Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nano-Biocomposite Films Preparation
2.3. Migration and Mathematical Diffusion Analysis
2.4. Determination of Antioxidant Activity
2.5. Bacterial Strains, Culture Conditions and Antibacterial Activity
2.6. Study of Disintegrability Under Composting Conditions
2.7. Statistical Analysis
3. Results
3.1. Migration Test and Antioxidant Activity of the Active PLA Nano-Biocomposite Films
Mathematical Modelling of Thymol Released from PLA Nano-Biocomposite Films
3.2. Antibacterial Activity
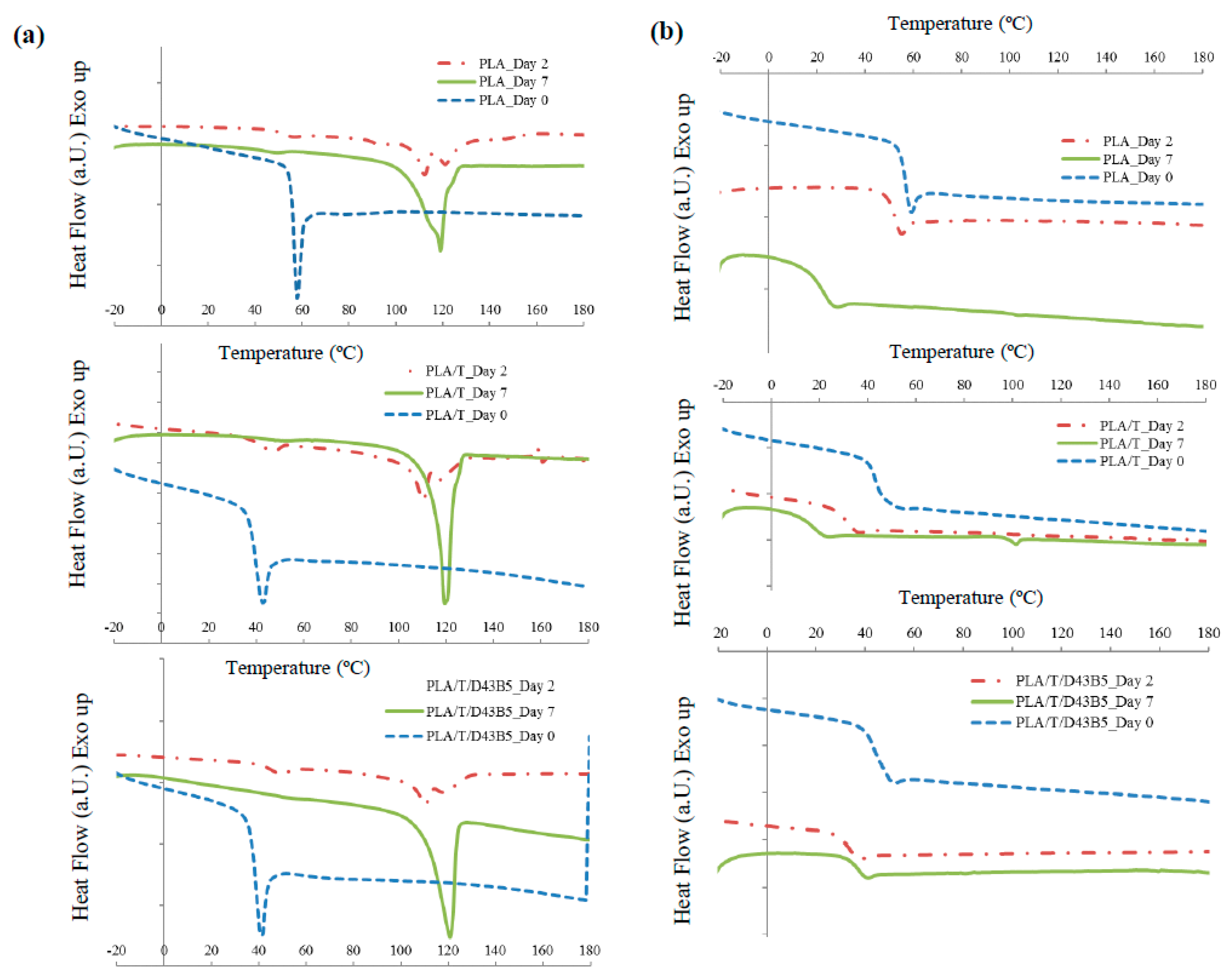
3.3. Disintegrability Under Composting Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clen. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Ramos, M.; Beltran, A.; Fortunati, E.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled release of thymol from poly(lactic acid)-based silver nanocomposite films with antibacterial and antioxidant activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B 2019, 176, 176. [Google Scholar] [CrossRef]
- Mayekar, P.C.; Castro-Aguirre, E.; Auras, R.; Selke, S.; Narayan, R. Effect of nano-clay and surfactant on the biodegradation of poly(lactic acid) films. Polymers 2020, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.; Morales, A.; Marin-Morales, M.; Mei, L. PLA and montmorillonite nanocomposites: Properties, biodegradation and potential toxicity. J. Polym. Environ. 2013, 21, 738–759. [Google Scholar] [CrossRef]
- Kalendova, A.; Smotek, J.; Stloukal, P.; Kracalik, M.; Slouf, M.; Laske, S. Transport properties of poly(lactic acid)/clay nanocomposites. Poly. Eng. Sci. 2019, 59, 2498–2501. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Garrigós, M.C. Active nanocomposites in food contact materials. In Nanoscience in Food and Agriculture 4; Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–44. [Google Scholar]
- Llanos, J.H.R.; Tadini, C.C. Preparation and characterization of bio-nanocomposite films based on cassava starch or chitosan, reinforced with montmorillonite or bamboo nanofibers. Int. J. Biol. Macromol. 2018, 107, 371–382. [Google Scholar] [CrossRef]
- Dobrucka, R.; Przekop, R. New perspectives in active and intelligent food packaging. J. Food Process. Preserv. 2019, 43, e14194. [Google Scholar] [CrossRef]
- Jahed, E.; Khaledabad, M.A.; Bari, M.R.; Almasi, H. Effect of cellulose and lignocellulose nanofibers on the properties of origanum vulgare ssp. Gracile essential oil-loaded chitosan films. React. Funct. Polym. 2017, 117, 70–80. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2017, 17, 165–199. [Google Scholar] [CrossRef]
- Tornuk, F.; Sagdic, O.; Hancer, M.; Yetim, H. Development of LLDPE based active nanocomposite films with nanoclays impregnated with volatile compounds. Food Res. Int. 2018, 107, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, N.; Ziani Cherif, H.; Borsali, F.; Benmansour, K.; Meghezzi, A. Preparation of active antimicrobial and antifungal alginate-montmorillonite/lemon essential oil nanocomposite films. Mater. Tech. 2020, 35, 383–394. [Google Scholar] [CrossRef]
- Valdes, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jimenez, A.; Garrigos, M.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef]
- Yahyaoui, M.; Gordobil, O.; Herrera Díaz, R.; Abderrabba, M.; Labidi, J. Development of novel antimicrobial films based on poly(lactic acid) and essential oils. React. Funct. Polym. 2016, 109, 1–8. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly(lactic acid)/poly(butylene-succinate-co-adipate) (PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Pack. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An active packaging film based on yam starch with eugenol and its application for pork preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Chen, M.; Jiang, S.; Cheng, J.; Li, X.; Zhang, M.; Jiang, S. Gelatin/zein fiber mats encapsulated with resveratrol: Kinetics, antibacterial activity and application for pork preservation. Food Hydrocoll. 2020, 101, 105577. [Google Scholar] [CrossRef]
- Wang, H.; Hao, L.; Wang, P.; Chen, M.; Jiang, S.; Jiang, S. Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocoll. 2017, 63, 437–446. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films based on κ-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Cran, M.J.; Bigger, S.W. Release of thymol from poly(lactic acid)-based antimicrobial films containing kenaf fibres as natural filler. LWT Food Sci. Technol. 2016, 66, 629–637. [Google Scholar] [CrossRef]
- Galotto, M.J.; López De Dicastillo, C.; Torres, A.; Guarda, A. Chpater 45. Thymol: Use in antimicrobial packaging. In Antimicrob Food Pack; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 553–562. [Google Scholar]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Song, S.; Luo, M.; Zhang, C.; Li, W.; Li, L.; Qin, Y. Effect of PLA nanocomposite films containing bergamot essential oil, TiO2 nanoparticles, and Ag nanoparticles on shelf life of mangoes. Sci. Hortic. 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated otolithes ruber fillets. Food Pack. Shelf Life 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Luzi, F.; Dominici, F.; Armentano, I.; Fortunati, E.; Burgos, N.; Fiori, S.; Jiménez, A.; Kenny, J.M.; Torre, L. Combined effect of cellulose nanocrystals, carvacrol and oligomeric lactic acid in PLA_PHB polymeric films. Carbohydr. Polym. 2019, 223, 115131. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Gulino, E.; Morreale, M.; Mantia, F. The effects of nanoclay on the mechanical properties, carvacrol release and degradation of a PLA/PBAT blend. Materials 2020, 13, 983. [Google Scholar] [CrossRef]
- Shebi, A.; Lisa, S. Evaluation of biocompatibility and bactericidal activity of hierarchically porous PLA/TiO2 nanocomposite films fabricated by breath-figure method. Mater. Chem. Phys. 2019, 230, 308–318. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef]
- Plastic materials and articles intended to come into contact with food. In Commission Regulation/(EU)/Nº-10/2011; European Commission: Brussels, Belgium, 2011; pp. 2–89.
- UNE-EN_13130-1. Materials and Articles in Contact with Foodstuffs-Plastics Substances Subject to Limitation-Part 1: Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and Food Simulants and the Determination of Substances in Plastics and the Selection of Conditions of Exposure to Food Simulants. Eur. Comm. Stand. 2005. [Google Scholar]
- Silva, A.S.; Cruz Freire, J.M.; Sendón, R.; Franz, R.; Paseiro Losada, P. Migration and diffusion of diphenylbutadiene from packages into foods. J. Agric. Food Chem. 2009, 57, 10225–10230. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; p. 414. [Google Scholar]
- ISO, UNEEN. Plastics-Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test. UNE-EN_20200. 2015. Available online: https://www.iso.org/standard/63367.html (accessed on 19 August 2020).
- Sanchez-Garcia, M.D.; Ocio, M.J.; Gimenez, E.; Lagaron, J.M. Novel polycaprolactone nanocomposites containing thymol of interest in antimicrobial film and coating applications. J. Plast. Film Sheeting 2008, 24, 239–251. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Sanfuentes, E.A. The synergistic antimicrobial effect of carvacrol and thymol in clay/polymer nanocomposite films over strawberry gray mold. LWT Food Sci. Technol. 2015, 64, 390–396. [Google Scholar] [CrossRef]
- Mastelic, J.; Jerkovic, I.; Blazevic, I.; Poljak-Blazi, M.; Borovic, S.; Ivancic-Bace, I.; Smrecki, V.; Zarkovic, N.; Brcic-Kostic, K.; Vikic-Topic, D.; et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Tehrany, E.A.; Desobry, S. Release of synthetic phenolic antioxidants from extruded poly lactic acid (PLA) film. Food Control. 2012, 28, 445–455. [Google Scholar] [CrossRef]
- Chung, D.; Papadakis, S.E.; Yam, K.L. Simple models for assessing migration from food-packaging films. Food Addit. Contam. 2002, 19, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Mascheroni, E.; Guillard, V.; Nalin, F.; Mora, L.; Piergiovanni, L. Diffusivity of propolis compounds in polylactic acid polymer for the development of anti-microbial packaging films. J. Food Eng. 2010, 98, 294–301. [Google Scholar] [CrossRef]
- Mhlanga, N.; Ray, S.S. Kinetic models for the release of the anticancer drug doxorubicin from biodegradable polylactide/metal oxide-based hybrids. Int. J. Biol. Macromol. 2015, 72, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, H.; Soto-Valdez, H.; Auras, R. Poly(lactic acid) film incorporated with marigold flower extract (tagetes erecta) intended for fatty-food application. Food Control. 2014, 46, 55–66. [Google Scholar] [CrossRef]
- Ramos, M.; Beltrán, A.; Peltzer, M.; Valente, A.J.M.; Garrigós, M.C. Release and antioxidant activity of carvacrol and thymol from polypropylene active packaging films. LWT Food Sci. Technol. 2014, 58, 470–477. [Google Scholar] [CrossRef]
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near critical and supercritical impregnation and kinetic release of thymol in LLDPE films used for food packaging. J. Supercrit. Fluids 2014, 85, 41–48. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Jiang, L.; Chuan, Y.; Yuan, M.; Chen, H. Characterization of active packaging films made from poly(lactic acid)/poly(trimethylene carbonate) incorporated with oregano essential oil. Molecules 2016, 21, 695. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef]
- de Azeredo, H.M.C. Antimicrobial nanostructures in food packaging. Trends Food Sci. Technol. 2013, 30, 56–69. [Google Scholar] [CrossRef]
- Hong, S.-I.; Rhim, J.-W. Antimicrobial activity of organically modified nano-clays. J. Nanosci. Nanotechnol. 2008, 8, 5818–5824. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and its blends with poly(butylene succinate) (PBS): A brief review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Puglia, D.; Dominici, F.; Santulli, C.; Kenny, J.M.; Torre, L. Investigation of thermo-mechanical, chemical and degradative properties of PLA-limonene films reinforced with cellulose nanocrystals extracted from phormium tenax leaves. Eur. Polym. J. 2014, 56, 77–91. [Google Scholar] [CrossRef]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(D,L-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Jimenez, A.; Kenny, J.M.; Garrigós, M.C. Characterization and disintegrability under composting conditions of PLA-based nanocomposite films with thymol and silver nanoparticles. Polym. Degrad. Stab. 2016, 132, 2–10. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.M.; Puglia, D. Effect of processing conditions and lignin content on thermal, mechanical and degradative behavior of lignin nanoparticles/polylactic (acid) bionanocomposites prepared by melt extrusion and solvent casting. Eur. Polym. J. 2015, 71, 126–139. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Kasperska, P.; Koter, I. Effect of poly(ε-caprolactone) as plasticizer on the properties of composites based on polylactide during hydrolytic degradation. React. Funct. Polym. 2016, 103, 99–107. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Sedničková, M.; Pekařová, S.; Kucharczyk, P.; Bočkaj, J.; Janigová, I.; Kleinová, A.; Jochec-Mošková, D.; Omaníková, L.; Perďochová, D.; Koutný, M.; et al. Changes of physical properties of PLA-based blends during early stage of biodegradation in compost. Int. J. Biol. Macromol. 2018, 113, 434–442. [Google Scholar] [CrossRef] [PubMed]


| PLA/T | PLA/T/D43B2.5 | PLA/T/D43B5 | |
|---|---|---|---|
| MP,0 (mg) | 16.60 ± 0.20 | 17.82 ± 0.09 | 17.21 ± 0.05 |
| MF,∞ (mg) | 6.25 ± 0.22 | 6.31 ± 0.27 | 5.29 ± 0.29 |
| l/cm | 0.0167 | 0.0215 | 0.0180 |
| A | 1.65 | 1.82 | 2.25 |
| KP,F | 60.3 | 42.4 | 41.1 |
| Equations (5) and (6): | |||
| D (cm2 s−1) | 3.36 × 10−11 | 4.86 × 10−11 | 2.25 × 10−11 |
| RMSE | 0.0773 | 0.0698 | 0.114 |
| Equations (6) and (8): | |||
| D’ (cm2 s−1) | 5.95 × 10−12 | 7.45 × 10−12 | 5.82 × 10−12 |
| RMSE | 0.00362 | 0.00306 | 0.00370 |
| Formulation | Cell Viability (%) | |||
|---|---|---|---|---|
| S. Aureus 8325-4 | E. Coli | |||
| 3 h | 24 h | 3 h | 24 h | |
| At 4 °C | ||||
| PLA | 100.0 ± 1.7 a | 100.0 ± 1.8 a | 100.0 ± 6.7 a | 100.0 ± 2.5 a |
| PLA/T | 83.5 ± 3.3 b | 85.5 ± 1.1 c | 65.4 ± 5.4 b | 60.6 ± 1.0 c |
| PLA/D43B2.5 | 98.0 ± 1.1 a | 88.6 ± 0.9 b,c | 83.5 ± 2.9 a | 74.0 ± 4.2 b |
| PLA/D43B5 | 91.1 ± 1.3 c | 94.4 ± 1.7 a,b | 88.0 ± 3.2 a | 77.8 ± 3.5 b |
| PLA/T/D43B2.5 | 68.0 ± 0.3 d | 60.9 ± 2.0 d | 53.7 ± 3.3 b | 52.0 ± 0.5 c,d |
| PLA//T/D43B5 | 70.0 ± 0.5 d | 61.6 ± 1.0 d | 54.4 ± 2.9 b | 50.0 ± 0.7 d |
| At 24 °C | ||||
| PLA | 100.0 ± 1.0 a | 100.0 ± 0.5 a | 100.0 ± 3.8 a | 100.0 ± 2.2 a |
| PLA/T | 72.8 ± 2.2 b | 62.4 ± 1.5 b | 69.5 ± 1.6 b | 66.2 ± 1.4 c |
| PLA/D43B2.5 | 83.4 ± 1.1 a | 83.4 ± 1.4 c | 75.3 ± 2.8 b | 82.6 ± 1.9 b |
| PLA/D43B5 | 75.4 ± 1.4 c | 81.2 ± 1.7 c | 70.3 ± 1.3 b | 80.0 ± 3.3 b |
| PLA/T/D43B2.5 | 56.1 ± 1.2 d | 59.4 ± 1.4 b | 53.1 ± 0.2 c | 57.9 ± 0.7 c,d |
| PLA//T/D43B5 | 56.6 ± 2.0 d | 58.7 ± 0.8 b | 59.6 ± 2.0 c | 60.2 ± 1.5 d |
| At 37 °C | ||||
| PLA | 100.0 ± 3.8 a | 100.0 ± 2.1 a | 100.0 ± 1.7 a | 100.0 ± 3.1 a |
| PLA/T | 57.9 ± 2.2 b | 73.3 ± 1.2 c | 62.9 ± 0.5 b | 71.2 ± 1.4 c |
| PLA/D43B2.5 | 63.8 ± 2.0 b | 84.6 ± 0.9 b | 67.6 ± 0.9 b | 79.4 ± 1.2 b |
| PLA/D43B5 | 64.3 ± 1.5 b | 89.8 ± 2.0 b | 64.8 ± 2.3 b | 71.7 ± 1.2 c |
| PLA/T/D43B2.5 | 44.3 ± 1.4 c | 51.6 ± 0.5 d | 47.2 ± 1.2 c | 49.3 ± 0.5 d |
| PLA/T/D43B5 | 48.5 ± 1.1 c | 59.6 ± 3.2 e | 47.3 ± 1.4 c | 53.2 ± 1.2 d |
| Time (days) | Disintegrability (%) | |||||
|---|---|---|---|---|---|---|
| PLA | PLA/T | PLA/T/D43B2.5 | PLA/T/D43B5 | PLA/D43B2.5 | PLA/D43B5 | |
| 2 | 0.3 ± 0.1 a | 5.1 ± 0.4 c | 8.4 ± 0.9 d | 7.2 ± 0.3 d | 2.4 ± 0.7 b | 1.2 ± 0.1 a,b |
| 4 | 0.4 ± 0.1 a | 3.5 ± 0.2 b,c | 5.2 ± 1.2 c,d | 6.0 ± 0.2 d | 2.3 ± 0.2 a,b | 2.0 ± 0.1 a,b |
| 7 | 42.2 ± 3.8 a | 51.3 ± 0.2 b | 57.9 ± 2.3 b | 49.8 ± 0.9 b | 53.0 ± 2.2 b | 54.5 ± 1.8 b |
| 10 | 56.3 ± 4.6 a | 72.0 ± 4.3 b | 76.0 ± 1.3 b | 77.3 ± 4.9 b | 67.8 ± 1.5 b | 69.9 ± 4.3 b |
| 14 | 72.4 ± 2.8 a | 65.6 ± 3.3 a | 68.9 ± 3.9 a | 65.5 ± 4.5 a | 64.9 ± 6.4 a | 64.0 ± 3.8 a |
| 21 | 73.2 ± 6.7 a | 79.5 ± 2.4 a | 82.4 ± 3.4 a | 81.8 ± 2.8 a | 82.2 ± 4.3 a | 78.2 ± 1.6 a |
| 28 | 77.3 ± 1.4 a | 81.9 ± 1.6 a | 82.2 ± 0.8 a | 79.7 ± 6.8 a | 76.2 ± 1.6 a | 77.5 ± 4.9 a |
| 35 | 95.7 ± 0.7 a | 98.0 ± 0.5 a | 95.5 ± 0.9 a | 97.8 ± 0.5 a | 97.2 ± 1.2 a | 96.5 ± 1.7 a |
| Formulation | Time (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 7 | 10 | 14 | 21 | 28 | |
| PLA |  |  |  |  |  |  |  |  |
| PLA/T |  |  |  |  |  |  |  |  |
| PLA/T/D43B2.5 |  |  |  |  |  |  |  |  |
| PLA/T/D43B5 |  |  |  |  |  |  |  |  |
| PLA/D43B2.5 |  |  |  |  |  |  |  |  |
| PLA/D43B5 |  |  |  |  |  |  |  |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, M.; Fortunati, E.; Beltrán, A.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites. Polymers 2020, 12, 1878. https://doi.org/10.3390/polym12091878
Ramos M, Fortunati E, Beltrán A, Peltzer M, Cristofaro F, Visai L, Valente AJM, Jiménez A, Kenny JM, Garrigós MC. Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites. Polymers. 2020; 12(9):1878. https://doi.org/10.3390/polym12091878
Chicago/Turabian StyleRamos, Marina, Elena Fortunati, Ana Beltrán, Mercedes Peltzer, Francesco Cristofaro, Livia Visai, Artur J.M. Valente, Alfonso Jiménez, José María Kenny, and María Carmen Garrigós. 2020. "Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites" Polymers 12, no. 9: 1878. https://doi.org/10.3390/polym12091878
APA StyleRamos, M., Fortunati, E., Beltrán, A., Peltzer, M., Cristofaro, F., Visai, L., Valente, A. J. M., Jiménez, A., Kenny, J. M., & Garrigós, M. C. (2020). Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites. Polymers, 12(9), 1878. https://doi.org/10.3390/polym12091878












