyaura Couplings in Water Facilitated by Nonionic Amphiphiles. Org. Lett. 2008, 10, 1333–1336. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Leong, W.W.Y.; Taft, B.R.; Krogstad, D.V. Manipulating Micellar Environments for Enhancing Transition Metal-Catalyzed Cross-Couplings in Water at Room Temperature. J. Org. Chem. 2011, 76, 5061–5073. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Hageman, M.; Fennewald, J.C.; Linstadt, R.; Slack, E.; Voigtritter, K. Selective Oxidations of Activated Alcohols in Water at Room Temperature. Chem. Commun. 2014, 50, 11378–11381. [Google Scholar] [CrossRef]
- Feng, J.; Handa, S.; Gallou, F.; Lipshutz, B.H. Safe and Selective Nitro Group Reductions Catalyzed by Sustainable and Recyclable Fe/Ppm Pd Nanoparticles in Water at Room Temperature. Angew. Chemie Int. Ed. 2016, 55, 8979–8983. [Google Scholar] [CrossRef]
- Cortes-Clerget, M.; Spink, S.E.; Gallagher, G.P.; Chaisemartin, L.; Filaire, E.; Berthon, J.Y.; Lipshutz, B.H. MC-1. A “Designer” Surfactant Engineered for Peptide Synthesis in Water at Room Temperature. Green Chem. 2019, 21, 2610–2614. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Gallou, F.; Handa, S. Evolution of Solvents in Organic Chemistry. ACS Sustain. Chem. Eng. 2016, 4, 5838–5849. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Aguinaldo, G.T.; Ghorai, S.; Voigtritter, K. Olefin Cross-Metathesis Reactions at Room Temperature Using the Nonionic Amphiphile “PTS”: Just Add Water. Org. Lett. 2008, 10, 1325–1328. [Google Scholar] [CrossRef]
- Cotanda, P.; Petzetakis, N.; O’reilly, R.K. Catalytic Polymeric Nanoreactors: More than a Solid Supported Catalyst. MRS Commun. 2012, 2, 119–126. [Google Scholar] [CrossRef]
- Lu, A.; O’Reilly, R.K. Advances in Nanoreactor Technology Using Polymeric Nanostructures. Curr. Opin. Biotechnol. 2013, 24, 639–645. [Google Scholar] [CrossRef]
- Lu, A.; Cotanda, P.; Patterson, J.P.; Longbottom, D.A.; O’Reilly, R.K. Aldol Reactions Catalyzed by L-Proline Functionalized Polymeric Nanoreactors in Water. Chem. Commun. 2012, 48, 9699–9701. [Google Scholar] [CrossRef]
- Liu, X.; Mu, S.; Long, Y.; Qiu, G.; Ling, Q.; Gu, H.; Lin, W. Gold Nanoparticles Stabilized by 1,2,3-Triazolyl Dendronized Polymers as Highly Efficient Nanoreactors for the Reduction of 4-Nitrophenol. Catal. Letters 2019, 149, 544–551. [Google Scholar] [CrossRef]
- Harrison, A.; Vuong, T.; Zeevi, M.; Hittel, B.; Wi, S.; Tang, C. Rapid Self-Assembly of Metal/Polymer Nanocomposite Particles as Nanoreactors and Their Kinetic Characterization. Nanomaterials 2019, 9, 318. [Google Scholar] [CrossRef]
- Choi, S.H.; Bates, F.S.; Lodge, T.P. Molecular Exchange in Ordered Diblock Copolymer Micelles. Macromolecules 2011, 44, 3594–3604. [Google Scholar] [CrossRef]
- Wunder, S.; Lu, Y.; Albrecht, M.; Ballauff, M. Catalytic Activity of Faceted Gold Nanoparticles Studied by a Model Reaction: Evidence for Substrate-Induced Surface Restructuring. ACS Catal. 2011, 1, 908–916. [Google Scholar] [CrossRef]
- Tang, C.; Amin, D.; Messersmith, P.B.; Anthony, J.E.; Prud’homme, R.K. Polymer Directed Self-Assembly of PH-Responsive Antioxidant Nanoparticles. Langmuir 2015, 31, 3612–3620. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by Metallic Nanoparticles in Aqueous Solution: Model Reactions. Chem. Soc. Rev. 2012, 41, 5577. [Google Scholar] [CrossRef]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic Analysis of the Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Pinkerton, N.M.; Gindy, M.E.; Calero-Ddelc, V.L.; Wolfson, T.; Pagels, R.F.; Adler, D.; Gao, D.; Li, S.; Wang, R.; Zevon, M.; et al. Single-Step Assembly of Multimodal Imaging Nanocarriers: MRI and Long-Wavelength Fluorescence Imaging. Adv. Healthc. Mater. 2015, 4, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Gindy, M.E.; Panagiotopoulos, A.Z.; Prud’Homme, R.K. Composite Block Copolymer Stabilized Nanoparticles: Simultaneous Encapsulation of Organic Actives and Inorganic Nanostructures. Langmuir 2008, 24, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lempke, L.; Ernst, A.; Kahl, F.; Weberskirch, R.; Krause, N. Sustainable Micellar Gold Catalysis-Poly(2-Oxazolines) as Versatile Amphiphiles. Adv. Synth. Catal. 2016, 358, 1491–1499. [Google Scholar] [CrossRef]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M.; Drechsler, M. Catalytic Activity of Palladium Nanoparticles Encapsulated in Spherical Poly Electrolyte Brushes and Core-Shell Microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kitchens, C.L. Modifying Ligand Chemistry to Enhance Reusability of PH-Responsive Colloidal Gold Nanoparticle Catalyst. J. Phys. Chem. C 2019, 123, 26450–26459. [Google Scholar] [CrossRef]
- Ansar, S.M.; Chakraborty, S.; Kitchens, C.L. PH-Responsive Mercaptoundecanoic Acid Functionalized Gold Nanoparticles and Applications in Catalysis. Nanomaterials 2018, 8, 339. [Google Scholar] [CrossRef]
- Teimouri, M.; Khosravi-Nejad, F.; Attar, F.; Saboury, A.A.; Kostova, I.; Benelli, G.; Falahati, M. Gold Nanoparticles Fabrication by Plant Extracts: Synthesis, Characterization, Degradation of 4-Nitrophenol from Industrial Wastewater, and Insecticidal Activity–A Review. J. Clean. Prod. 2018, 184, 740–753. [Google Scholar] [CrossRef]
- Kong, X.K.; Sun, Z.Y.; Chen, M.; Chen, C.L.; Chen, Q.W. Metal-Free Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol by N-Doped Graphene. Energy Environ. Sci. 2013, 6, 3260–3266. [Google Scholar] [CrossRef]
- Vaidya, M.J.; Kulkarni, S.M.; Chaudhari, R.V. Synthesis of P-Aminophenol by Catalytic Hydrogenation of p-Nitrophenol. Org. Process Res. Dev. 2003, 7, 202–208. [Google Scholar] [CrossRef]
- Nigra, M.M.; Ha, J.M.; Katz, A. Identification of Site Requirements for Reduction of 4-Nitrophenol Using Gold Nanoparticle Catalysts. Catal. Sci. Technol. 2013, 3, 2976–2983. [Google Scholar] [CrossRef]
- 3Gu, S.; Kaiser, J.; Marzun, G.; Ott, A.; Lu, Y.; Ballauff, M.; Zaccone, A.; Barcikowski, S.; Wagener, P. Ligand-Free Gold Nanoparticles as a Reference Material for Kinetic Modelling of Catalytic Reduction of 4-Nitrophenol. Catal. Letters 2015, 145, 1105–1112. [Google Scholar]
- Neal, R.D.; Inoue, Y.; Hughes, R.A.; Neretina, S. Catalytic Reduction of 4-Nitrophenol by Gold Catalysts: The Influence of Borohydride Concentration on the Induction Time. J. Phys. Chem. C 2019, 123, 12894–12901. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, L.; Yang, B.; Du, J. Multifunctional Homopolymer Vesicles for Facile Immobilization of Gold Nanoparticles and Effective Water Remediation. ACS Nano 2014, 8, 5022–5031. [Google Scholar] [CrossRef] [PubMed]
- Antonels, N.C.; Meijboom, R. Preparation of Well-Defined Dendrimer Encapsulated Ruthenium Nanoparticles and Their Evaluation in the Reduction of 4-Nitrophenol According to the Langmuir-Hinshelwood Approach. Langmuir 2013, 29, 13433–13442. [Google Scholar] [CrossRef]
- Gregor, L.; Reilly, A.K.; Dickstein, T.A.; Mazhar, S.; Bram, S.; Morgan, D.G.; Losovyj, Y.; Pink, M.; Stein, B.D.; Matveeva, V.G.; et al. Facile Synthesis of Magnetically Recoverable Pd and Ru Catalysts for 4-Nitrophenol Reduction: Identifying Key Factors. ACS Omega 2018, 3, 14717–14725. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, X.; Cao, M. Hybrids of Gold Nanoparticles with Core-Shell Hyperbranched Polymers: Synthesis, Characterization, and Their High Catalytic Activity for Reduction of 4-Nitrophenol. Catalysts 2015, 6. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, X.; Dai, X.; Qin, Y.; Duan, A.; Yu, Y.; Zhuo, H.; Zhao, H.; Zhang, P.; Jiang, Y.; et al. Morphology-Selective Synthesis of Active and Durable Gold Catalysts with High Catalytic Performance in the Reduction of 4-Nitrophenol. Nano Res. 2016, 9, 3099–3115. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, Z.M.; Cao, C.Y.; He, W.D.; Jiang, L.; Song, W.G. Temperature-Responsive Smart Nanoreactors: Poly(N-Isopropylacrylamide)- Coated Au@mesoporous-SiO2 Hollow Nanospheres. Langmuir 2012, 28, 13452–13458. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Chen, G. A New Type of Raspberry-like Polymer Composite Sub-Microspheres with Tunable Gold Nanoparticles Coverage and Their Enhanced Catalytic Properties. J. Mater. Chem. A 2013, 1, 930–937. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Q.; Cui, Y.; Gao, D.; Kang, J.; Sun, H.; Zhu, L.; Chen, S. Highly Stable and Biocompatible Dendrimer-Encapsulated Gold Nanoparticle Catalysts for the Reduction of 4-Nitrophenol. New J. Chem. 2017, 41, 8399–8406. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Yuan, P.; Sun, Q.; Jia, Y.; Yan, W.; Chen, Z.; Xu, Q. Au Nanoparticle Decorated N-Containing Polymer Spheres: Additive-Free Synthesis and Remarkable Catalytic Behavior for Reduction of 4-Nitrophenol. J. Mater. Sci. 2015, 50, 1323–1332. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Astruc, D.; Lin, W.; Gu, H. Highly-Branched Amphiphilic Organometallic Dendronized Diblock Copolymer: ROMP Synthesis, Self-Assembly and Long-Term Au and Ag Nanoparticle Stabilizer for High-Efficiency Catalysis. Polymer (Guildf). 2019, 173, 1–10. [Google Scholar] [CrossRef]
- Guo, M.; He, J.; Li, Y.; Ma, S.; Sun, X. One-Step Synthesis of Hollow Porous Gold Nanoparticles with Tunable Particle Size for the Reduction of 4-Nitrophenol. J. Hazard. Mater. 2016, 310, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ansar, S.M.; Stroud, J.G.; Kitchens, C.L. Comparison of Colloidal versus Supported Gold Nanoparticle Catalysis. J. Phys. Chem. C 2018, 122, 7749–7758. [Google Scholar] [CrossRef]
- Blanco, E.; Atienzar, P.; Hernández, P.; Quintana, C. The Langmuir-Hinshelwood Approach for Kinetic Evaluation of Cucurbit [7]Uril-Capped Gold Nanoparticles in the Reduction of the Antimicrobial Nitrofurantoin. Phys. Chem. Chem. Phys. 2017, 19, 18913–18923. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.; Wu, C.; Fu, G.; Zhao, H.; He, B. Synthesis, Characterization and Application of Well-Defined Environmentally Responsive Polymer Brushes on the Surface of Colloid Particles. Polymer (Guildf). 2007, 48, 1989–1997. [Google Scholar] [CrossRef]
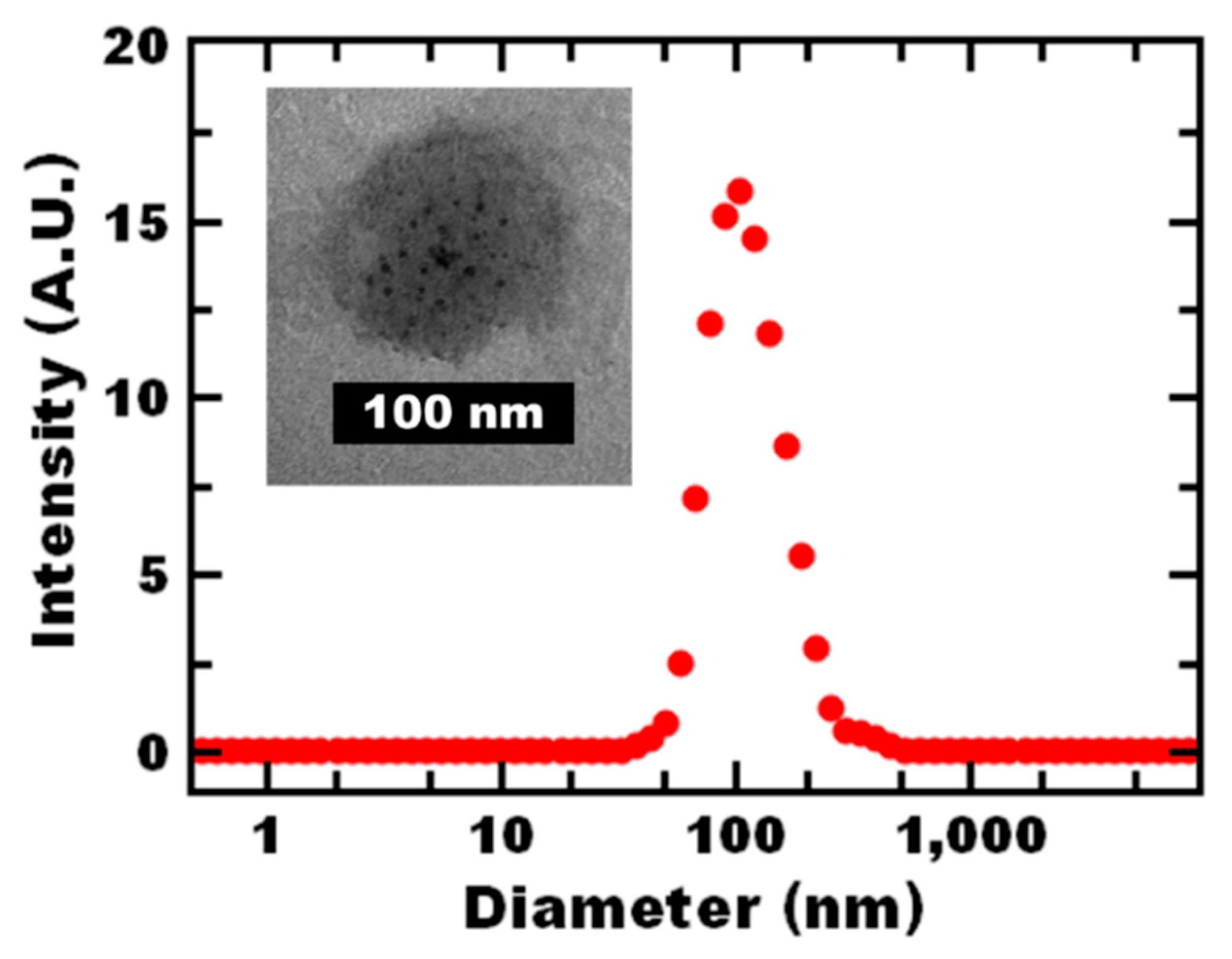

| System | Size (nm) | PDI | Stability * |
|---|---|---|---|
| CO NR | 113 ± 10 | 0.184 ± 0.011 | Yes |
| PS 750 NR | 124 ± 6 | 0.131 ± 0.012 | Yes |
| Dodecane NR | 168 ± 6 | 0.108 ± 0.003 | No |
| Dodecylamine NR | 143 ± 23 | 0.263 ± 0.019 | Yes |
| Dodecanethiol NR | 101 ± 6 | 0.087 ± 0.028 | Yes |
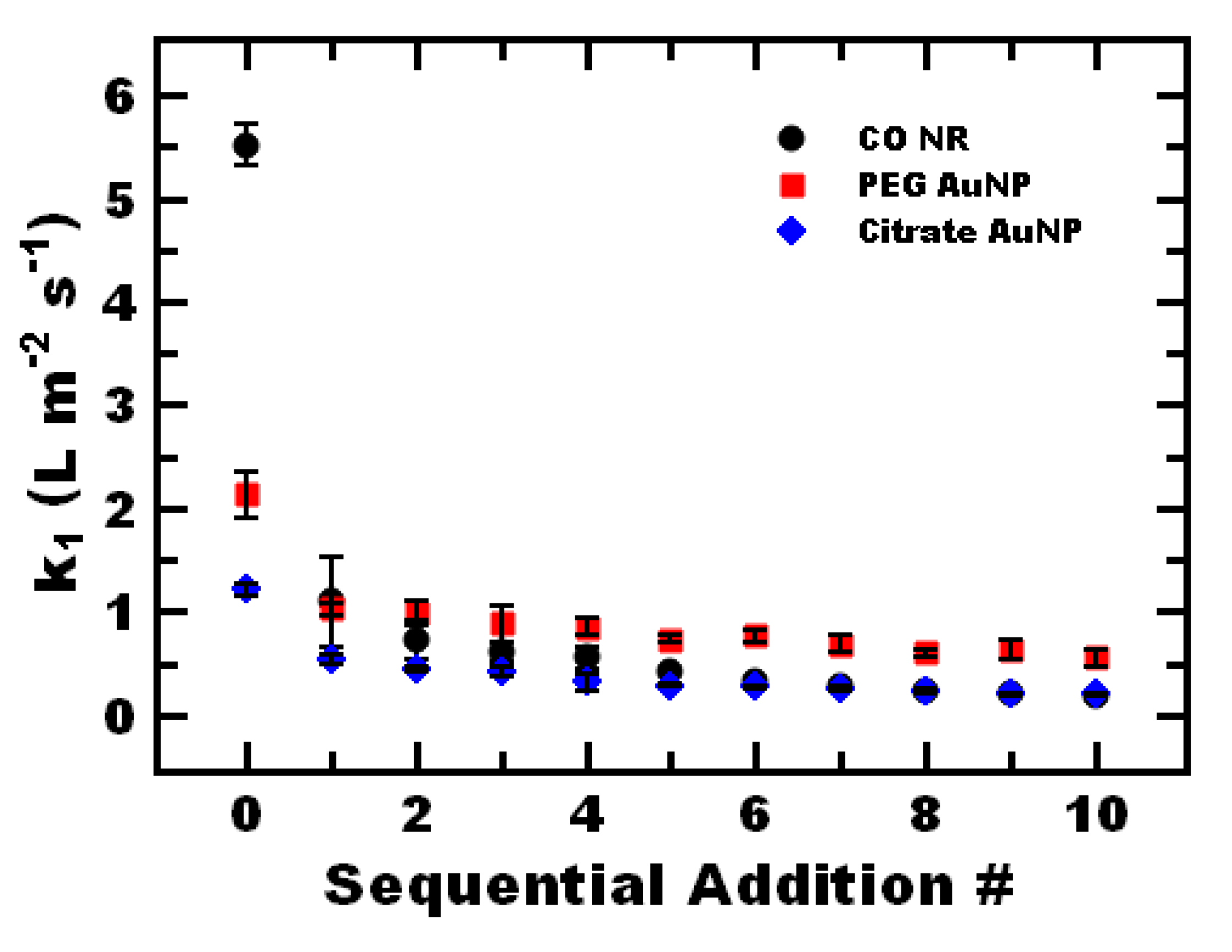
| Nanoreactor | k1 (L m−2 s−1) |
|---|---|
| CO NR | 5.7 ± 0.7 |
| CO NP w AuNP | 1.6 ± 0.4 |
| PEG-coated AuNP | 2.1 ± 0.2 |
| Citrate-stabilized AuNP | 1.2 ± 0.1 |
| System | k0 (mol m−2 s−1) |
|---|---|
| CO NR | 0.0103 ± 0.0015 |
| CO NP w AuNP | 0.0084 ± 0.0001 |
| PEG AuNP | 0.0151 ± 0.0008 |
| Citrate AuNP | 0.0100 ± 0.0005 |
| Gold nanoparticles coated on polystyrene microspheres grafted with poly([2-aminoethyl]-methacrylate hydrochloride) from ref [18] | 2.27 ± 0.34 × 10−4 |
| Nanoreactor | k1 (L m−2 s−1) |
|---|---|
| CO NR | 5.7 ± 0.7 |
| PS 750 NR | 0.7 ± 0.1 |
| Dodecylamine NR | <0.001 ± 0.001 |
| Dodecanethiol NR | <0.001 ± 0.001 |
| PS 750 NP without AuNP | 0.001 ± 0.001 |
| Core Material Organic Phase | Core Material: Water Partition Coefficient of 4NP |
|---|---|
| Castor Oil | 7.81 ± 0.16 |
| Toluene | 0.09 ± 0.01 |
| System | TOF (min−1) | Reference |
|---|---|---|
| CO NR | 29.7 ± 3.8 × 104 | This Paper |
| PS 750 NR | 3.3 ± 0.2 × 104 | This Paper |
| Gold nanoparticles (NP) coated on poly(glycidyl methacrylate) microspheres with poly(allylamine hydrochloride) | 1.5 × 104 | [40] |
| Gold NPs stabilized by amphiphilic dendronized diblock copolymer (hydrophilic triethylene glycol (TEG) and hydrophobic ferrocenyl (Fc) groups) | 440 | [43] |
| Gold NPs stabilized by polyamidoamine dendrimer | 2 × 103 | [41] |
| Gold NPs stabilized by PEI derivatives | 1.5 × 104 | [37] |
| PVP-stabilized gold NPs | 0.8 | [44] |
| Citrate-stabilized gold NPs | 3.0 | [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrison, A.; P. Zeevi, M.; L. Vasey, C.; D. Nguyen, M.; Tang, C. Accelerated Reaction Rates within Self-Assembled Polymer Nanoreactors with Tunable Hydrophobic Microenvironments. Polymers 2020, 12, 1774. https://doi.org/10.3390/polym12081774
Harrison A, P. Zeevi M, L. Vasey C, D. Nguyen M, Tang C. Accelerated Reaction Rates within Self-Assembled Polymer Nanoreactors with Tunable Hydrophobic Microenvironments. Polymers. 2020; 12(8):1774. https://doi.org/10.3390/polym12081774
Chicago/Turabian StyleHarrison, Andrew, Michael P. Zeevi, Christopher L. Vasey, Matthew D. Nguyen, and Christina Tang. 2020. "Accelerated Reaction Rates within Self-Assembled Polymer Nanoreactors with Tunable Hydrophobic Microenvironments" Polymers 12, no. 8: 1774. https://doi.org/10.3390/polym12081774
APA StyleHarrison, A., P. Zeevi, M., L. Vasey, C., D. Nguyen, M., & Tang, C. (2020). Accelerated Reaction Rates within Self-Assembled Polymer Nanoreactors with Tunable Hydrophobic Microenvironments. Polymers, 12(8), 1774. https://doi.org/10.3390/polym12081774