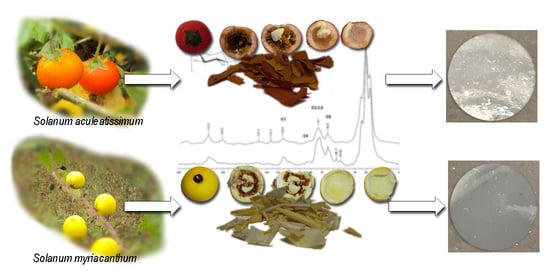
Cutin from Solanum Myriacanthum Dunal and Solanum Aculeatissimum Jacq. as a Potential Raw Material for Biopolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
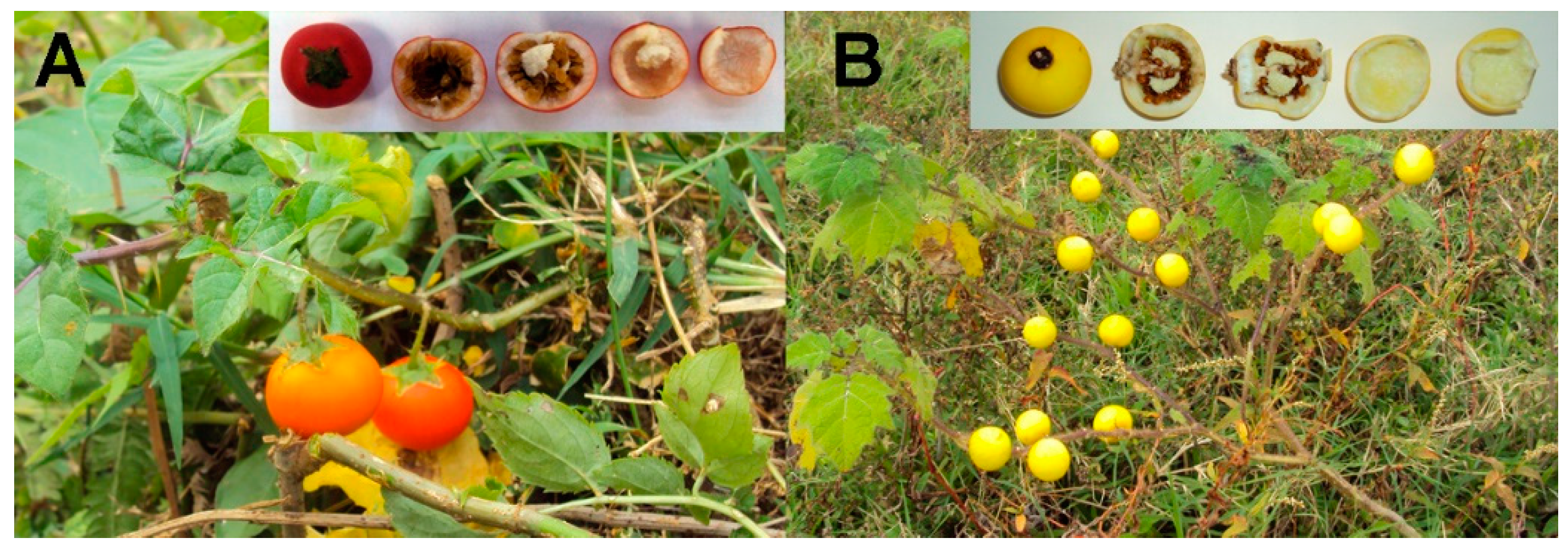
2.2. Isolation of Cutin
2.3. Treatment of Cutin with Trifluoroacetic Acid (TFA)
2.4. Alkaline Hydrolysis of the Cutin with KOH/MeOH
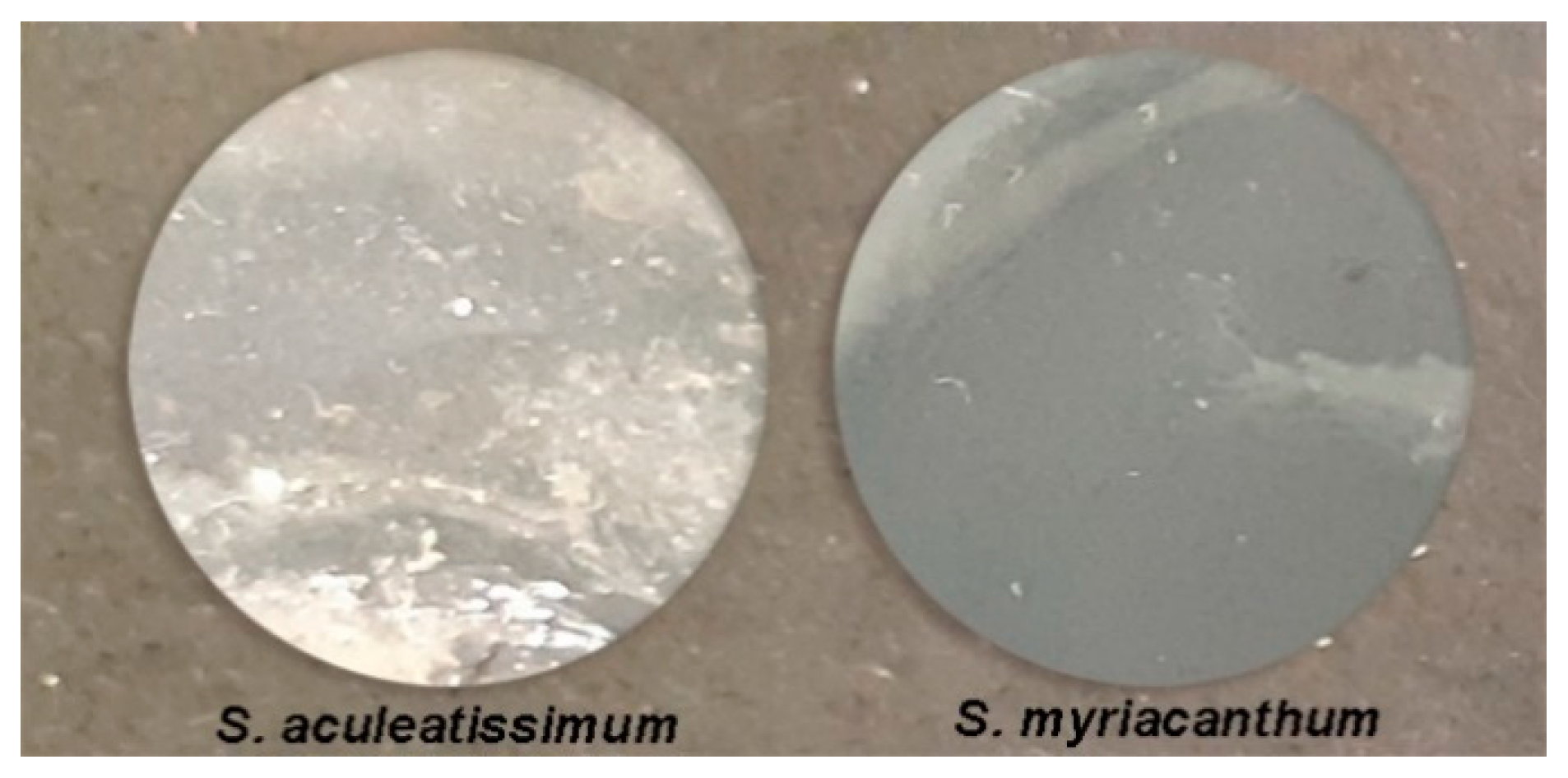
2.5. Preparation of Cutin Films
2.6. NMR Spectroscopy
2.7. ATR-FTIR Spectroscopy
2.8. Atomic Force Microscopy
2.9. Mass Spectrometry
3. Results
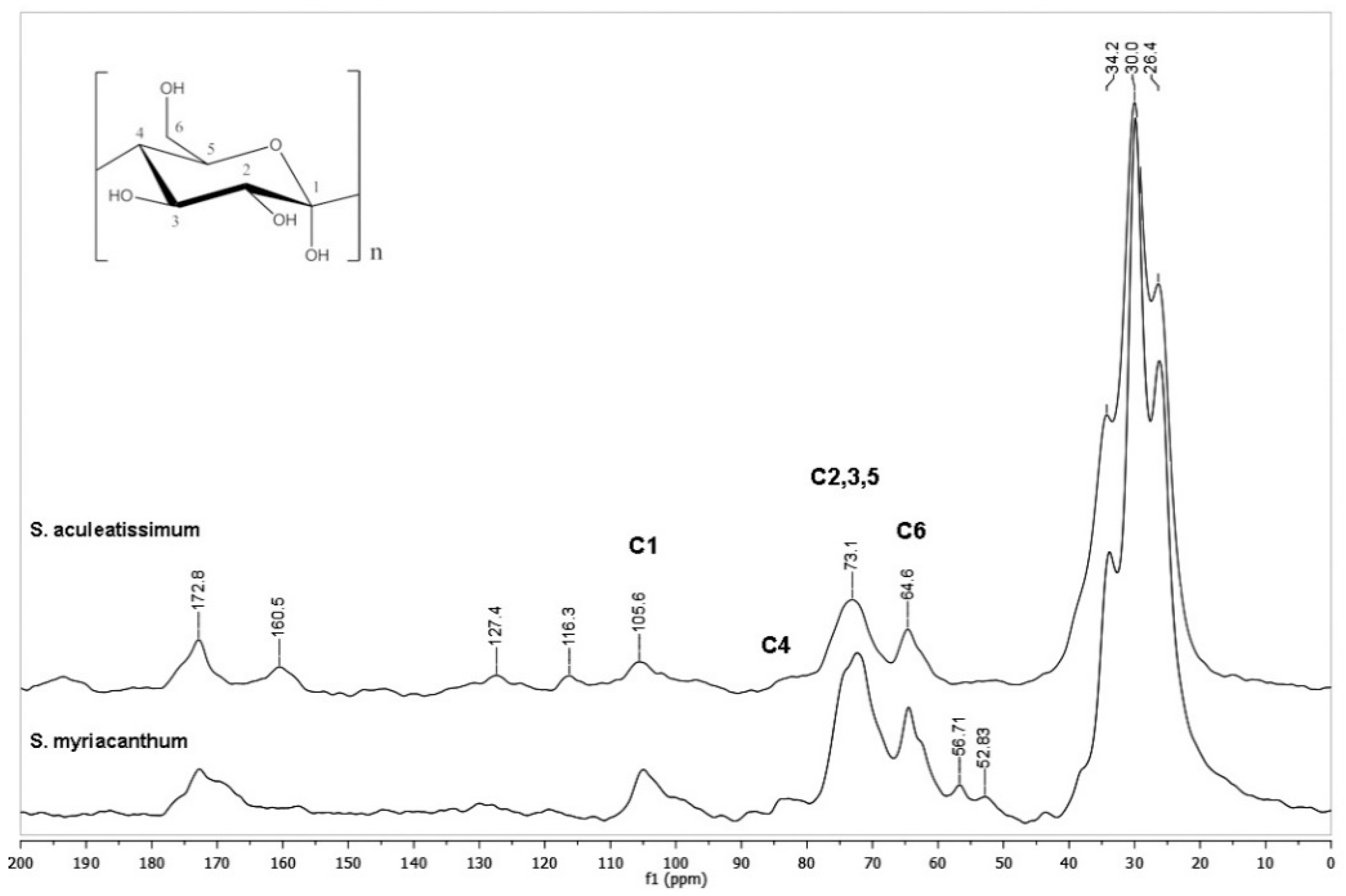
3.1. CPMAS 13C NMR Analysis
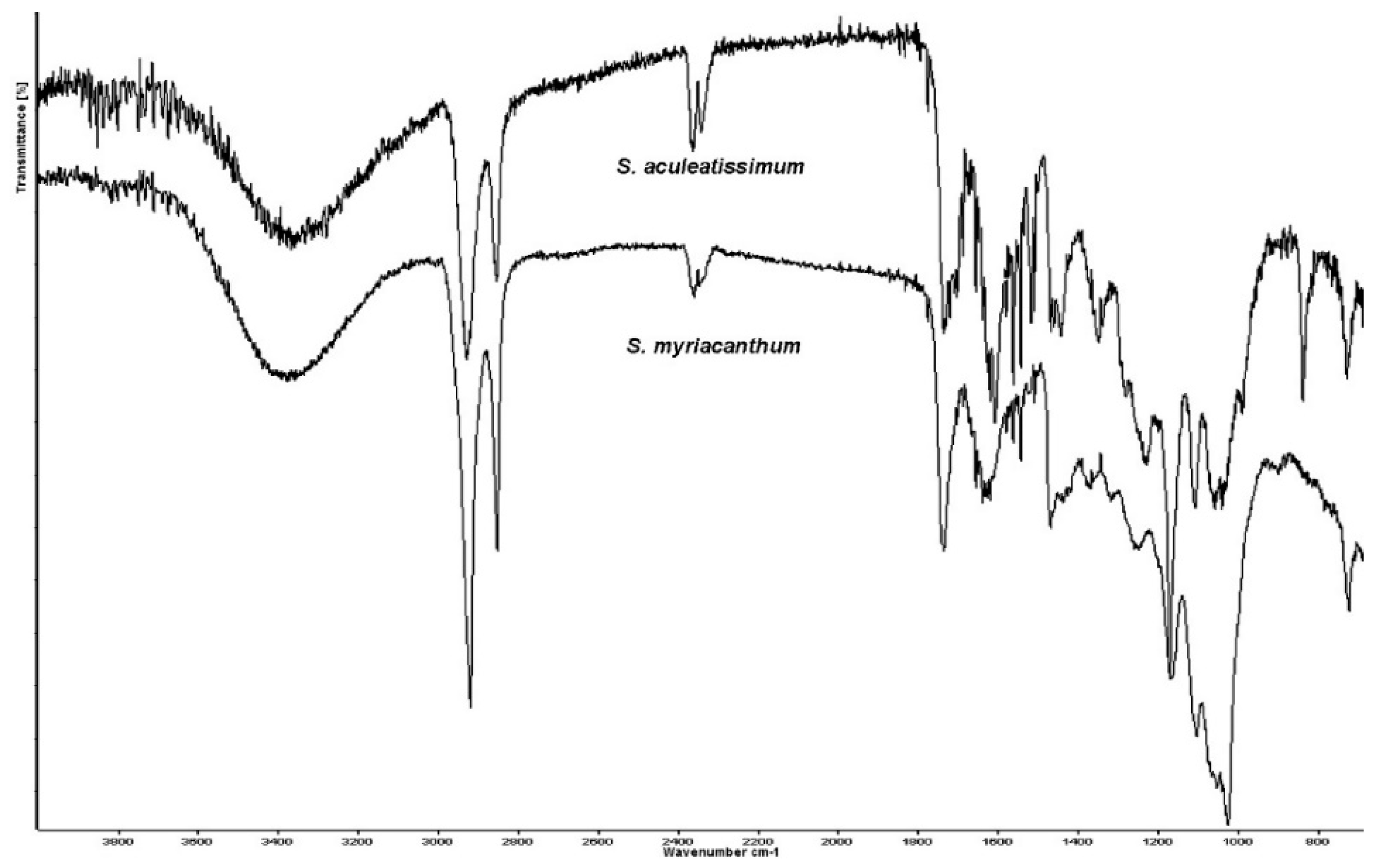
3.2. Infrared Spectroscopy Analysis of the S. Aculeatissimum and S. Myriacanthum Cutins
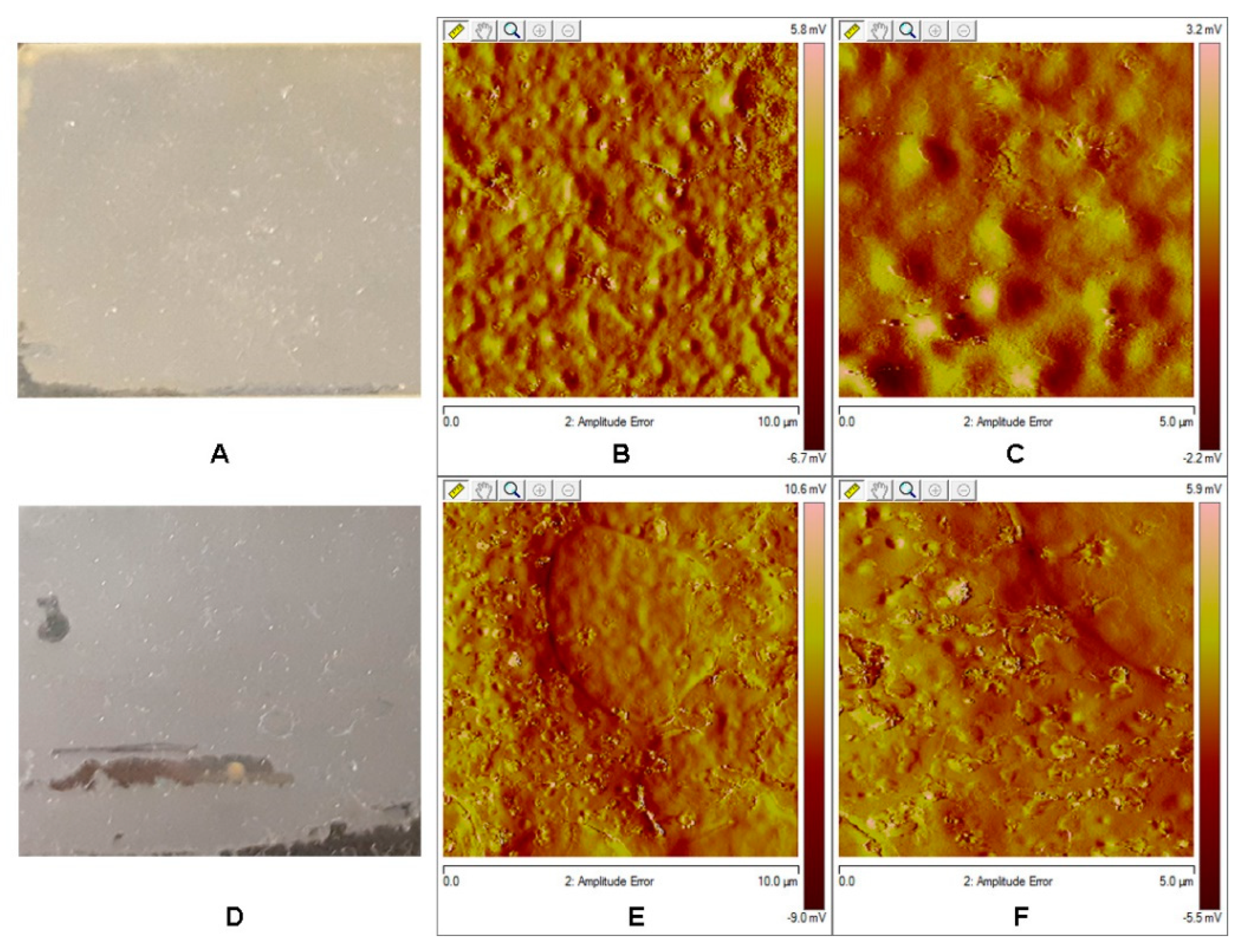
3.3. Atomic Force Microscopy Analysis
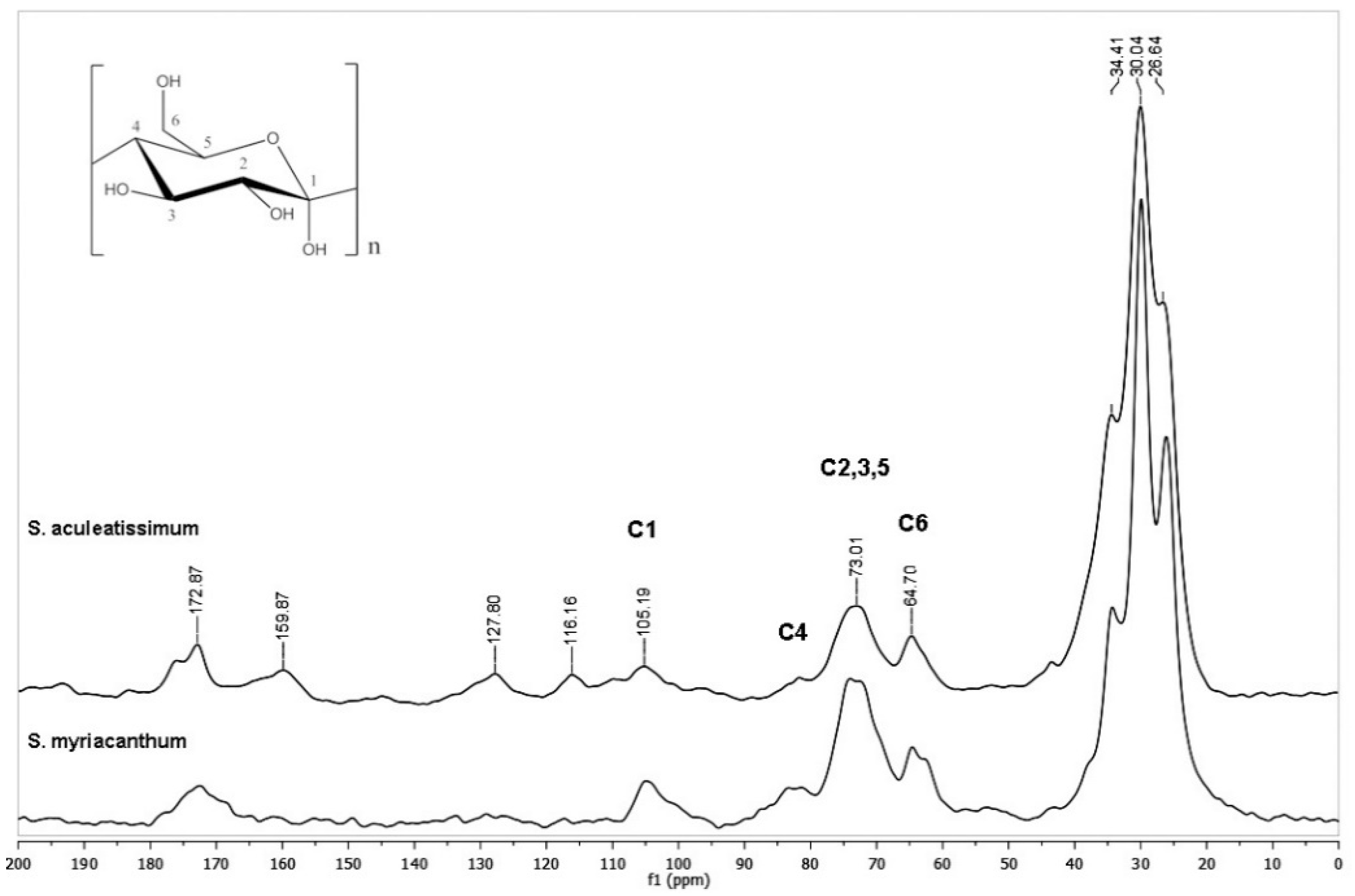
3.4. Alkaline Hydrolysis (KOH/MeOH)
3.5. Analysis of the Films Prepared from Hydrolyzed Cutins
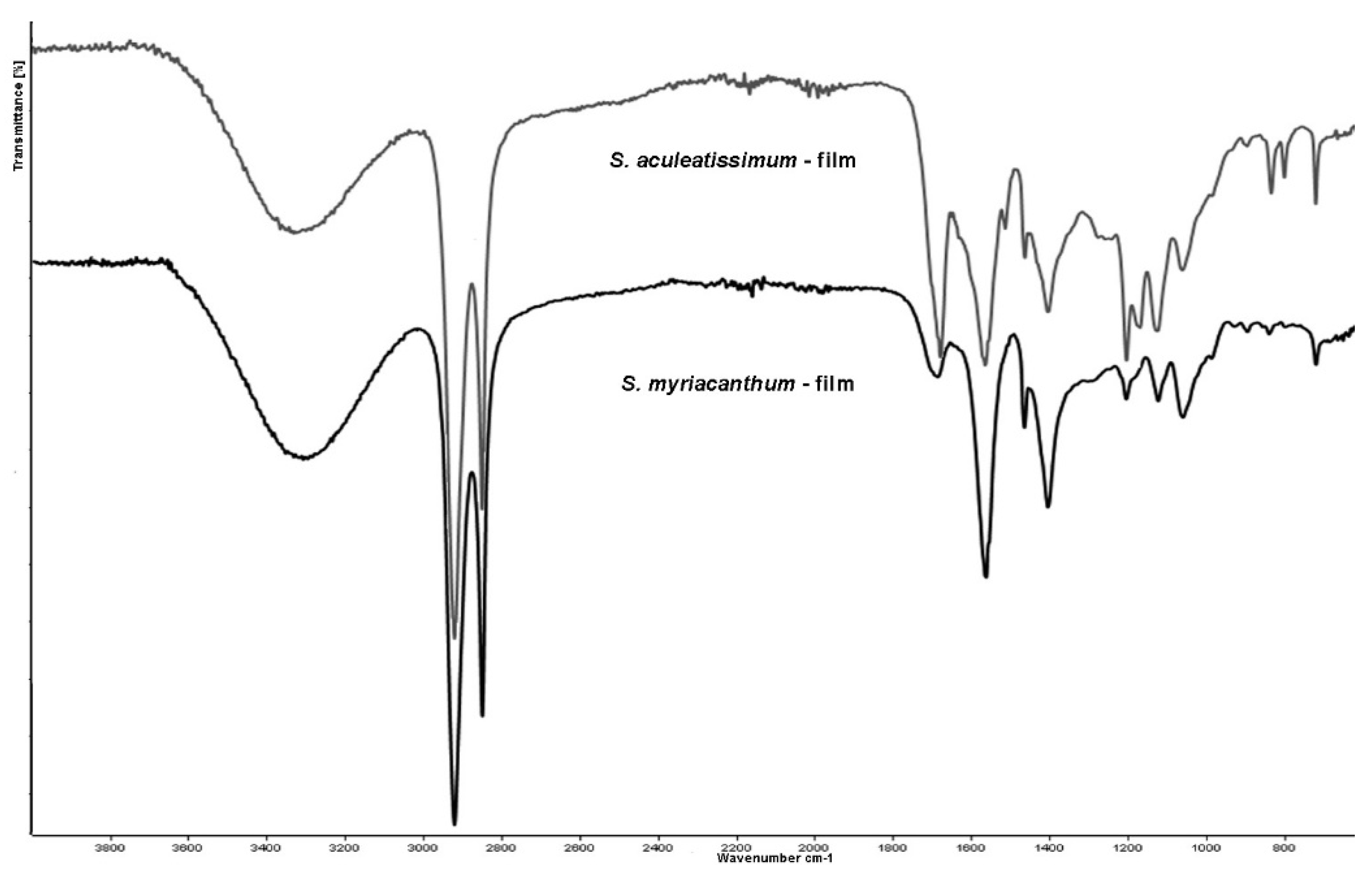
3.6. ATR-FTIR Analysis of the Films
3.7. AFM Analysis of the Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bargel, H.; Koch, K.; Cerman, Z.; Neinhuis, C. Structure-Function Relationships of the Plant Cuticle and Cuticular Waxes—A Smart Material? Funct. Plant Biol. 2006, 33, 893–910. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The Biophysical Design of Plant Cuticles: An Overview. N. Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef]
- Fich, E.A.; Segerson, N.A.; Rose, J.K. The Plant Polyester Cutin: Biosynthesis, Structure, and Biological Roles. Annu. Rev. Plant Biol. 2016, 67, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P.E. Polyesters in Higher Plants. Adv. Biochem. Eng. Biotechnol. 2001, 71, 1–49. [Google Scholar] [PubMed]
- Burghardt, M.; Riederer, M. Cuticular transpiration. In Biology of the Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 292–311. [Google Scholar]
- Walton, T.J.; Kolattukudy, P.E. Determination of the structures of cutin monomers by a novel depolymerization procedure and combined gas chromatography and mass spectrometry. Biochemistry 1972, 11, 1885–1897. [Google Scholar] [CrossRef]
- Waltson, T. Waxes, cutin and suberin. In Lipids, Membranes and Aspects of Photobiology; Harwood, E.J., Boyer, J., Eds.; Academic Press: London, UK, 1990; pp. 105–158. [Google Scholar]
- Heredia-Guerrero, J.A.; Benitez, J.J.; Dominguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Cruz-Carrillo, M.; Gómez-Patiño, M.B.; Zepeda-Vallejo, L.G. Derivatives of 10,16-dihydroxyhexadecanoicacid isolated from tomato (Solanum lycopersicum) as potential material for aliphatic polyesters. Molecules 2011, 16, 4923–4936. [Google Scholar] [CrossRef]
- Tedeschi, G.; Guzman-Puyol, S.; Ceseracciu, L.; Paul, U.C.; Picone, P.; Di, C.M.; Athanassiou, A.; Heredia-Guerrero, J.A. Multifunctional Bioplastics Inspired by Wood Composition: Effect of Hydrolyzed Lignin Addition to Xylan-Cellulose Matrices. Biomacromolecules 2020, 21, 910–920. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martinez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Liu, D.; Yan, X.; Si, M.; Deng, X.; Min, X.; Shi, Y.; Chai, L. Bioconversion of Lignin into Bioplastics by Pandoraea Sp. B-6: Molecular Mechanism. Environ. Sci. Pollut. Res. Int. 2019, 26, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, P. Exploring the Potential for Adopting Alternative Materials to Reduce Marine Plastic Litter; United Nations Environment Programme: Nairobi, Kenya, 2018; pp. 1–124. [Google Scholar]
- Coles, R.; Kay, M.; Song, J. Bioplastics. In Food and Beverage Packaging Technology, 2nd ed.; Coles, R., Kirwan, C.M., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 295–319. [Google Scholar]
- Mostafa, N.A.; Farag, A.A.; Abo-dief, H.L.; Tayeb, A.M. Production of biodegradable plastic from agricultural wastes. Arab. J. Chem. 2018, 11, 546–553. [Google Scholar] [CrossRef]
- Giosafatto, C.V.; Di, P.P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of Citrus Pectin Edible Films Containing Transglutaminase-Modified Phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.D.; Perez, L.L.; Salcedo, J.M.; Cordoba, L.P.; Sobral, P.J. Production and Characterization of Films Based on Blends of Chitosan from Blue Crab (Callinectes Sapidus) Waste and Pectin from Orange (Citrus Sinensis Osbeck) Peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Ahmad, N.; Akhtar, J.; Ahmad, N.M.; Ali, A.; Zia, M. Management of Citrus Waste by Switching in the Production of Nanocellulose. IET Nanobiotechnol. 2016, 10, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Baez, D.; Hernandez Ortiz, J.V.; Teran, J.C.; Torres, E.; Gomez-Patino, M.B. Aliphatic Diacidic Long-Chain C16 Polyesters From 10,16-Dihydroxyhexadecanoic Acid Obtained from Tomato Residual Wastes. Molecules 2019, 24, 1524. [Google Scholar] [CrossRef]
- Gómez-Patiño, M.B.; López-Simeón, R.; Espinosa-Domínguez, S.; Hernández-Guerrero, M.; Arrieta-Baez, D.; Beltrán-Conde, H.; Campos-Terán, J.; Reyes-Duarte, D. Aprovechamiento de residuos agroindustriales: Composición, modificación enzimática y evaluación de sus potenciales aplicaciones in Obtención Enzimática de Ingredientes Funcionales, Compuestos Bioactivos y Nutraceúticos a Partir de Recursos Naturales Iberoamericanos. In Biblioteca de las Ciencias, 40th ed.; Fabián, G.C.S., Gasca, F.J.P., Eds.; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2012. [Google Scholar]
- Benitez, J.J.; Osbild, S.; Guzman-Puyol, S.; Heredia, A.; Heredia-Guerrero, J.A. Bio-Based Coatings for Food Metal Packaging Inspired in Biopolyester Plant Cutin. Polymers 2020, 12, 942. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Heredia, A.; Dominguez, E.; Cingolani, R.; Bayer, I.S.; Athanassiou, A.; Benitez, J.J. Cutin From Agro-Waste As a Raw Material for the Production of Bioplastics. J. Exp. Bot. 2017, 68, 5401–5410. [Google Scholar] [CrossRef]
- Cuevas-Reyes, L. Taxonomía de la Familia Solanaceae en el Municipio de Coacoatzintla. Bachelor’s Thesis, Facultad de Biología, Universidad Veracruzana, Veracruz, Mexico, 2018. [Google Scholar]
- Kohara, A.; Nakajima, C.; Hashimoto, K.; Ikenaga, T.; Tanaka, H.; Shoyama, Y.; Yoshida, S.; Muranaka, T. A Novel Glucosyltransferase Involved in Steroid Saponin Biosynthesis in Solanum Aculeatissimum. Plant Mol. Biol. 2005, 57, 225–239. [Google Scholar] [CrossRef]
- Ikenaga, T.; Kikuta, S.; Itimura, K.; Nakashima, K.; Matsubara, T. Growth and Production of Steroid Saponin in Solanum Aculeatissimum during One Vegetation Period. Planta Med. 1988, 54, 140–142. [Google Scholar] [CrossRef]
- Yadav, A.K.; Tangpu, V. Anthelmintic Activity of Ripe Fruit Extract of Solanum Myriacanthum Dunal (Solanaceae) Against Experimentally Induced Hymenolepis Diminuta (Cestoda) Infections in Rats. Parasitol. Res. 2012, 110, 1047–1053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arrieta-Baez, D.; Stark, R.E. Using Trifluoroacetic Acid to Augment Studies of Potato Suberin Molecular Structure. J. Agric. Food Chem. 2006, 54, 9636–9641. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Patiño, M.B.; Cassani, J.; Jaramillo-Flores, M.E.; Zepeda-Vallejo, L.G.; Sandoval, G.; Jimenez-Estrada, M.; Arrieta-Baez, D. Oligomerization of 10,16-Dihydroxyhexadecanoic Acid and Methyl 10,16-Dihydroxyhexadecanoate Catalyzed by Lipases. Molecules 2013, 18, 9317–9333. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, V.B.L.; Arrieta-Baez, D.; Cortez, S.P.I.; Méndez-Méndez, J.V.; Berdeja, M.B.M.; Gómez-Patiño, M.B. Comparative studies of cutins from lime (Citrus aurantifolia) and grapefruit (Citrus paradisi) after TFA hydrolysis. Phytochemistry 2017, 144, 78–86. [Google Scholar] [CrossRef]
- Luque, P.; Ramírez, F.J.; Heredia, A.; Bukovac, M.J. Fouriertrans-form IR studies on the interaction of selected chemicals with isolates cuticles. In Air Pollutants and the Leaf Cuticle, NATO ASI Series, G36; Percy, K.E., Cape, J.N., Jagels, R., Simpson, C.J., Eds.; Springer: Berlin, Germany, 1994; pp. 217–223. [Google Scholar]
- Gerard, H.C.; Osman, S.F.; Fett, W.F.; Moreau, R.A. Separation, identification and quantification of monomers from cutin polymers by high performance liquid chromatography and evaporative light scattering detection. Phytochem. Anal. 1992, 3, 139–144. [Google Scholar] [CrossRef]
- Posé, S.; Paniagua, C.; Matas, A.J.; Gunning, A.P.; Morris, V.M.; Quesada, M.A.; Mercado, J.A. A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest storage by atomic force microscopy. Trends Food Sci. Technol. 2019, 87, 47–58. [Google Scholar] [CrossRef]

| Name | [M − H]−obs | [M − H]−exact | Formula | Error | %RA | |
|---|---|---|---|---|---|---|
| SA | SM | |||||
| Coumaric acid | 163.0386 | 163.0389 | C9H8O3 | 2.1 | 0.18 | --- |
| Capric acid | 171.1352 | 171.1379 | C10H20O2 | 3.5 | 0.36 | 0.25 |
| Coniferaldehyde | 177.0557 | 177.0546 | C10H10O3 | 3.6 | 0.54 | --- |
| n-Nonanedioic acid | 187.1001 | 187.0964 | C9H16O4 | 4.1 | 0.18 | 0.25 |
| Ferulic acid | 193.0473 | 193.0495 | C10H10O4 | 2.5 | 0.18 | --- |
| Lauric acid | 199.1689 | 199.1692 | C12H24O2 | 2.5 | 0.18 | 0.25 |
| Myristic acid | 227.2003 | 227.2005 | C14H28O2 | 1.9 | 0.72 | 1.02 |
| n-Pentadecanoic acid | 241.2144 | 241.2162 | C15H30O2 | 3.9 | 0.72 | 1.02 |
| Palmitic acid | 255.2322 | 255.2318 | C16H32O2 | 2.0 | 2.88 | 3.29 |
| Hexyl 2-(4-hydroxy-3-methoxy-phenyl) acetate | 265.1481 | 265.1434 | C15H22O4 | 4.2 | --- | 1.77 |
| 16-hydroxypalmitic acid | 271.2257 | 271.2267 | C16H32O3 | 2.6 | 6.12 | 1.52 |
| Linoleic acid | 279.2330 | 279.2318 | C18H32O2 | 3.1 | 2.02 | |
| 10,16-DHPA | 287.2209 | 287.2216 | C16H32O4 | 2.6 | 69.84 | 44.02 |
| Heptadecanedioic acid | 299.2228 | 299.2216 | C17H32O4 | 3.4 | 2.88 | 1.26 |
| 8-hydroxyhexadecane dioic acid | 301.2017 | 301.2009 | C16H30O5 | 4.2 | 5.76 | 1.52 |
| 18-hydroxy-9S,10R-epoxy-octadecanoic acid | 313.2387 | 313.2373 | C18H34O4 | 3.9 | --- | 9.36 |
| 9,10,18-trihydroxy-octadecanoic acid | 331.2487 | 331.2479 | C18H36O5 | 2.4 | 2.88 | 24.03 |
| 2,3-Divanillyl-1,4-butanediol | 361.1563 | 361.1645 | C20H26O6 | 4.5 | 2.16 | 4.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Patiño, M.B.; Estrada-Reyes, R.; Vargas-Diaz, M.E.; Arrieta-Baez, D. Cutin from Solanum Myriacanthum Dunal and Solanum Aculeatissimum Jacq. as a Potential Raw Material for Biopolymers. Polymers 2020, 12, 1945. https://doi.org/10.3390/polym12091945
Gómez-Patiño MB, Estrada-Reyes R, Vargas-Diaz ME, Arrieta-Baez D. Cutin from Solanum Myriacanthum Dunal and Solanum Aculeatissimum Jacq. as a Potential Raw Material for Biopolymers. Polymers. 2020; 12(9):1945. https://doi.org/10.3390/polym12091945
Chicago/Turabian StyleGómez-Patiño, Mayra Beatriz, Rosa Estrada-Reyes, María Elena Vargas-Diaz, and Daniel Arrieta-Baez. 2020. "Cutin from Solanum Myriacanthum Dunal and Solanum Aculeatissimum Jacq. as a Potential Raw Material for Biopolymers" Polymers 12, no. 9: 1945. https://doi.org/10.3390/polym12091945
APA StyleGómez-Patiño, M. B., Estrada-Reyes, R., Vargas-Diaz, M. E., & Arrieta-Baez, D. (2020). Cutin from Solanum Myriacanthum Dunal and Solanum Aculeatissimum Jacq. as a Potential Raw Material for Biopolymers. Polymers, 12(9), 1945. https://doi.org/10.3390/polym12091945