3D Printing of a Reactive Hydrogel Bio-Ink Using a Static Mixing Tool
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bio-Ink Formulation
2.3. 3D Printing with a Static Mixing Tool
2.4. Bio-Ink and 3D Printed Scaffolds Characterization
2.4.1. Rheological Analysis
2.4.2. Attenuated Total Reflection Fourier Transform Infra-Red (ATR-FTIR) Spectroscopy
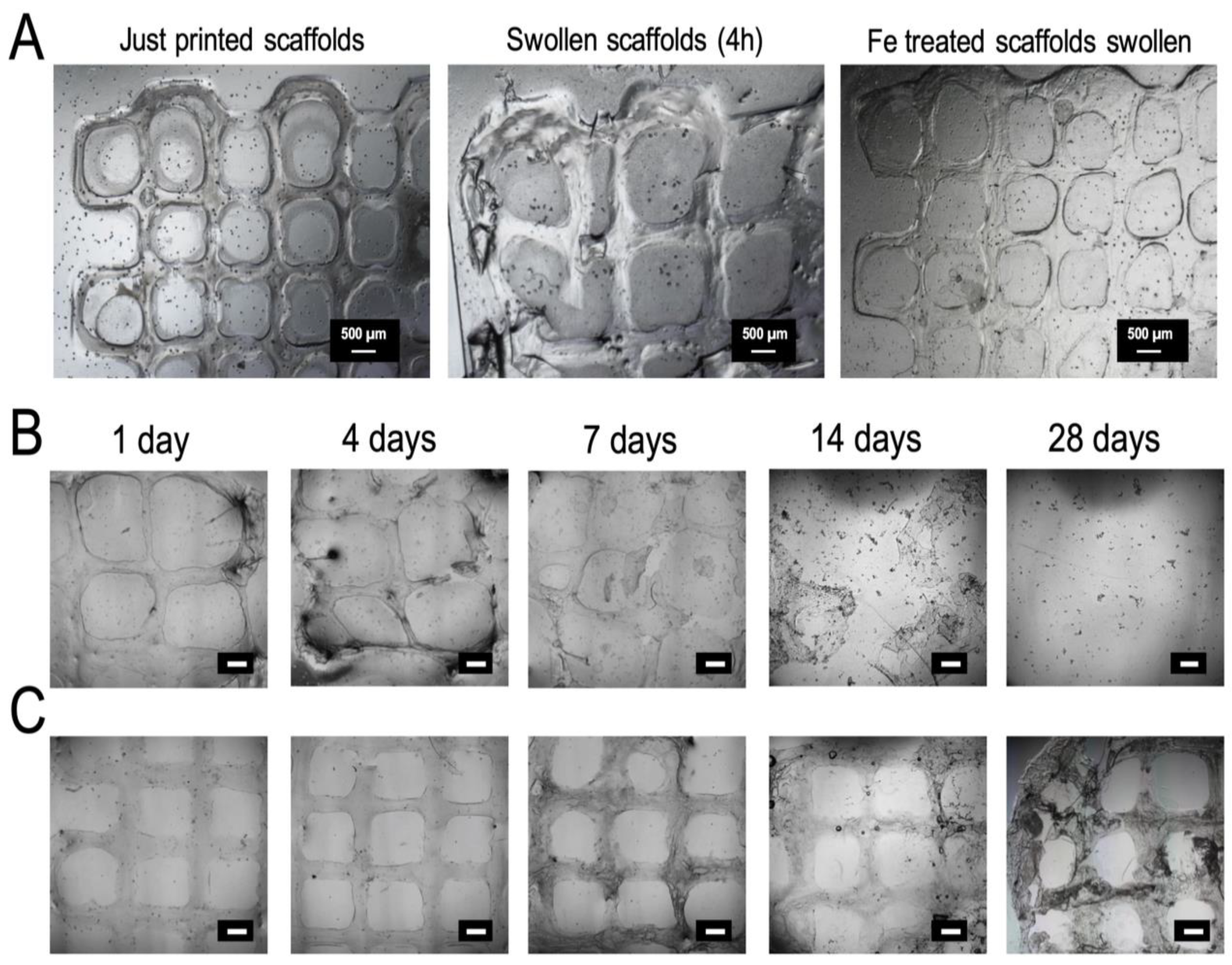
2.4.3. Scaffolds Morphology
2.4.4. In Vitro Swelling and Degradation Studies
2.5. CMCh2/HAox4-HA0.4 Based Bioprinting
2.5.1. Cell Culture
2.5.2. Bio-Ink Preparation
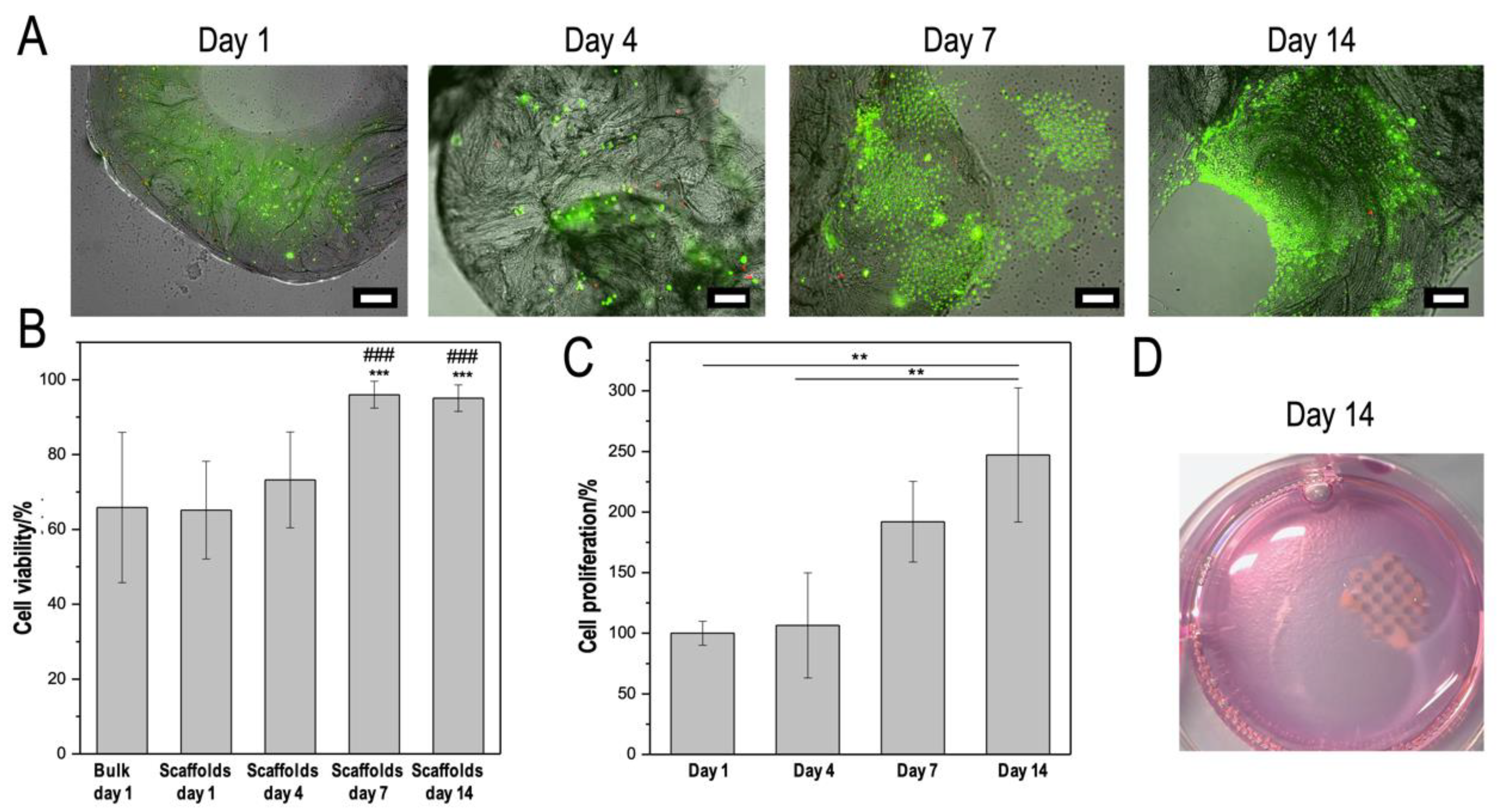
2.5.3. Cell Assays and Staining
3. Results and Discussion
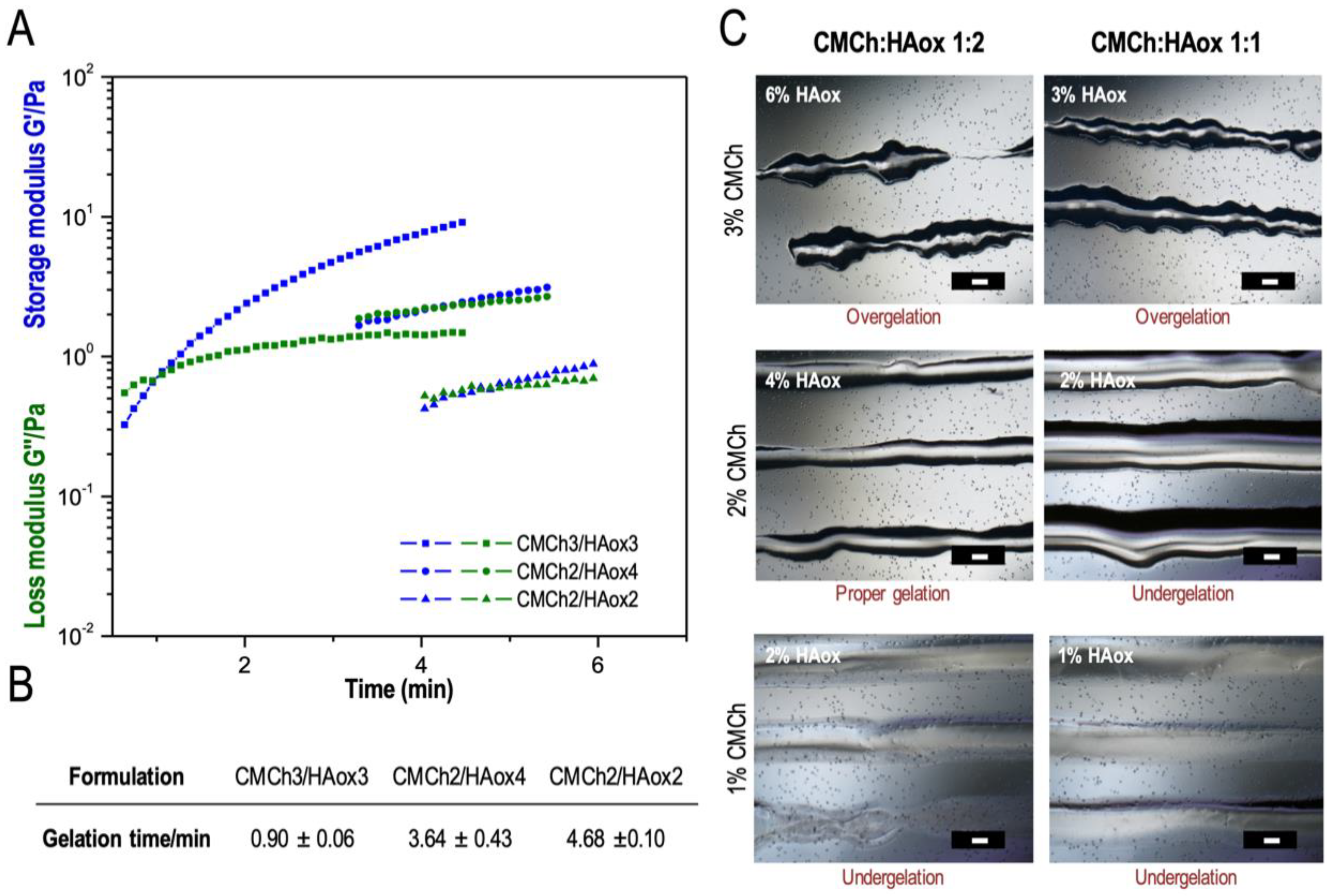
3.1. 3D Printing with a Static Mixing Tool
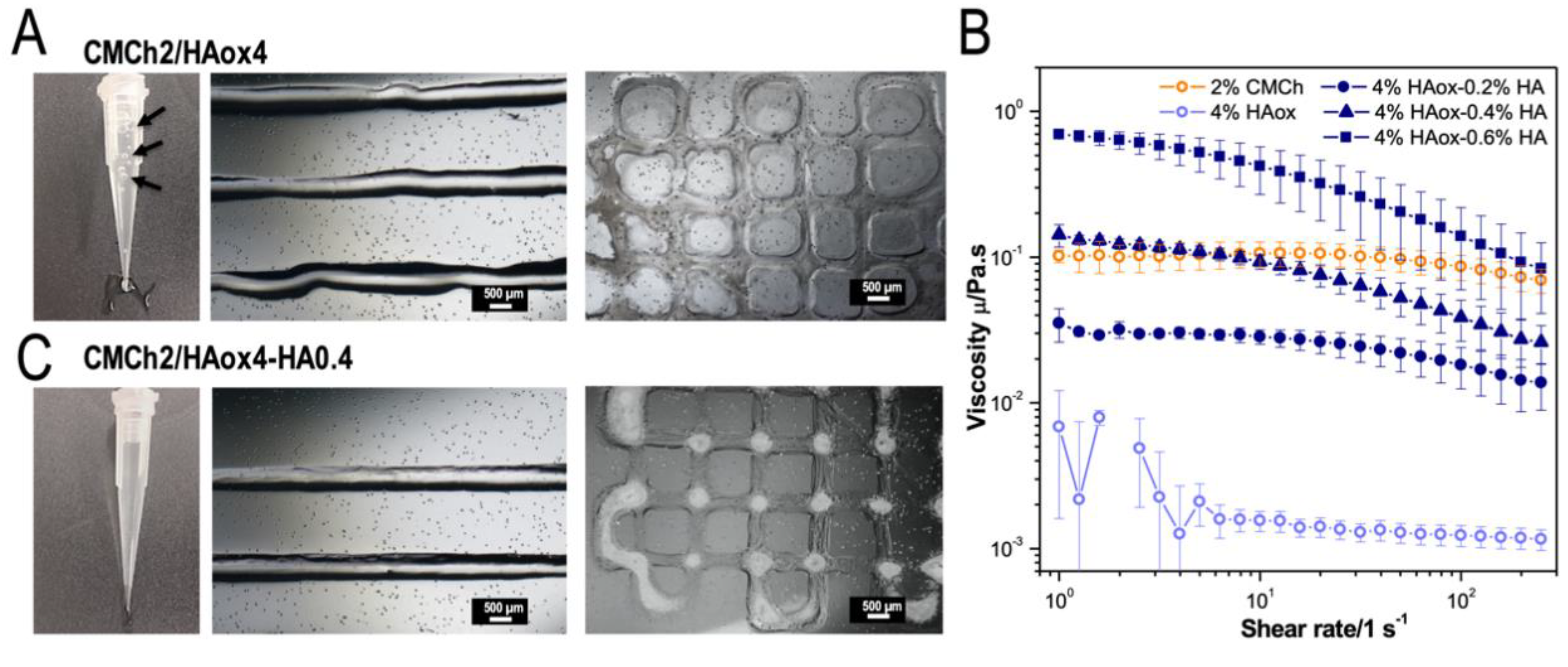
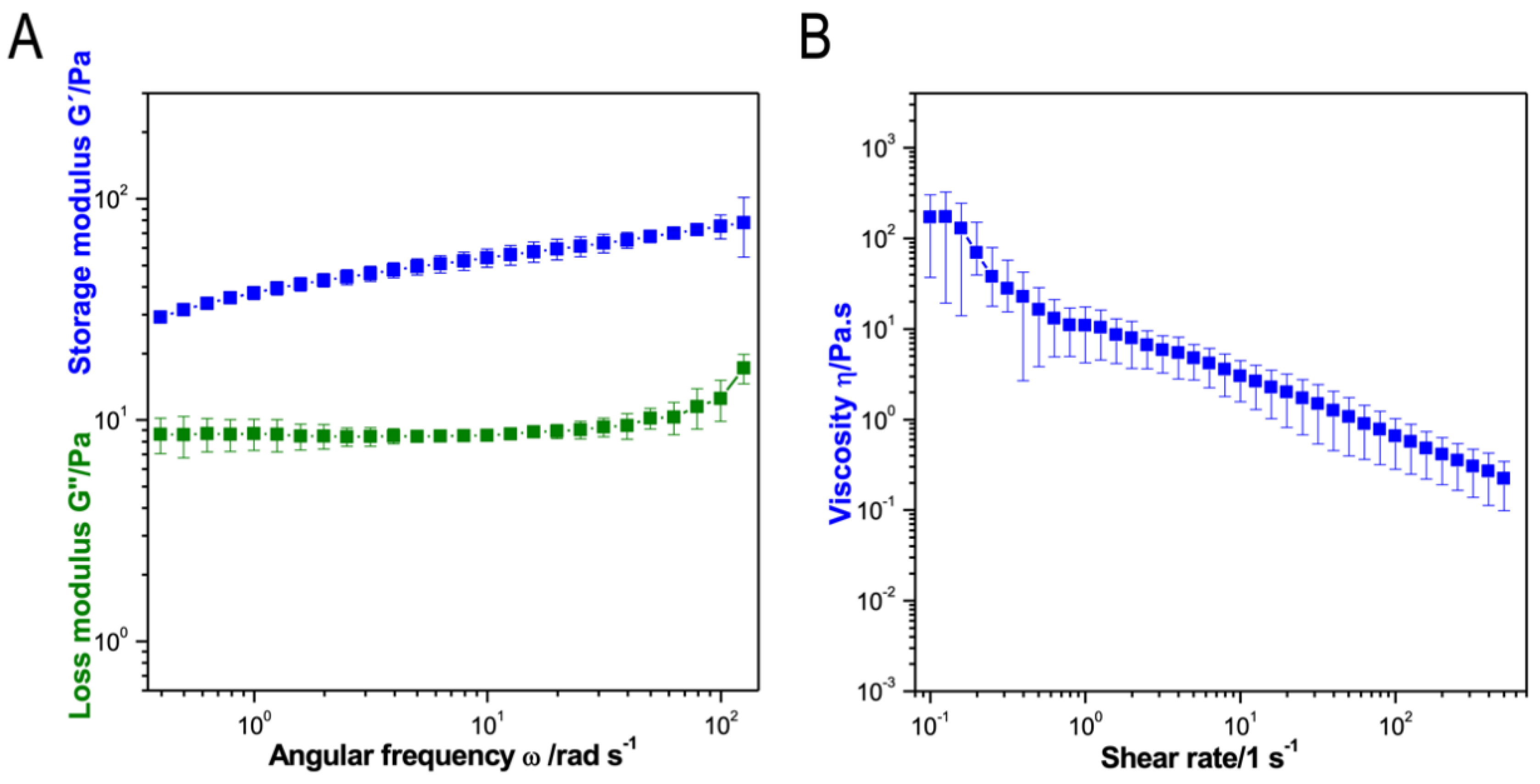
3.2. Characterization of the Optimized Bio-Ink
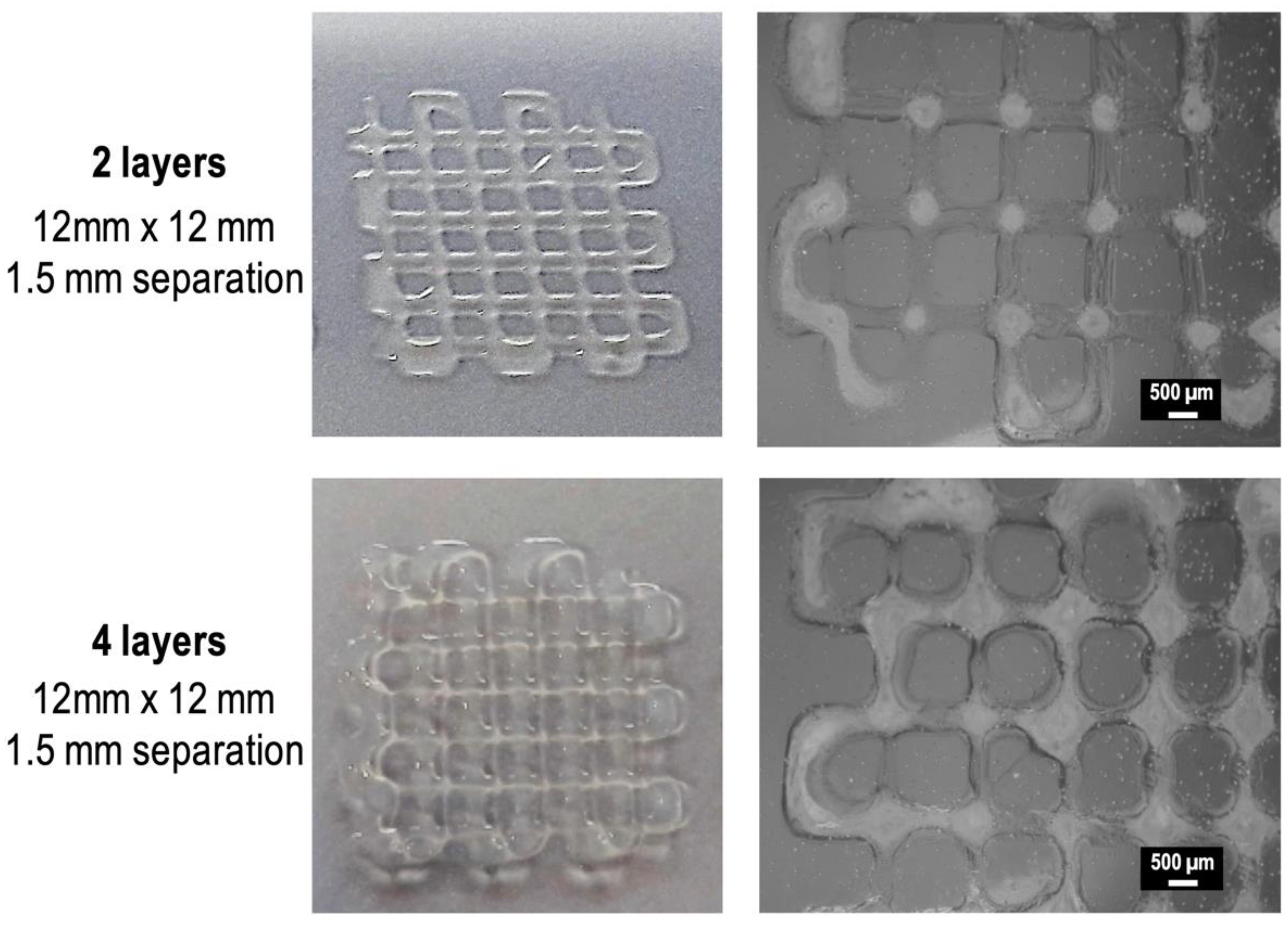
3.3. Characterization of the 3D Printed Hydrogel Scaffolds
3.4. CMCh2/HAox4-HA0.4 Based Bio-Printing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk-Biegun, M.K.; del Campo, A. 3D bioprinting of structural proteins. Biomaterials 2017, 134, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. Part A 2013, 101, 272–284. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Chaudhari, A.; Vig, K.; Baganizi, D.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.; Pillai, S. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Mora-Boza, A.; Puertas-Bartolomé, M.; Vázquez-Lasa, B.; Román, J.S.; Pérez-Caballer, A.; Olmeda-Lozano, M. Contribution of bioactive hyaluronic acid and gelatin to regenerative medicine. Methodologies of gels preparation and advanced applications. Eur. Polym. J. 2017, 95, 11–26. [Google Scholar] [CrossRef]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Zhao, Y.; Dilley, R.J.; Redmond, S.L.; Wang, X.; Liu, X. 3D printing of silk particle-reinforced chitosan hydrogel structures and their properties. ACS Biomater. Sci. Eng. 2018, 4, 3036–3046. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Oner, E.T.; Ekren, N.; Erdemir, G.; Kuruca, S.E.; Yuca, E.; Bostan, M.S.; Eroglu, M.S.; Ikram, F.; Uzun, M. Comparative characterization of the hydrogel added PLA/β-TCP scaffolds produced by 3D bioprinting. Bioprinting 2019, 13, e00046. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhang, X.; Zhao, X.; Ma, J.; Pu, X.; Wang, Y.; Ran, F.; Wang, Y.; Leng, F. Snakegourd root/Astragalus polysaccharide hydrogel preparation and application in 3D printing. Int. J. Biol. Macromol. 2019, 121, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jang, T.-S.; Pan, H.M.; Jung, H.-D.; Sia, M.W.; Xie, S.; Hang, Y.; Chong, S.K.M.; Wang, D.; Song, J.J.I.J.o.B. 3D Freeform Printing of Nanocomposite Hydrogels Through in situ Precipitation in Reactive Viscous Fluid. Int. J. Bioprinting 2020, 6, 258. [Google Scholar]
- Jang, T.-S.; Jung, H.-D.; Pan, M.H.; Han, W.T.; Chen, S.; Song, J. 3D printing of hydrogel composite systems: Recent advances in technology for tissue engineering. Int. J. Bioprinting 2018. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Ahlfeld, T.; Lode, A.; Gelinsky, M. A versatile method for combining different biopolymers in a core/shell fashion by 3D plotting to achieve mechanically robust constructs. Biofabrication 2016, 8, 045001. [Google Scholar] [CrossRef]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef]
- Huang, J.; Fu, H.; Wang, Z.; Meng, Q.; Liu, S.; Wang, H.; Zheng, X.; Dai, J.; Zhang, Z. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv. 2016, 6, 108423–108430. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.-W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 035004. [Google Scholar] [CrossRef]
- Haring, A.P.; Thompson, E.G.; Tong, Y.; Laheri, S.; Cesewski, E.; Sontheimer, H.; Johnson, B.N. Process-and bio-inspired hydrogels for 3D bioprinting of soft free-standing neural and glial tissues. Biofabrication 2019, 11, 025009. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2018, 14, 025006. [Google Scholar] [CrossRef] [PubMed]
- Mora-Boza, A.; Włodarczyk-Biegun, M.K.; del Campo, A.; Vázquez-Lasa, B.; Román, J.S. Glycerylphytate as an ionic crosslinker for 3D printing of multi-layered scaffolds with improved shape fidelity and biological features. Biomater. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bakarich, S.E.; Gorkin, R., III; Gately, R.; Naficy, S.; in het Panhuis, M.; Spinks, G.M. 3D printing of tough hydrogel composites with spatially varying materials properties. Addit. Manuf. 2017, 14, 24–30. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Gorkin Iii, R. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019, 95, 176–187. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and properties of chitosan Inks for 3D printing of hydrogel microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Liu, S.; Li, L. Three-dimensional bioprinting of oppositely charged hydrogels with super strong Interface bonding. ACS Appl. Mater. Interfaces 2018, 10, 11164–11174. [Google Scholar] [CrossRef]
- Highley, C.B.; Song, K.H.; Daly, A.C.; Burdick, J.A. Jammed microgel inks for 3D printing applications. Adv. Sci. 2019, 6, 1801076. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2011, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.D.; Di Bella, C.; Thompson, F.; Augustine, C.; Beirne, S.; Cornock, R.; Richards, C.J.; Chung, J.; Gambhir, S.; Yue, Z. Development of the Biopen: A handheld device for surgical printing of adipose stem cells at a chondral wound site. Biofabrication 2016, 8, 015019. [Google Scholar] [CrossRef]
- Gregor, A.; Hośek, J. 3D printing methods of biological scaffolds used in tissue engineering. Rom Rev Precis Mech Opt Mechatron. 2011, 3, 143–148. [Google Scholar]
- Zimmermann, R.; Hentschel, C.; Schrön, F.; Moedder, D.; Büttner, T.; Atallah, P.; Wegener, T.; Gehring, T.; Howitz, S.; Freudenberg, U. High resolution bioprinting of multi-component hydrogels. Biofabrication 2019, 11, 045008. [Google Scholar] [CrossRef]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R., III; Stewart, E.M.; in het Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef]
- Bootsma, K.; Fitzgerald, M.M.; Free, B.; Dimbath, E.; Conjerti, J.; Reese, G.; Konkolewicz, D.; Berberich, J.A.; Sparks, J.L. 3D printing of an interpenetrating network hydrogel material with tunable viscoelastic properties. J. Mech. Behav. Biomed. Mater. 2017, 70, 84–94. [Google Scholar] [CrossRef]
- Deepthi, S.; Jayakumar, R. Alginate nanobeads interspersed fibrin network as in situ forming hydrogel for soft tissue engineering. Bioact. Mater. 2018, 3, 194–200. [Google Scholar] [CrossRef]
- Hozumi, T.; Ohta, S.; Ito, T. Analysis of the Calcium Alginate Gelation Process Using a Kenics Static Mixer. Ind. Eng. Chem. Res. 2015, 54, 2099–2107. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Zhang, T.; Zan, Y.; Ni, T.; Liu, M.; Pei, R. Fast-forming BMSC-encapsulating hydrogels through bioorthogonal reaction for osteogenic differentiation. Biomater. Sci. 2018, 6, 2578–2581. [Google Scholar] [CrossRef] [PubMed]
- Whitely, M.; Cereceres, S.; Dhavalikar, P.; Salhadar, K.; Wilems, T.; Smith, B.; Mikos, A.; Cosgriff-Hernandez, E. Improved in situ seeding of 3D printed scaffolds using cell-releasing hydrogels. Biomaterials 2018, 185, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Moglia, R.S.; Whitely, M.; Dhavalikar, P.; Robinson, J.; Pearce, H.; Brooks, M.; Stuebben, M.; Cordner, N.; Cosgriff-Hernandez, E. Injectable polymerized high internal phase emulsions with rapid in situ curing. Biomacromolecules 2014, 15, 2870–2878. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable hydrogel with slow degradability composed of gelatin and hyaluronic acid cross-linked by Schiff’s base formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef]
- Wu, H.-D.; Yang, J.-C.; Tsai, T.; Ji, D.-Y.; Chang, W.-J.; Chen, C.-C.; Lee, S.-Y. Development of a chitosan–polyglutamate based injectable polyelectrolyte complex scaffold. Carbohydr. Polym. 2011, 85, 318–324. [Google Scholar] [CrossRef]
- Kohn, C.; Klemens, J.; Kascholke, C.; Murthy, N.; Kohn, J.; Brandenburger, M.; Hacker, M. Dual–component collagenous peptide/reactive oligomer hydrogels as potential nerve guidance materials–from characterization to functionalization. Biomater. Sci. 2016, 4, 1605–1621. [Google Scholar] [CrossRef]
- Caballero, S.S.R.; Saiz, E.; Montembault, A.; Tadier, S.; Maire, E.; David, L.; Delair, T.; Grémillard, L. 3-D printing of chitosan-calcium phosphate inks: Rheology, interactions and characterization. J. Mater. Sci. Mater. Med. 2019, 30, 6. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R. 3D-printed chitosan-based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Highley, C.B.; Rodell, C.B.; Sun, W.; Burdick, J.A. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater. Sci. Eng. 2016, 2, 1743–1751. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Nguyen, N.T.-P.; Nguyen, L.V.-H.; Tran, N.M.-P.; Nguyen, T.-H.; Huynh, C.-K.; Van, T.V. Synthesis of cross-linking chitosan-hyaluronic acid based hydrogels for tissue engineering applications. In Proceedings of the International Conference on the Development of Biomedical Engineering in Vietnam; Springer: Singapore, 2017; 63, pp. 671–675. [Google Scholar] [CrossRef]
- Qian, C.; Zhang, T.; Gravesande, J.; Baysah, C.; Song, X.; Xing, J. Injectable and self-healing polysaccharide-based hydrogel for pH-responsive drug release. Int. J. Biol. Macromol. 2019, 123, 140–148. [Google Scholar] [CrossRef]
- Ye, K.; Felimban, R.; Traianedes, K.; Moulton, S.E.; Wallace, G.G.; Chung, J.; Quigley, A.; Choong, P.F.; Myers, D.E. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS ONE 2014, 9, e99410. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Choi, B.; Wu, B.; Lee, M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013, 5, 045003. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, L.; He, T.; Wang, N.; Yang, S.; Yang, X.; Zeng, Y.; Zhang, W.; Yang, L.; Wu, Q.; et al. Peritoneal adhesion prevention with a biodegradable and injectable N,O-carboxymethyl chitosan-aldehyde hyaluronic acid hydrogel in a rat repeated-injury model. Sci. Rep. 2016, 6, 37600. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, S.; Karkhaneh, A. A self-crosslinking tri-component hydrogel based on functionalized polysaccharides and gelatin for tissue engineering applications. Mater. Lett. 2016, 164, 468–471. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Benito-Garzón, L.; Fung, S.; Kohn, J.; Vázquez-Lasa, B.; San Román, J. Bioadhesive functional hydrogels: Controlled release of catechol species with antioxidant and antiinflammatory behavior. Mater. Sci. Eng. C 2019, 105, 110040. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.-P.; Nguyen, L.V.-H.; Tran, N.M.-P.; Nguyen, D.T.; Nguyen, T.N.-T.; Tran, H.A.; Dang, N.N.-T.; Van Vo, T.; Nguyen, T.-H. The effect of oxidation degree and volume ratio of components on properties and applications of in situ cross-linking hydrogels based on chitosan and hyaluronic acid. Mater. Sci. Eng. C 2019, 103, 109670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Heindel, N.D. Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef]
- Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Zhang, K.; Chen, X.; Cui, L.; Yin, J. Injectable in situ self-cross-linking hydrogels based on poly (L-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules 2014, 15, 4495–4508. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Wang, K.; Jana, S.; Wood, D.L.; Sytsma, S.K.; Sham, J.G.; Kievit, F.M.; Zhang, M. Porous chitosan-hyaluronic acid scaffolds as a mimic of glioblastoma microenvironment ECM. Biomaterials 2013, 34, 10143–10150. [Google Scholar] [CrossRef]
- Kim, Y.; Larkin, A.L.; Davis, R.M.; Rajagopalan, P. The design of in vitro liver sinusoid mimics using chitosan–hyaluronic acid polyelectrolyte multilayers. Tissue Eng. Part A 2010, 16, 2731–2741. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Keum, J.K.; Zhou, H.; Gelfer, M.; Avila-Orta, C.A.; Pan, H.; Chen, W.; Chiao, S.M.; Hsiao, B.S. Water soluble complexes of chitosan-g-MPEG and hyaluronic acid. J. Biomed. Mater. Res. Part A 2007, 80, 800–812. [Google Scholar] [CrossRef]
- Weng, L.; Chen, X.; Chen, W. Rheological characterization of in situ crosslinkable hydrogels formulated from oxidized dextran and N-carboxyethyl chitosan. Biomacromolecules 2007, 8, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Purcell, B.P.; Lobb, D.; Charati, M.B.; Dorsey, S.M.; Wade, R.J.; Zellars, K.N.; Doviak, H.; Pettaway, S.; Logdon, C.B.; Shuman, J.A. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat. Mater. 2014, 13, 653. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L.; Wu, Q.; Wei, Y.; Gong, C. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar] [CrossRef]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-inflammatory and anti-oxidant properties of laccase-synthesized phenolic-O-carboxymethyl chitosan hydrogels. New Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, J. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Peng, X.; Peng, Y.; Han, B.; Liu, W.; Zhang, F.; Linhardt, R.J. Stimulated crosslinking of catechol-conjugated hydroxyethyl chitosan as a tissue adhesive. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 582–593. [Google Scholar] [CrossRef]
- Barbucci, R.; Magnani, A.; Consumi, M. Swelling behavior of carboxymethylcellulose hydrogels in relation to cross-linking, pH, and charge density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- Mercê, A.L.R.; Carrera, L.C.M.; Romanholi, L.K.S.; Recio, M.a.Á.L. Aqueous and solid complexes of iron (III) with hyaluronic acid: Potentiometric titrations and infrared spectroscopy studies. J. Inorg. Biochem. 2002, 89, 212–218. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puertas-Bartolomé, M.; Włodarczyk-Biegun, M.K.; del Campo, A.; Vázquez-Lasa, B.; San Román, J. 3D Printing of a Reactive Hydrogel Bio-Ink Using a Static Mixing Tool. Polymers 2020, 12, 1986. https://doi.org/10.3390/polym12091986
Puertas-Bartolomé M, Włodarczyk-Biegun MK, del Campo A, Vázquez-Lasa B, San Román J. 3D Printing of a Reactive Hydrogel Bio-Ink Using a Static Mixing Tool. Polymers. 2020; 12(9):1986. https://doi.org/10.3390/polym12091986
Chicago/Turabian StylePuertas-Bartolomé, María, Małgorzata K. Włodarczyk-Biegun, Aránzazu del Campo, Blanca Vázquez-Lasa, and Julio San Román. 2020. "3D Printing of a Reactive Hydrogel Bio-Ink Using a Static Mixing Tool" Polymers 12, no. 9: 1986. https://doi.org/10.3390/polym12091986
APA StylePuertas-Bartolomé, M., Włodarczyk-Biegun, M. K., del Campo, A., Vázquez-Lasa, B., & San Román, J. (2020). 3D Printing of a Reactive Hydrogel Bio-Ink Using a Static Mixing Tool. Polymers, 12(9), 1986. https://doi.org/10.3390/polym12091986






