Facile Synthesis of Lacunary Keggin-Type Phosphotungstates-Decorated g-C3N4 Nanosheets for Enhancing Photocatalytic H2 Generation
Abstract
1. Introduction
2. Experimental
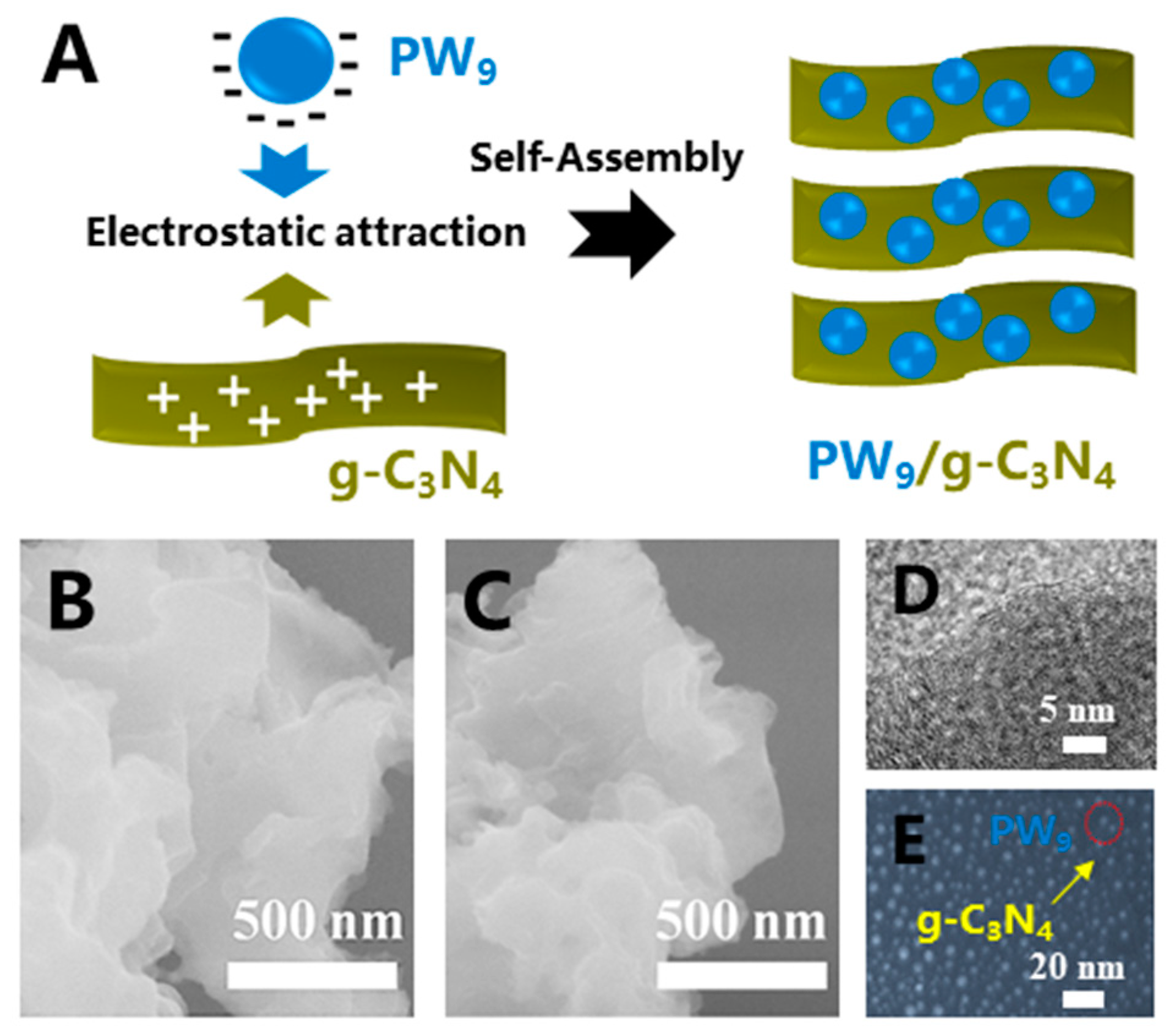
2.1. Synthesis of the PW9, g-C3N4 NS, and PW9/g-C3N4 Heterojunction NSs
2.2. Characterization
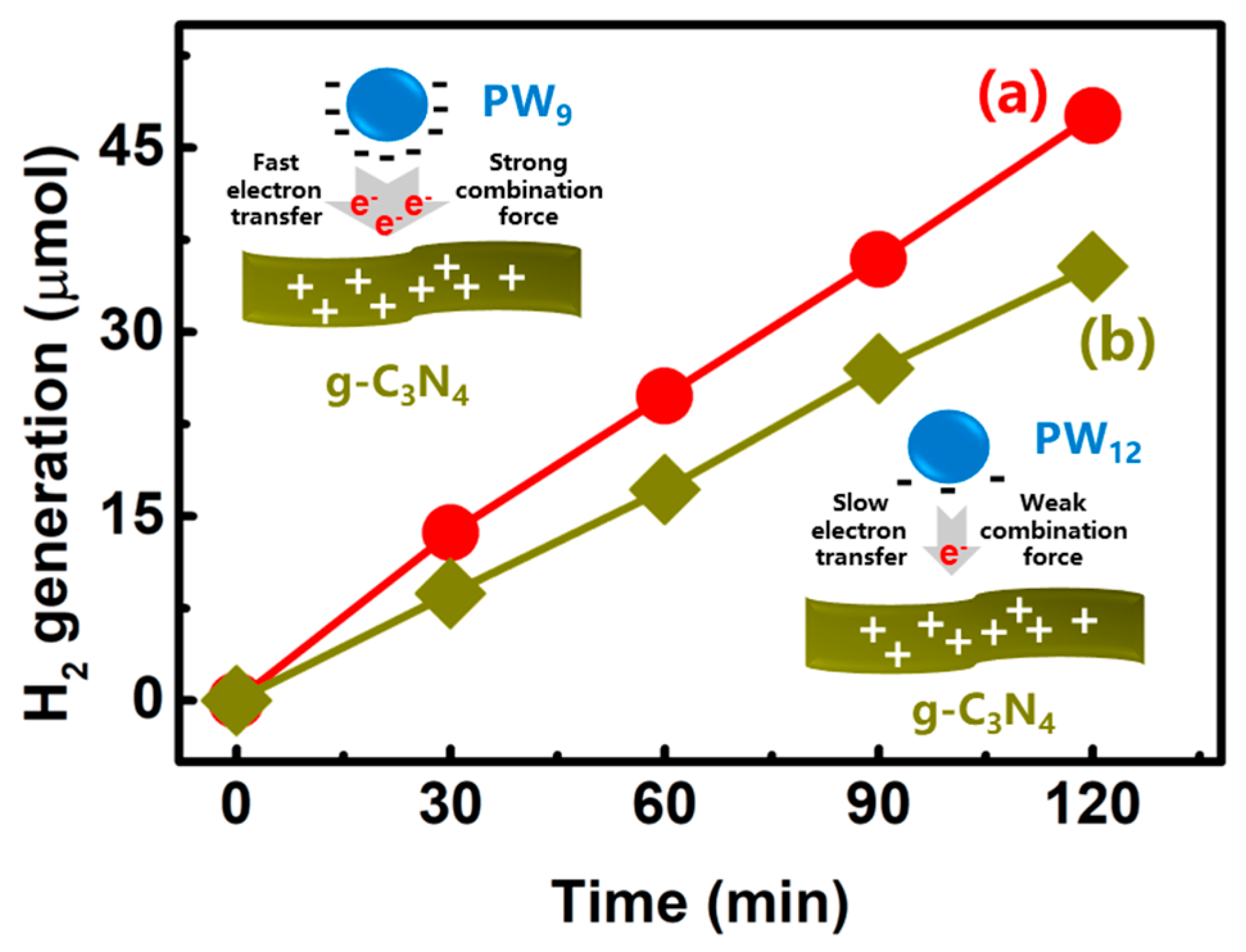
2.3. Photocatalytic H2 Generation
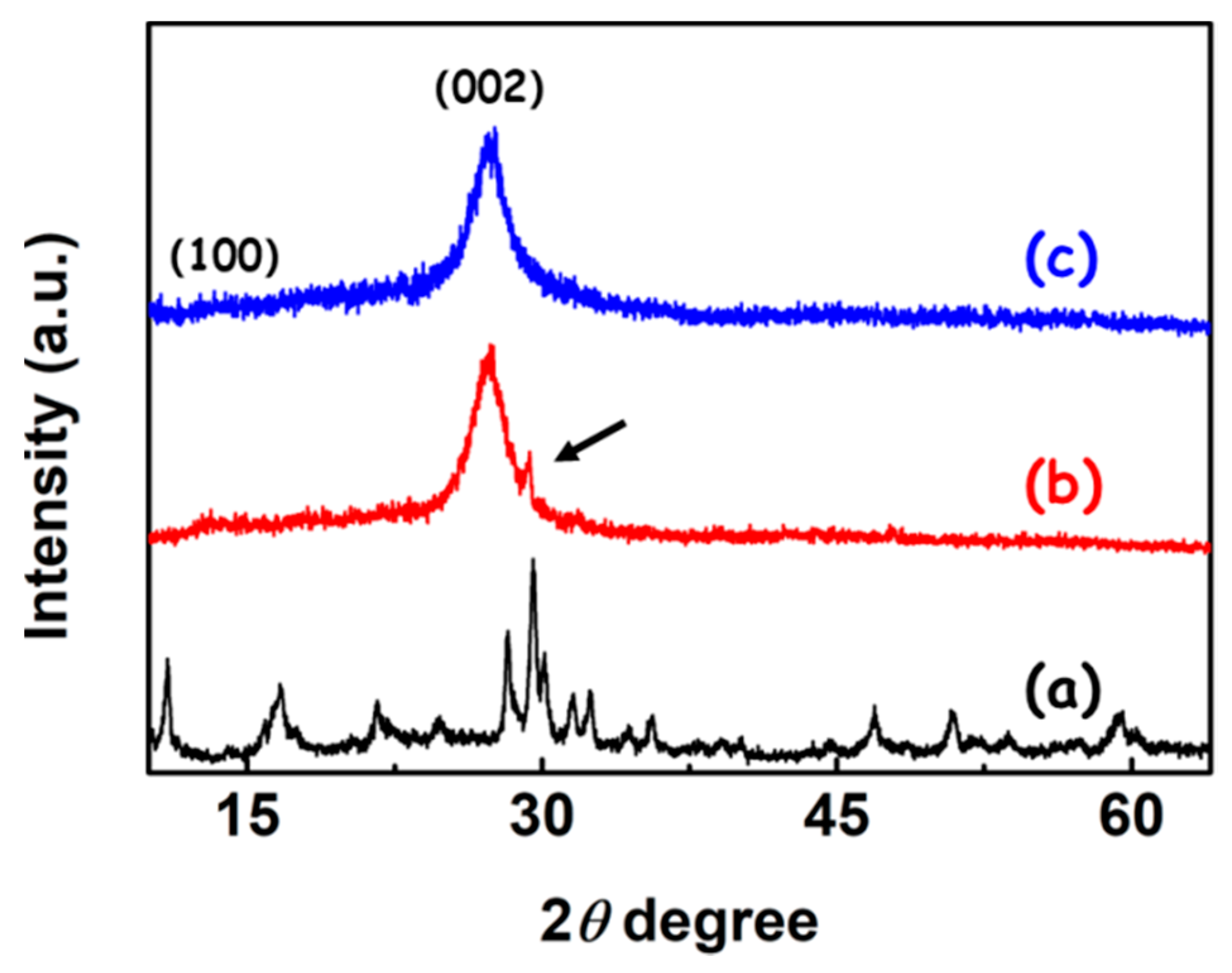
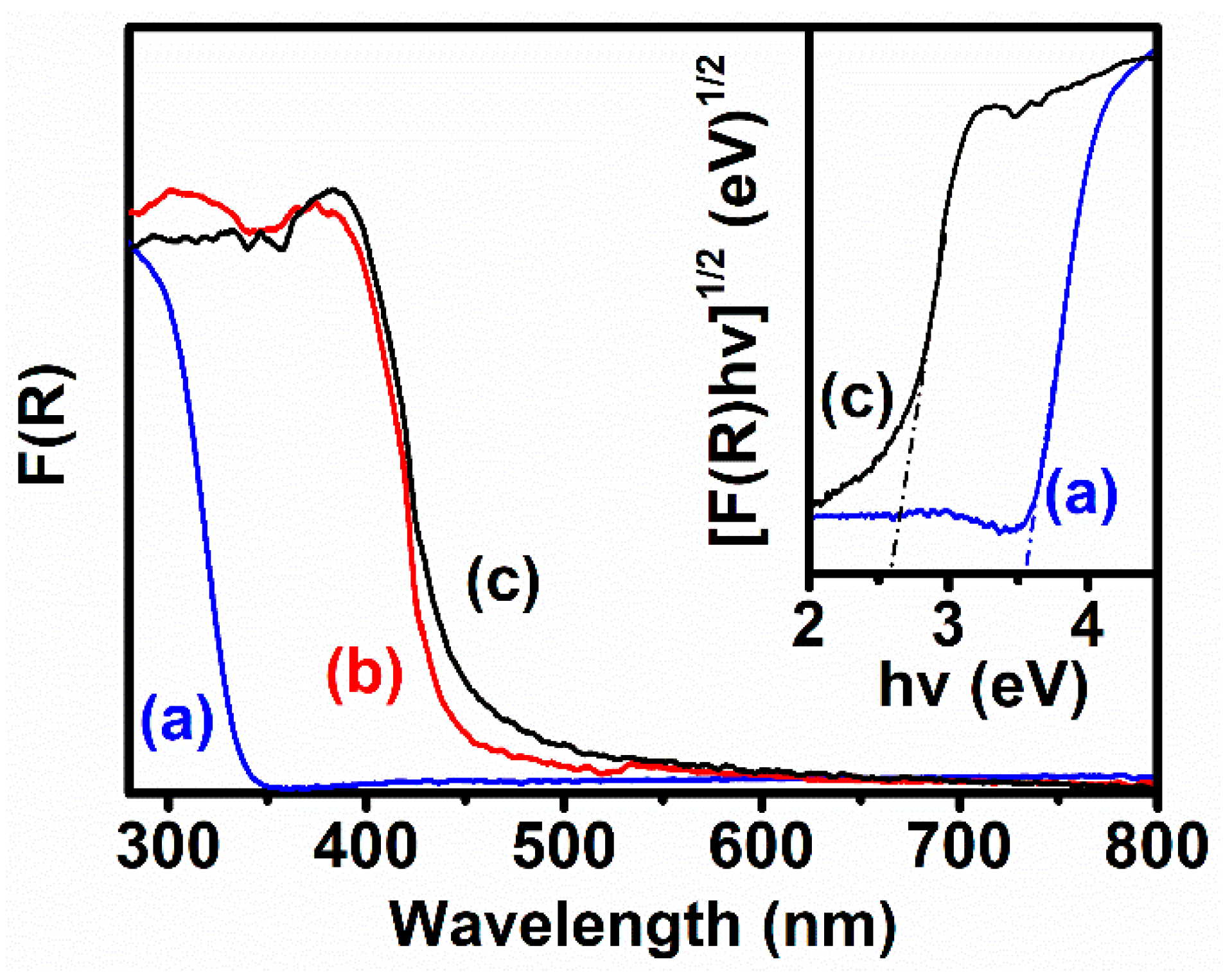
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Amirav, L.; Alivisatos, A.P. Photocatalytic Hydrogen Production with Tunable Nanorod Heterostructures. J. Phys. Chem. Lett. 2010, 1, 1051–1054. [Google Scholar] [CrossRef]
- Bai, Y.; Wilbraham, L.; Slater, B.J.; Zwijnenburg, M.A.; Sprick, R.S.; Cooper, A.I. Accelerated Discovery of Organic Polymer Photocatalysts for Hydrogen Evolution from Water through the Integration of Experiment and Theory. J. Am. Chem. Soc. 2019, 141, 9063–9071. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Gul, I.; Sayed, M.; Shah, N.S.; Ali Khan, J.; Polychronopoulou, K.; Iqbal, J.; Rehman, F. Solar light responsive bismuth doped titania with Ti3+ for efficient photocatalytic degradation of flumequine: Synergistic role of peroxymonosulfate. Chem. Eng. J. 2020, 384, 123255. [Google Scholar] [CrossRef]
- Sayed, M.; Arooj, A.; Shah, N.S.; Khan, J.A.; Shah, L.A.; Rehman, F.; Arandiyan, H.; Khan, A.M.; Khan, A.R. Narrowing the band gap of TiO2 by co-doping with Mn2+ and Co2+ for efficient photocatalytic degradation of enoxacin and its additional peroxidase like activity: A mechanistic approach. J. Mol. Liq. 2018, 272, 403–412. [Google Scholar] [CrossRef]
- Sayed, M.; Gul, M.; Shah, N.S.; Khan, J.A.; Khan, Z.U.H.; Rehman, F.; Khan, A.R.; Rauf, S.; Arandiyan, H.; Yang, C.P. In-situ dual applications of ionic liquid coated Co2+ and Fe3+ co-doped TiO2: Superior photocatalytic degradation of ofloxacin at pilot scale level and enhanced peroxidase like activity for calorimetric biosensing. J. Mol. Liq. 2019, 282, 275–285. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Selli, E. TiO2-based materials for photocatalytic hydrogen production. J. Energy Chem. 2017, 26, 250–258. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, Z.; Li, Y.; Peng, S.; Liu, B. The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts. Appl. Catal. B Environ. 2020, 269. [Google Scholar] [CrossRef]
- Hu, C.; Lin, Y.-R.; Yang, H.-C. Recent Developments in Graphitic Carbon Nitride Based Hydrogels as Photocatalysts. ChemSusChem 2019, 12, 1794–1806. [Google Scholar] [CrossRef] [PubMed]
- Balula, S.S.; Santos, I.C.M.S.; Cunha-Silva, L.; Carvalho, A.P.; Pires, J.; Freire, C.; Cavaleiro, J.A.S.; de Castro, B.; Cavaleiro, A.M.V. Phosphotungstates as catalysts for monoterpenes oxidation: Homo- and heterogeneous performance. Catal. Today 2013, 203, 95–102. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X.; Yang, X.; Hu, A.; Guo, Y.; Yang, Y. Enhanced gas-phase photocatalytic removal of aromatics over direct Z-scheme-dictated H3PW12O40/g-C3N4 film-coatedoptical fibers. Appl. Catal. B Environ. 2019, 251, 168–180. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, Y.; Zhang, M. CO2 photoreduction to CO/CH4 over Bi2W0.5Mo0.5O6 solid solution nanotubes under visible light. RSC Adv. 2020, 10, 8821–8824. [Google Scholar] [CrossRef]
- Gagea, B.C.; Lorgouilloux, Y.; Altintas, Y.; Jacobs, P.A.; Martens, J.A. Bifunctional conversion of n-decane over HPW heteropoly acid incorporated into SBA-15 during synthesis. J. Catal. 2009, 265, 99–108. [Google Scholar] [CrossRef]
- Llanos, A.; Melo, L.; Avendano, F.; Montes, A.; Brito, J.L. Synthesis and characterization of HPW/MCM-41 (Si) and HPW/MCM-41 (Si/Al) catalysts: Activity for toluene alkylation with 1-dodecene. Catal. Today 2008, 133, 20–27. [Google Scholar] [CrossRef]
- Song, Y.X.; Xin, F.; Zhang, L.X.; Wang, Y. Oxidation of Cyclohexene in the Presence of Transition-Metal-Substituted Phosphotungstates and Hydrogen Peroxide: Catalysis and Reaction Pathways. ChemCatChem 2017, 9, 4139–4147. [Google Scholar] [CrossRef]
- Domaille, P.J.; Hervéa, G.; Téazéa, A. Vanadium(V) Substituted Dodecatungstophosphates. In Inorganic Syntheses; Ginsberg, A.P., Ed.; Wiley: New York, NY, USA, 1990; Volume 27, Chapter 17; pp. 96–104. [Google Scholar]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Li, X.Y.; Ding, C.; Zhao, C.X.; Wang, F.; Li, C.L.; Yang, X.F. Activation of graphitic carbon nitride by solvent -mediated supramolecular assembly for enhanced hydrogen evolution. Appl. Surf. Sci. 2020, 525. [Google Scholar] [CrossRef]
- Huang, J.; Liu, T.; Wang, R.; Zhang, M.; Wang, L.; She, H.; Wang, Q. Facile loading of cobalt oxide on bismuth vanadate: Proved construction of p-n junction for efficient photoelectrochemical water oxidation. J. Colloid Interface Sci. 2020, 570, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Zhao, S.; Gong, Y.; Djellabi, R.; Lin, S.; Zhao, X. Highly-efficient photocatalytic hydrogen peroxide production over polyoxometalates covalently immobilized onto titanium dioxide. Appl. Catal. A Gen. 2020, 591, 117271. [Google Scholar] [CrossRef]
- Dante, R.C.; Chamorro-Posada, P.; Vázquez-Cabo, J.; Rubi?os-López, ó.; Sánchez-árevalo, F.M.; Huerta, L.; Martín-Ramos, P.; Lartundo-Rojas, L.; ávila-Vega, C.F.; Rivera-Tapia, E.D. Nitrogen-carbon graphite-like semiconductor synthesized from uric acid. Carbon 2017, 121, 368–379. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, L.; Xu, J.; Liu, Y.; Jiang, D.; Du, P. Nitrogen photofixation on holey g-C3N4 nanosheets with carbon vacancies under visible-light irradiation. Chin. Chem. Lett. 2020, 31, 792–796. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, B.; Yao, Q.; Zhou, Y.; Yang, H.; Liu, Y. H3PW12O40/mpg-C3N4 as an efficient and reusable catalyst in the alkylation of o-xylene and styrene. Appl. Organomet. Chem. 2019, 33. [Google Scholar] [CrossRef]
- Li, K.; Yan, L.; Zeng, Z.; Luo, S.; Luo, X.; Liu, X.; Guo, H.; Guo, Y. Fabrication of H3PW12O40-doped carbon nitride nanotubes by one-step hydrothermal treatment strategy and their efficient visible-light photocatalytic activity toward representative aqueous persistent organic pollutants degradation. Appl. Catal. B Environ. 2014, 156, 141–152. [Google Scholar] [CrossRef]
- Li, K.; Hu, J.; Li, W.; Ma, F.; Xu, L.; Guo, Y. Design of mesostructured H3PW12O40-silica materials with controllable ordered and disordered pore geometries and their application for the synthesis of diphenolic acid. J. Mater. Chem. 2009, 19, 8628–8638. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, X. Nanostructure Engineering and Doping of Conjugated Carbon Nitride Semiconductors for Hydrogen Photosynthesis. Angew. Chem. Int. Ed. 2013, 52, 1735–1738. [Google Scholar] [CrossRef]
- Li, X.-H.; Wang, X.; Antonietti, M. Solvent-Free and Metal-Free Oxidation of Toluene Using O2 and g-C3N4 with Nanopores: Nanostructure Boosts the Catalytic Selectivity. ACS Catal. 2012, 2, 2082–2086. [Google Scholar] [CrossRef]
- Pinto, T.; Arquilliere, P.; Dufaud, V.; Lefebvre, F. Isomerization of n-hexane over Pt-H3PW12O40/SBA-15 bifunctional catalysts: Effect of the preparation method on catalytic performance. Appl. Catal. A Gen. 2016, 528, 44–51. [Google Scholar] [CrossRef]
- Cao, S.-W.; Liu, X.-F.; Yuan, Y.-P.; Zhang, Z.-Y.; Liao, Y.-S.; Fang, J.; Loo, S.C.J.; Sum, T.C.; Xue, C. Solar-to-fuels conversion over In2O3/g-C3N4 hybrid photocatalysts. Appl. Catal. B Environ. 2014, 147, 940–946. [Google Scholar] [CrossRef]
- Tian, B.; Tian, B.; Smith, B.; Scott, M.C.; Hua, R.; Lei, Q.; Tian, Y. Supported black phosphorus nanosheets as hydrogen-evolving photocatalyst achieving 5.4% energy conversion efficiency at 353K. Nat. Commun. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Pablo, M.-R.; Jesús, M.-G.; Manuela, R.S. Polymeric Carbon Nitride-Based Composites for Visible-Light-Driven Photocatalytic Hydrogen Generation. In Hydrogen Production Technologies; Scrivener Publishing: Beverly, MA, USA, 2017; pp. 579–621. [Google Scholar] [CrossRef]
- Cao, S.-W.; Liu, X.-F.; Yuan, Y.-P.; Zhang, Z.-Y.; Fang, J.; Loo, S.C.J.; Barber, J.; Sum, T.C.; Xue, C. Artificial photosynthetic hydrogen evolution over g-C3N4 nanosheets coupled with cobaloxime. Phys. Chem. Chem. Phys. 2013, 15, 18363–18366. [Google Scholar] [CrossRef]
- Guo, Y.; Li, K.; Yu, X.; Clark, J.H. Mesoporous H3PW12O40-silica composite: Efficient and reusable solid acid catalyst for the synthesis of diphenolic acid from levulinic acid. Appl. Catal. B Environ. 2008, 81, 182–191. [Google Scholar] [CrossRef]
- Lu, N.; Zhao, Y.H.; Liu, H.B.; Guo, Y.H.; Yuan, X.; Xu, H.; Peng, H.F.; Qin, H.W. Design of polyoxometallate-titania composite film (H3PW12O40/TiO2) for the degradation of an aqueous dye Rhodamine B under the simulated sunlight irradiation. J. Hazard. Mater. 2012, 199, 1–8. [Google Scholar] [CrossRef]
- Svoboda, L.; Licciardello, N.; Dvorsky, R.; Bednar, J.; Henych, J.; Cuniberti, G. Design and Performance of Novel Self-Cleaning g-C3N4/PMMA/PUR Membranes. Polymers 2020, 12, 850. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, T.; Lv, Z.; Cui, M.; Zhao, Z.; Cao, X.; Wei, Q. TiO2 Sol-Gel Coated PAN/O-MMT Multi-Functional Composite Nanofibrous Membrane Used as the Support for Laccase Immobilization: Synergistic Effect between the Membrane Support and Enzyme for Dye Degradation. Polymers 2020, 12, 139. [Google Scholar] [CrossRef]
- Li, K.; Guo, Y.; Ma, F.; Li, H.; Chen, L.; Guo, Y. Design of ordered mesoporous H3PW12O40-titania materials and their photocatalytic activity to dye methyl orange degradation. Catal. Commun. 2010, 11, 839–843. [Google Scholar] [CrossRef]
- Wei, X.; Shao, C.; Li, X.; Lu, N.; Wang, K.; Zhang, Z.; Liu, Y. Facile in situ synthesis of plasmonic nanoparticles-decorated g-C3N4/TiO2 heterojunction nanofibers and comparison study of their photosynergistic effects for efficient photocatalytic H2 evolution. Nanoscale 2016, 8, 11034–11043. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Xiao, F.; Zhang, L.; Yue, F.; Liang, X.; Wang, J.; Su, X. W18O49 nanowires grown on g-C3N4 sheets with enhanced photocatalytic hydrogen evolution activity under visible light. J. Mol. Catal. A Chem. 2016, 418–419, 95–102. [Google Scholar] [CrossRef]
- Liu, J.; Liu, N.; Li, H.; Wang, L.; Wu, X.; Huang, H.; Liu, Y.; Bao, F.; Lifshitz, Y.; Lee, S. A critical study of the generality of the two step two electron pathway for water splitting by application of a C3N4/MnO2 photocatalyst. Nanoscale 2016, 8, 11956–11961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, K.; Feng, Z.; Bao, Y.; Dong, B. Hierarchical Sheet-on-Sheet ZnIn2S4/g-C3N4 Heterostructure with Highly Efficient Photocatalytic H2 production Based on Photoinduced Interfacial Charge Transfer. Sci. Rep. 2016, 6, 19221. [Google Scholar] [CrossRef]
- Li, A.; Peng, Z.; Fu, X. Exfoliated, mesoporous W18O49/g-C3N4 composites for efficient photocatalytic H2 evolution. Solid State Sci. 2020, 106, 106298. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, B.; Gong, G.; Li, D.; Liu, Y.; Cao, W.; Xing, L.; Zeng, J.; Shi, W.; Yuan, S. In-situ growth of Zn–AgIn5S8 quantum dots on g-C3N4 towards 0D/2D heterostructured photocatalysts with enhanced hydrogen production. Int. J. Hydrogen Energy 2019, 44, 15882–15891. [Google Scholar] [CrossRef]
- Jin, X.; Fan, X.; Tian, J.; Cheng, R.; Li, M.; Zhang, L. MoS2 quantum dot decorated g-C3N4 composite photocatalyst with enhanced hydrogen evolution performance. RSC Adv. 2016, 6, 52611–52619. [Google Scholar] [CrossRef]
- Li, L.; Wu, Q.Y.; Guo, Y.H.; Hu, C.W. Nanosize and bimodal porous polyoxotungstate-anatase TiO2 composites: Preparation and photocatalytic degradation of organophosphorus pesticide using visible-light excitation. Microporous Mesoporous Mater. 2005, 87, 1–9. [Google Scholar] [CrossRef]
- Blanco, M.; Monteserin, C.; Angulo, A.; Perez-Marquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-Doped Electrospun Nanofibrous Membrane for Photocatalytic Water Treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, M.Y.; Ma, X.Z.; Li, L.; Zhou, X.J.; Zhang, Z.Y. Asymmetric supercapacitors by integrating high content Na+/K+-inserted MnO2 nanosheets and layered Ti3C2Tx paper. Electrochim. Acta 2020, 332, 135497. [Google Scholar] [CrossRef]


| Catalyst | Precursor | Light Source | Activity | Ref. |
|---|---|---|---|---|
| Ag/g-C3N4/TiO2 | Melamine | 300 W Xe lamp with AM 1.5 filter | 1.5 μmol/h | [44] |
| W18O49/g-C3N4 | Urea | 300 W Xe lamp with λ > 420 nm filter | 3.69 μmol/h | [45] |
| g-C3N4/MnO2 | Urea | 300 W Xe lamp with λ > 420 nm filter | 5.53 μmol/h | [46] |
| ZnIn2S4/g-C3N4 | Melamine | 300 W Xe lamp with λ > 420 nm filter | 14.1 μmol/h | [47] |
| W18O49/g-C3N4 | Melamine | 300 W Xe lamp with λ > 420 nm filter | 18.25 μmol/h | [48] |
| Zn-AgIn5S8/g-C3N4 | Urea | 300 W Xe lamp with λ > 420 nm filter | 17.32 μmol/h | [49] |
| MoS2/g-C3N4 | Urea | 300 W Xe lamp with λ > 420 nm filter | 19.66 μmol/h | [50] |
| PW9/g-C3N4 | Urea | 300 W Xe lamp with AM 1.5 filter | 23.8 μmol/h | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Sun, M.; Wei, X.; Zhang, P.; Zhang, Z. Facile Synthesis of Lacunary Keggin-Type Phosphotungstates-Decorated g-C3N4 Nanosheets for Enhancing Photocatalytic H2 Generation. Polymers 2020, 12, 1961. https://doi.org/10.3390/polym12091961
Lu N, Sun M, Wei X, Zhang P, Zhang Z. Facile Synthesis of Lacunary Keggin-Type Phosphotungstates-Decorated g-C3N4 Nanosheets for Enhancing Photocatalytic H2 Generation. Polymers. 2020; 12(9):1961. https://doi.org/10.3390/polym12091961
Chicago/Turabian StyleLu, Na, Menghan Sun, Xiaoming Wei, Peng Zhang, and Zhenyi Zhang. 2020. "Facile Synthesis of Lacunary Keggin-Type Phosphotungstates-Decorated g-C3N4 Nanosheets for Enhancing Photocatalytic H2 Generation" Polymers 12, no. 9: 1961. https://doi.org/10.3390/polym12091961
APA StyleLu, N., Sun, M., Wei, X., Zhang, P., & Zhang, Z. (2020). Facile Synthesis of Lacunary Keggin-Type Phosphotungstates-Decorated g-C3N4 Nanosheets for Enhancing Photocatalytic H2 Generation. Polymers, 12(9), 1961. https://doi.org/10.3390/polym12091961







