Crystallization Induced Enhanced Emission in Two New Zn(II) and Cd(II) Supramolecular Coordination Complexes with the 1-(3,4-Dimethylphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carboxylate Ligand
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
- (1)
- Colorless parallelepipeds, 34.3 mg (yield 31.0%, based on C12H12N3NaO2). FT-IR (KBr pellet, cm−1): 3417(vs) ν(O–H), 3169(w), 3043(w) ν(Csp2–H), 2949(m), 2924(m) and 2854(w) ν(Csp3–H), 1614(vs) ν(C=O and/or C=C), 1576(s) ν(C–N), 1400(vs) ν(N=N and/or -CH3), 1290(m) ν(C–O), 1248(w) ν(Csp2–N), 532 (vw) ν(Zn–O).
- (2)
- Colorless blocks, 47.7 mg (yield 40.0%, based on C12H12N3NaO2). FT-IR (KBr pellet, cm−1): 3412(vs) ν(O–H), 3198(w) ν(Csp2–H), 2945(m), 2924(m) and 2860(w) ν(Csp3–H), 1605(vs) ν(C=O and/or C=C), 1572(s) ν(C–N), 1402(vs) ν(N=N and/or -CH3), 1286(m) ν(C–O), 1246(w) ν(Csp2–N), 808 (vw) ν(Cd–O).
2.2. Characterization
2.3. X-ray Structural Determination
2.4. Computational Details
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. FT-IR Spectroscopy
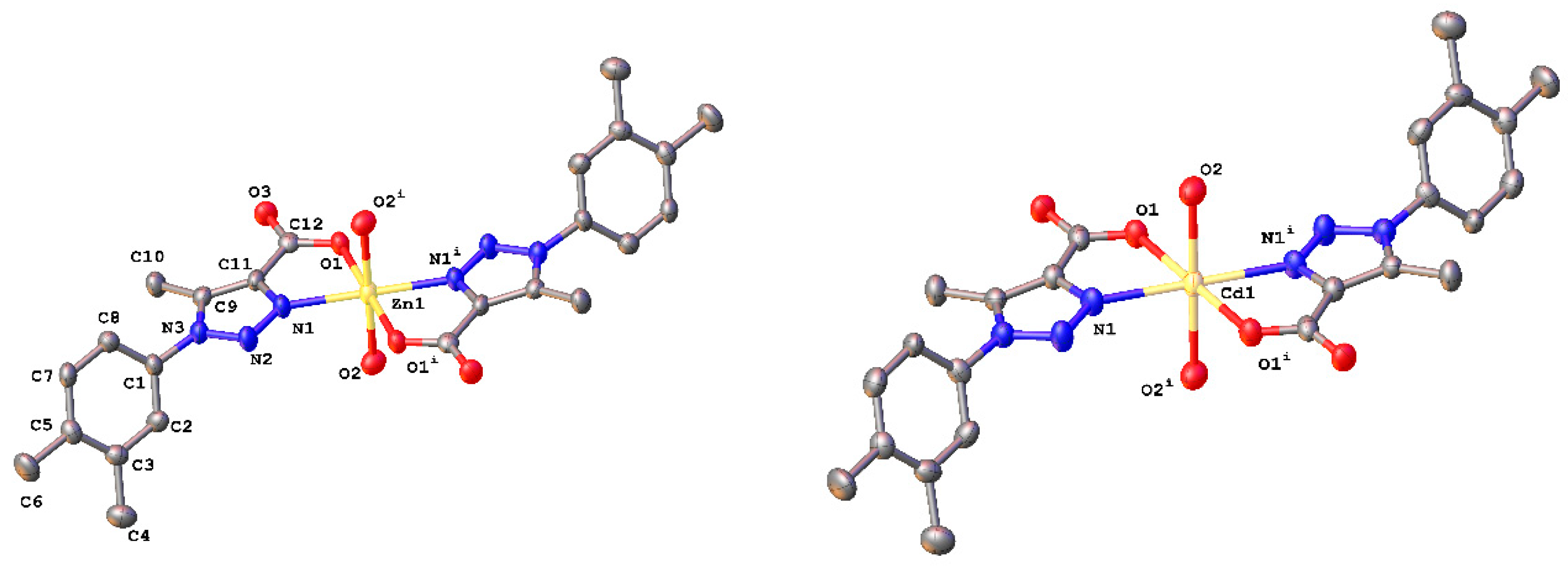
3.3. X-ray Crystallographic Studies
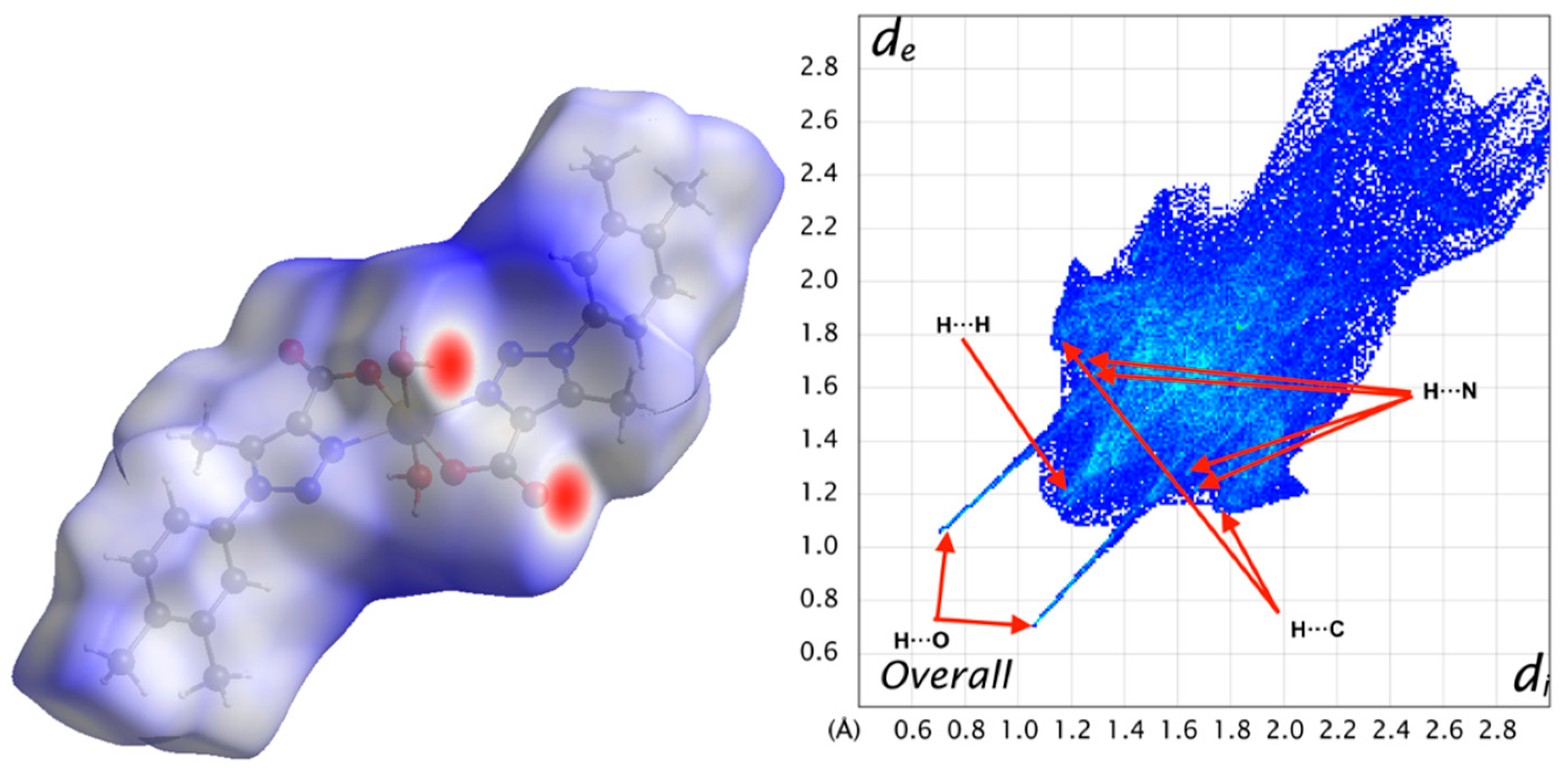
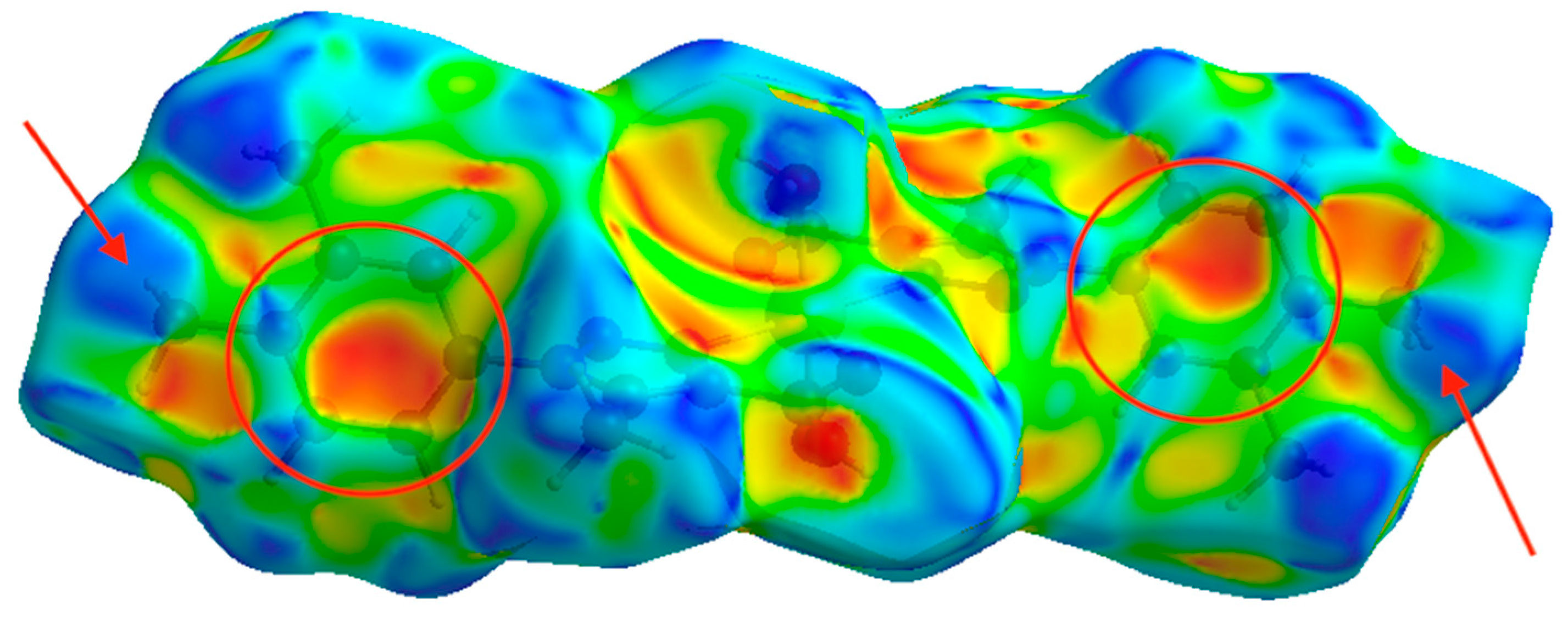
3.4. Hirshfeld Surface Analysis
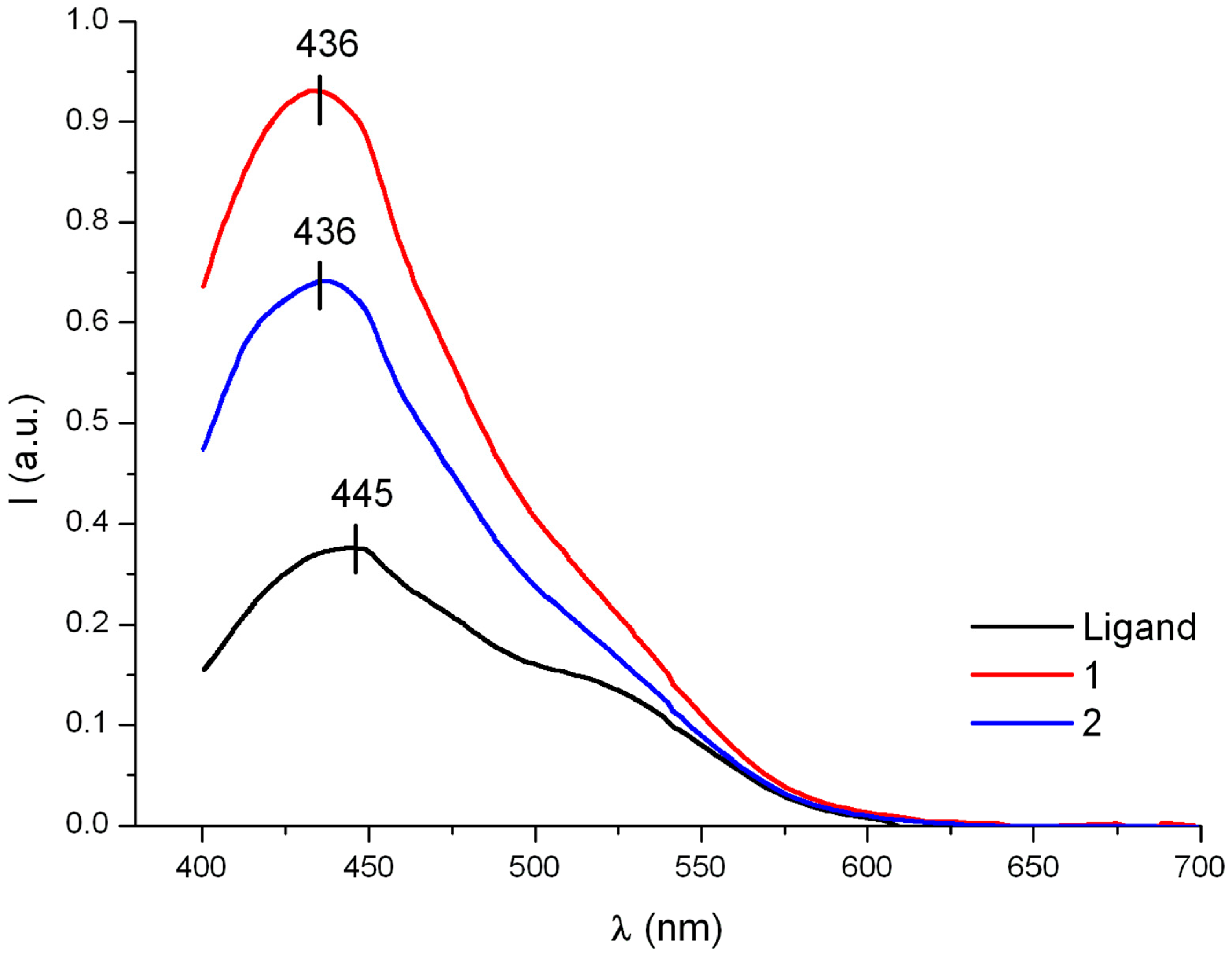
3.5. Electronic Emission Spectra
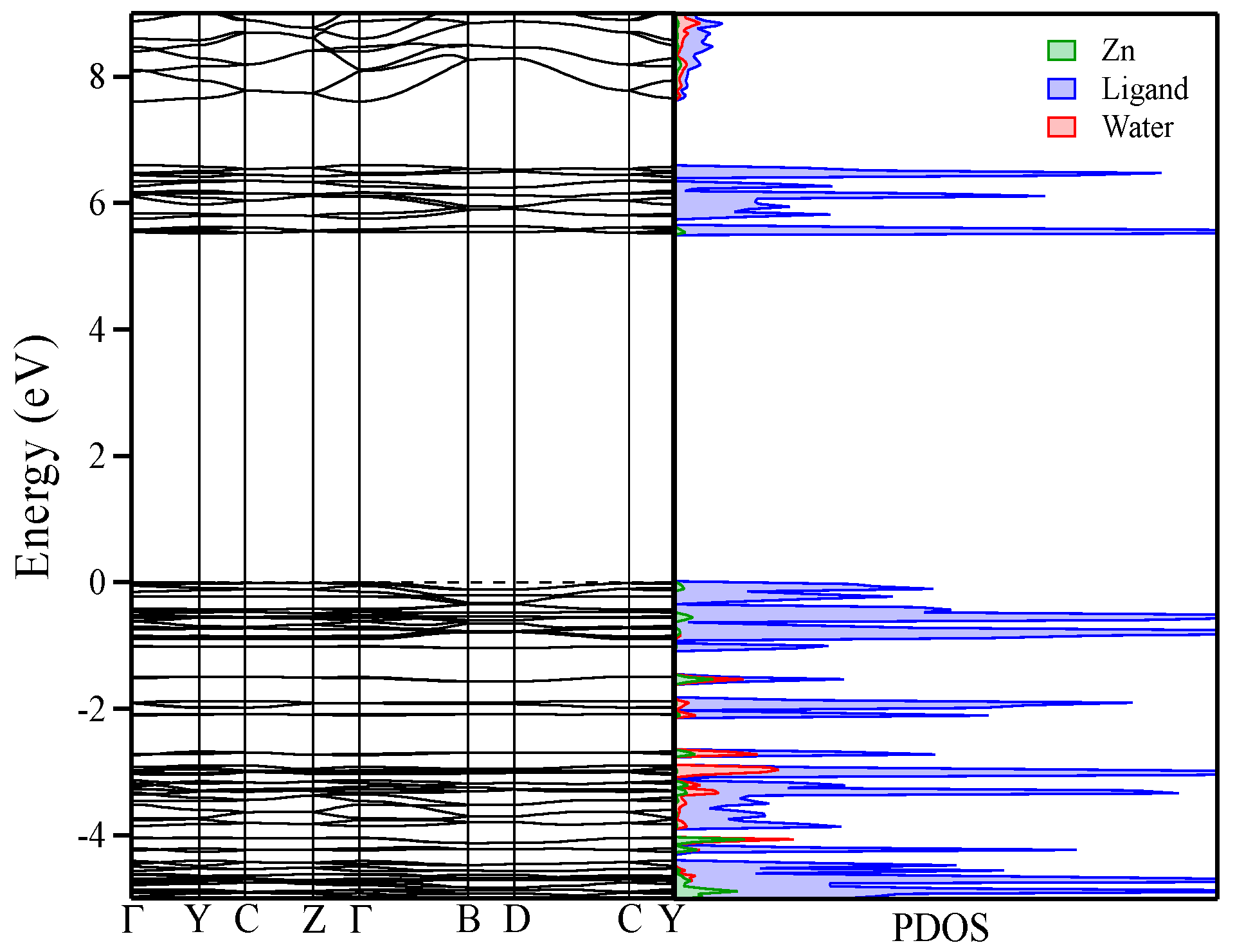
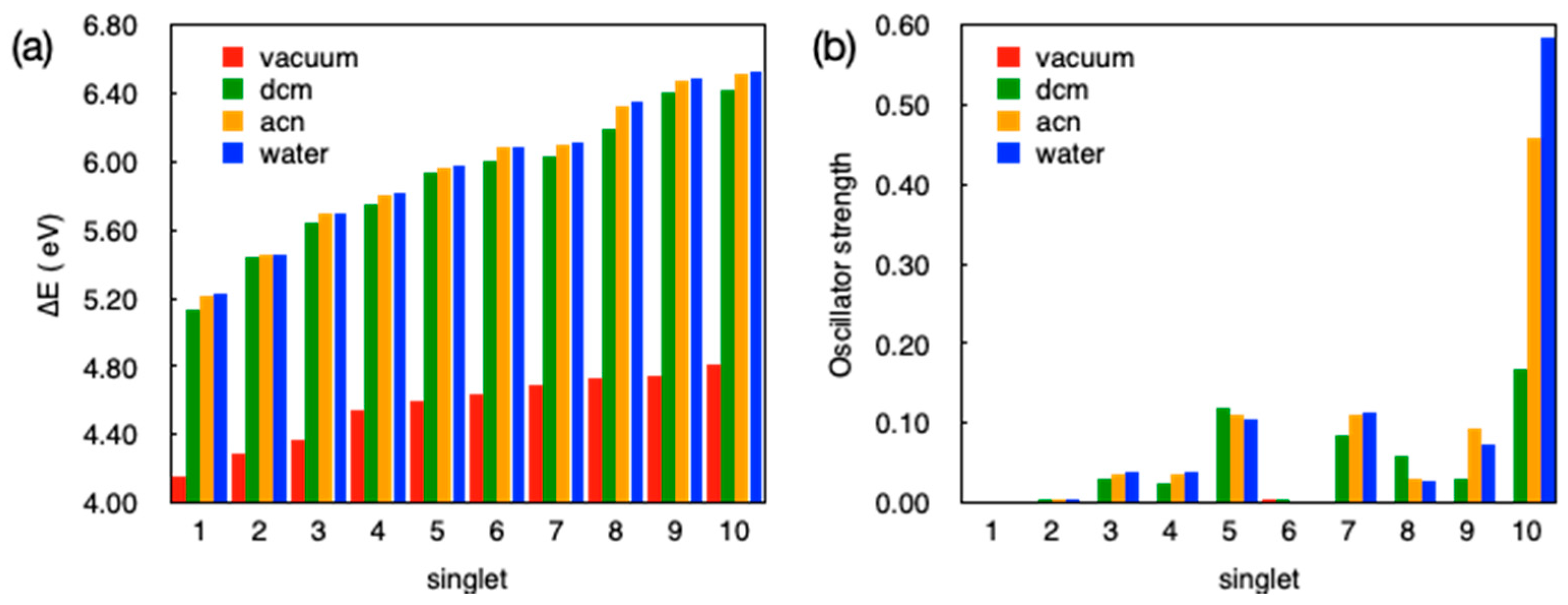
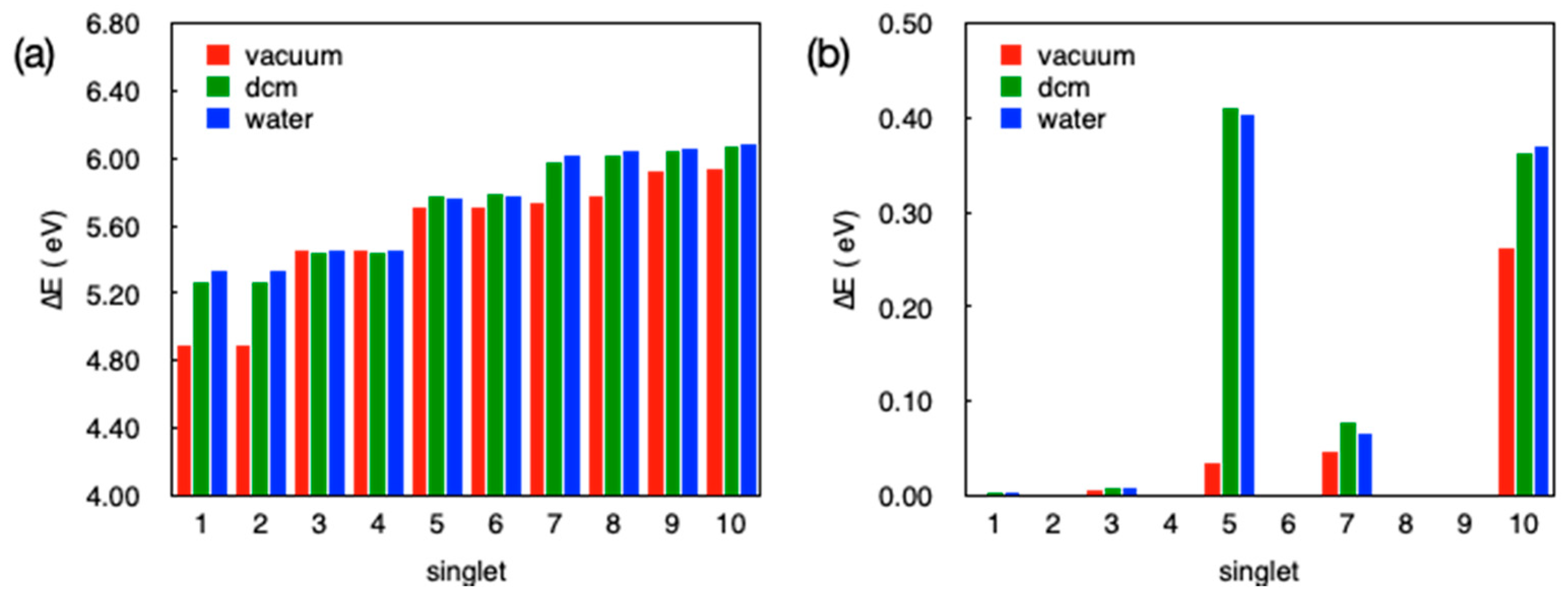
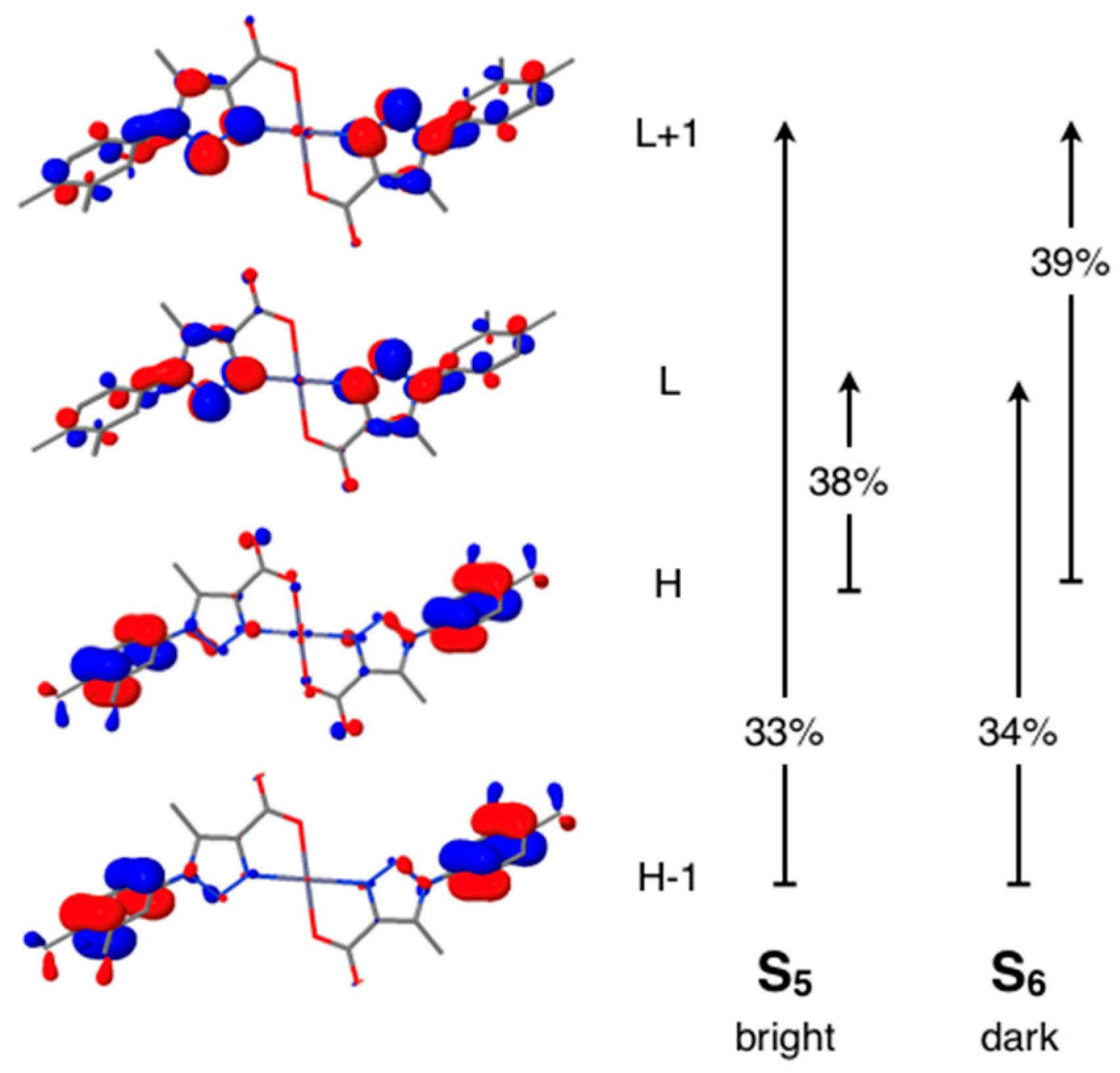
3.6. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yersin, H. Highly Efficient OLEDs with Phosphorescent Materials; Wiley Online Library: Hoboken, NJ, USA, 2008; ISBN 9783527405947. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527621309 (accessed on 18 August 2019).
- Wong, W.Y.; Ho, C.L. Heavy metal organometallic electrophosphors derived from multi-component chromophores. Coord. Chem. Rev. 2009, 253, 1709–1758. [Google Scholar] [CrossRef]
- Sasabe, H.; Kido, J. Multifunctional materials in high-performance OLEDs: Challenges for solid-state lighting. Chem. Mater. 2011, 23, 621–630. [Google Scholar] [CrossRef]
- Wu, W.C.; Chen, C.Y.; Tian, Y.; Jang, S.H.; Hong, Y.; Liu, Y.; Hu, R.; Tang, B.Z.; Lee, Y.T.; Chen, C.T.; et al. Enhancement of aggregation-induced emission in dye-encapsulating polymeric micelles for bioimaging. Adv. Funct. Mater. 2010, 20, 1413–1423. [Google Scholar] [CrossRef]
- Lo, K.K.-W.; Choi, A.W.-T.; Law, W.H.-T. Applications of luminescent inorganic and organometallic transition metal complexes as biomolecular and cellular probes. Dalton Trans. 2012, 41, 6021–6047. [Google Scholar] [CrossRef] [PubMed]
- You, Y. Phosphorescence bioimaging using cyclometalated Ir (III) complexes. Curr. Opin. Chem. Biol. 2013, 17, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.M.C.; Yam, V.W.W. Luminescence platinum (II) terpyridyl complexes-From fundamental studies to sensory functions. Coord. Chem. Rev. 2007, 251, 2477–2488. [Google Scholar] [CrossRef]
- Jung, H.S.; Chen, X.; Kim, J.S.; Yoon, J. Recent progress in luminescent and colorimetric chemosensors for detection of thiols. Chem. Soc. Rev. 2013, 42, 6019–6031. [Google Scholar] [CrossRef]
- Zhou, Y.; Yoon, J. Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids. Chem. Soc. Rev. 2012, 41, 52–67. [Google Scholar] [CrossRef]
- Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M. New fluorescent chemosensors for metal ions in solution. Coord. Chem. Rev. 2012, 256, 170–192. [Google Scholar] [CrossRef]
- Kato, T. Hydrogen-Bonded Liquid Crystals: Molecular Self-Assembly for Dynamically Functional Materials. In Molecular Self-Assembly Organic Versus Inorganic Approaches; Springer: Berlin/Heidelberg, Germany, 2000; pp. 95–146. Available online: https://link.springer.com/chapter/10.1007%2F3-540-46591-X_4 (accessed on 25 May 2020).
- Ulbricht, C.; Beyer, B.; Friebe, C.; Winter, A.; Schubert, U.S. Recent Developments in the Application of Phosphorescent Iridium (III) Complex Systems. Adv. Mater. 2009, 21, 4418–4441. [Google Scholar] [CrossRef]
- Ning, Z.; Chen, Z.; Zhang, Q.; Yan, Y.; Qian, S.; Cao, Y.; Tian, H. Aggregation-induced Emission (AIE)-active Starburst Triarylamine Fluorophores as Potential Non-doped Red Emitters for Organic Light-emitting Diodes and Cl2 Gas Chemodosimeter. Adv. Funct. Mater. 2007, 17, 3799–3807. [Google Scholar] [CrossRef]
- Slifkin, M.A. Charge Transfer and Excimer Formation. Nature 1963, 200, 766–767. [Google Scholar] [CrossRef]
- Chandross, E.A.; Dempster, C.J. Excimer fluorescence and dimer phosphorescence from a naphthalene sandwich pair. J. Am. Chem. Soc. 1970, 92, 704–706. [Google Scholar] [CrossRef]
- Hoche, J.; Schmitt, H.-C.; Humeniuk, A.; Fischer, I.; Mitrić, R.; Röhr, M.I.S. The mechanism of excimer formation: An experimental and theoretical study on the pyrene dimer. Phys. Chem. Chem. Phys. 2017, 19, 25002–25015. [Google Scholar] [CrossRef]
- Cook, R.E.; Phelan, B.T.; Kamire, R.J.; Majewski, M.B.; Young, R.M.; Wasielewski, M.R. Excimer Formation and Symmetry-Breaking Charge Transfer in Cofacial Perylene Dimers. J. Phys. Chem. A 2017, 121, 1607–1615. [Google Scholar] [CrossRef]
- Hecht, S.; Fréchet, J.M.J. Dendritic encapsulation of function: Applying nature’s site isolation principle from biomimetics to materials science. Angew. Chem.-Int. Ed. 2001, 40, 74–91. [Google Scholar] [CrossRef]
- Freeman, A.W.; Koene, S.C.; Malenfant, P.R.L.; Thompson, M.; Fréchet, J.M.J. Dendrimer-Containing Light-Emitting Diodes: Toward Site-Isolation of Chromophores. J. Am. Chem. Soc. 2000, 122, 12385–12386. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Tang, B.Z.; Chen, H.; Qiu, C.; Kwok, H.-S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Zhao, Z.; He, B.; Tang, B.Z. Aggregation-induced emission of siloles. Chem. Sci. 2015, 6, 5347–5365. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Jiang, X.-M.; Ma, X.-Q.; Liu, J.; Yao, H.; Zhang, Y.; Wei, T.-B. Novel bispillar [5]arene-based AIEgen and its’ application in mercury(II) detection. Sens. Actuators B Chem. 2018, 272, 139–145. [Google Scholar] [CrossRef]
- Fan, Y.-Q.; Liu, J.; Chen, Y.-Y.; Guan, X.-W.; Wang, J.; Yao, H.; Zhang, Y.; Wei, T.; Lin, Q. An easy-to-make strong white AIE supramolecular polymer as a colour tunable photoluminescence material. J. Mater. Chem. C 2018, 6, 13331–13335. [Google Scholar] [CrossRef]
- Alam, P.; Climent, C.; Alemany, P.; Laskar, I.R. “Aggregation-induced emission” of transition metal compounds: Design, mechanistic insights, and applications. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100317. [Google Scholar] [CrossRef]
- Sathish, V.; Ramdass, A.; Thanasekaran, P.; Lu, K.-L.; Rajagopal, S. Aggregation-induced phosphorescence enhancement (AIPE) based on transition metal complexes—An overview. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 25–44. [Google Scholar] [CrossRef]
- Parke, S.M.; Rivard, E. Aggregation Induced Phosphorescence in the Main Group. Isr. J. Chem. 2018, 58, 915–926. [Google Scholar] [CrossRef]
- Ravotto, L.; Ceroni, P. Aggregation induced phosphorescence of metal complexes: From principles to applications. Coord. Chem. Rev. 2017, 346, 62–76. [Google Scholar] [CrossRef]
- Mauro, M.; Cebrián, C. Aggregation-induced Phosphorescence Enhancement in IrIII Complexes. Isr. J. Chem. 2018, 58, 901–914. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2012, 113, 734–777. [Google Scholar] [CrossRef]
- Wang, A.; Fan, R.; Wang, P.; Fang, R.; Hao, S.; Zhou, X.; Zheng, X.; Yang, Y. Research on the Mechanism of Aggregation-Induced Emission through Supramolecular Metal–Organic Frameworks with Mechanoluminescent Properties and Application in Press-Jet Printing. Inorg. Chem. 2017, 56, 12881–12892. [Google Scholar] [CrossRef]
- Morsali, A.; Masoomi, M.Y. Structures and properties of mercury(II) coordination polymers. Coord. Chem. Rev. 2009, 253, 1882–1905. [Google Scholar] [CrossRef]
- Tang, M.; Guo, W.; Zhang, S.-Z.; Du, M. Group IIB metal complexes with a multidentate N-donor tecton 3,4-bis(2-pyridyl)-5-(3-pyridyl)-1,2,4-triazole: Metal-directed assemblies, crystal structures and properties. Inorg. Chem. Commun. 2011, 14, 1217–1220. [Google Scholar] [CrossRef]
- Connell, T.; Schieber, C.; Silvestri, I.P.; White, J.M.; Williams, S.J.; Donnelly, P.S. Copper and Silver Complexes of Tris(triazole)amine and Tris(benzimidazole)amine Ligands: Evidence that Catalysis of an Azide–Alkyne Cycloaddition (“Click”) Reaction by a Silver Tris(triazole)amine Complex Arises from Copper Impurities. Inorg. Chem. 2014, 53, 6503–6511. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Patel, B.K. Molecular structures and fluorescence property of Zn(II), Cd(II) complexes of 3-pyridyl-5-aryl-(1 H)-1,2,4-triazoles. Polyhedron 2017, 132, 112–122. [Google Scholar] [CrossRef]
- Cisterna, J.; Araneda, C.; Narea, P.; Cárdenas, A.; Llanos, J.; Brito, I. The Positional Isomeric Effect on the Structural Diversity of Cd(II) Coordination Polymers, Using Flexible Positional Isomeric Ligands Containing Pyridyl, Triazole, and Carboxylate Fragments. Molecules 2018, 23, 2634. [Google Scholar] [CrossRef] [PubMed]
- Granifo, J.; Garland, M.T.; Baggio, R. The effect in the assembly and node nuclearity of the long and rigid character of bis-pyridyl exo-bidentate spacers when react with zinc acetate: Crystal structures of the high nuclearity coordination polymers [Zn7(μ4-O)2(OAc)10(3pdb)]n (3pdb=1,4-bis(3-pyridyl)-2,3-diaza-1,3-butadiene) and [Zn7(μ4-O)2(OAc)10(4pdb)]n (4pdb=1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene). Polyhedron 2006, 25, 2277–2283. [Google Scholar] [CrossRef]
- Xu, B.; Xie, J.; Hu, H.-M.; Yang, X.-L.; Dong, F.-X.; Yang, M.-L.; Xue, G. Synthesis, Crystal Structure, and Luminescence of Zn/Cd Coordination Polymers with a New Fuctionalized Terpyridyl Carboxylate Ligand. Cryst. Growth Des. 2014, 14, 1629–1641. [Google Scholar] [CrossRef]
- Zhou, Y.; Ge, X.; Zhang, Z.; Luo, W.; Xu, H.; Li, W.; Zhu, J. Design and realization on orange-red emitting of samarium activated sodium lanthanum metaphosphate with low CCT and high CP. J. Alloys Compd. 2019, 811, 152020. [Google Scholar] [CrossRef]
- Na Kim, H.; Guo, Z.; Zhu, W.; Yoon, J.; Tian, H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem. Soc. Rev. 2011, 40, 79–93. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline metal-organic frameworks (MOFs): Synthesis, structure and function. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2013, 70, 3–10. [Google Scholar] [CrossRef]
- Liu, Q.; Wan, F.; Qiu, L.-X.; Sun, Y.-Q.; Chen, Y.-P. Four 2D Ln–Cd heterometal–organic coordination polymers based on tetranuclear Ln–Cd oxo-cluster with highly selective luminescent sensing of organic molecules and metal cations. RSC Adv. 2014, 4, 27013–27021. [Google Scholar] [CrossRef]
- Feng, R.; Jiang, F.-L.; Chen, L.; Yan, C.-F.; Wu, M.; Hong, M.-C. A luminescent homochiral 3D Cd(ii) framework with a threefold interpenetrating uniform net 86. Chem. Commun. 2009, 5296. [Google Scholar] [CrossRef]
- Wang, G.-H.; Li, Z.-G.; Jia, H.-Q.; Hu, N.-H.; Xu, J.-W. Metal–organic frameworks based on the pyridine-2,3-dicarboxylate and a flexible bispyridyl ligand: Syntheses, structures, and photoluminescence. CrystEngComm 2009, 11, 292–297. [Google Scholar] [CrossRef]
- Zou, J.-P.; Peng, Q.; Wen, Z.; Zeng, G.-S.; Xing, Q.-J.; Guo, G.-C. Two Novel Metal−Organic Frameworks (MOFs) with (3,6)-Connected Net Topologies: Syntheses, Crystal Structures, Third-Order Nonlinear Optical and Luminescent Properties. Cryst. Growth Des. 2010, 10, 2613–2619. [Google Scholar] [CrossRef]
- Vallejos, J.; Brito, I.; Cárdenas, A.; Bolte, M.; Conejeros, S.; Alemany, P.; Llanos, J. Self-Assembly of Discrete Metallocycles versus Coordination Polymers Based on Cu(I) and Ag(I) Ions and Flexible Ligands: Structural Diversification and Luminescent Properties. Polymer 2016, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-X.; Hubbard, T.A.; Cockroft, S.L. Can non-polar hydrogen atoms accept hydrogen bonds? Chem. Commun. 2014, 50, 5212–5214. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.F.; Hernández-Trujillo, J.; Tang, T.-H.; Bader, R.F.W. Hydrogen–Hydrogen Bonding: A Stabilizing Interaction in Molecules and Crystals. Chem.-A Eur. J. 2003, 9, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals; Butterworth Heinemann: Burlington, MA, USA, 1997. [Google Scholar]
- Bruker AXS Inc. APEX3 Package, APEX3, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. SADABS, Software for Empirical Absorption Correction. Univ. Gott. Inst. Fur Anorg. Chem. Univ. Tammanstrasse 1996, 4, 1999–2003. [Google Scholar]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.A.; Grimwood, S.K.; McKinnon, D.J.; Turner, J.J.; Jayatilaka, M.J.; Spackman, D. Crystal Explorer (Version 17.5); University of Western Australia: Perth, Australia, 2012. [Google Scholar]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Details on the CRYSTAL Code Gaussian, Basis Sets, Computational Schemes, etc. Available online: http://www.crystal.unito.it (accessed on 1 October 2019).
- Dovesi, R.; Saunders, V.R.; Roetti, C.; Orlando, R.; Zicovich-Wilson, C.M.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.J.; et al. CRYSTAL17 User’s Manual; University of Torino: Torino, Italy, 2017. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Gatti, C.; Saunders, V.R.; Roetti, C. Crystal field effects on the topological properties of the electron density in molecular crystals: The case of urea. J. Chem. Phys. 1994, 101, 10686–10696. [Google Scholar] [CrossRef]
- Valenzano, L.; Torres, F.J.; Doll, K.; Pascale, F.; Zicovich-Wilson, C.; Dovesi, R. Ab InitioStudy of the Vibrational Spectrum and Related Properties of Crystalline Compounds; the Case of CaCO3Calcite. Z. Für Phys. Chem. 2006, 220, 893–912. [Google Scholar] [CrossRef]
- Heyd, J.; Peralta, J.E.; Scuseria, G.E.; Martin, R.L. Energy band gaps and lattice parameters evaluated with the Heyd-Scuseria-Ernzerhof screened hybrid functional. J. Chem. Phys. 2005, 123, 174101. [Google Scholar] [CrossRef]
- Laun, J.; Oliveira, D.V.; Bredow, T. Consistent gaussian basis sets of double- and triple-zeta valence with polarization quality of the fifth period for solid-state calculations. J. Comput. Chem. 2018, 39, 1285–1290. [Google Scholar] [CrossRef]
- Pack, J.D.; Monkhorst, H.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1977, 16, 1748–1749. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute’s charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Liao, S.-Y.; Gu, W.; Yang, L.-Y.; Li, T.-H.; Zhang, M.; Wang, L.; Liu, X. Three new metal–organic frameworks constructed from triazol-phenyl polycarboxyl acid: Synthesis, crystal structures and properties. Polyhedron 2012, 36, 38–44. [Google Scholar] [CrossRef]
- Ma, C.; Liang, S.-K.; Zhao, F.-C.; Meng, Y.; Li, Y.-Y.; Zhu, M.-C.; Gao, E.-J. Cadmium(II) complex with 2-methyl-1H-4,5-imidazoledicarboxylic acid ligand: Synthesis, characterization, and biological activity. J. Coord. Chem. 2014, 67, 3551–3564. [Google Scholar] [CrossRef]
- Yeşilel, O.Z.; Erer, H.; Büyükgüngör, O. Supramolecular architectures of cadmium(ii)-orotate complexes containing water clusters. CrystEngComm 2011, 13, 1339–1349. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.-M.; Lin, J.-R.; Wang, W.-X. Hydrothermal syntheses and crystal structures of two Zn(II) complexes with 1-substituted-1H-[1,2,3]-triazole-4-carboxylic acid. J. Coord. Chem. 2011, 64, 2735–2745. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Mondal, A.; Bhattacharya, B.; Das, S.; Bhunia, S.; Chowdhury, R.; Dey, S.; Reddy, C.M. Metal-like Ductility in Organic Plastic Crystals: Role of Molecular Shape and Dihydrogen Bonding Interactions in Aminoboranes. Angew. Chem. 2020, 132, 11064–11073. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Hestand, N.; Spano, F.C. Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors upon request. |


| Compound | 1 | 2 |
|---|---|---|
| Empirical Formula | C24H28N6O6Zn | C24H28N6O6Cd |
| Formula mass, g mol−1 | 561.89 | 608.92 |
| Collection T, K | 295.48 | 295.62 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c |
| a (Å) | 11.6896(4) | 11.8854(5) |
| b (Å) | 16.7231(7) | 16.8493(7) |
| c (Å) | 7.2911(3) | 7.4316(3) |
| β (°) | 102.539(4) | 105.219(3) |
| V (Å3) | 1391.31(10) | 1436.06(10) |
| Z | 2 | 2 |
| ρcalcd (gcm−3) | 1.341 | 1.408 |
| Crystal size (mm) | 0.227 × 0.087 × 0.050 | 0.099 × 0.077 × 0.068 |
| F (000) | 584.0 | 620.0 |
| Abs coeff (mm−1) | 1.628 | 6.481 |
| θ range (°) | 7.748/136.318 | 7.708/117.936 |
| Range h,k,l | −14/12, −19/20, −8/8 | −13/13, −18/18, −7/8 |
| No. total refl. | 12,232 | 12,407 |
| No. unique refl. | 2491 | 2057 |
| Comp. θmax (%) | 98 | 100 |
| Max/min transmission | 0.923/0.709 | 0.577/0.643 |
| Data/Restraints/Parameters | 2491/0/174 | 2057/0/174 |
| Final R [I>2σ(I)] | 0.0686 | 0.056 |
| R indices (all data) | 0.1271 | 0.081 |
| Goodness of fit/F2 | 1.029 | 1.085 |
| Largest diff. Peak/hole (eÅ−3) | 0.37/−0.34 | 0.82/−0.60 |
| Contact | 1 | 2 |
|---|---|---|
| H···H | 45.2 | 41.5 |
| H···C | 16.3 | 15.7 |
| H···N | 9.9 | 10.0 |
| H···O | 25.3 | 24.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narea, P.; Cisterna, J.; Cárdenas, A.; Amo-Ochoa, P.; Zamora, F.; Climent, C.; Alemany, P.; Conejeros, S.; Llanos, J.; Brito, I. Crystallization Induced Enhanced Emission in Two New Zn(II) and Cd(II) Supramolecular Coordination Complexes with the 1-(3,4-Dimethylphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carboxylate Ligand. Polymers 2020, 12, 1756. https://doi.org/10.3390/polym12081756
Narea P, Cisterna J, Cárdenas A, Amo-Ochoa P, Zamora F, Climent C, Alemany P, Conejeros S, Llanos J, Brito I. Crystallization Induced Enhanced Emission in Two New Zn(II) and Cd(II) Supramolecular Coordination Complexes with the 1-(3,4-Dimethylphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carboxylate Ligand. Polymers. 2020; 12(8):1756. https://doi.org/10.3390/polym12081756
Chicago/Turabian StyleNarea, Pilar, Jonathan Cisterna, Alejandro Cárdenas, Pilar Amo-Ochoa, Félix Zamora, Clàudia Climent, Pere Alemany, Sergio Conejeros, Jaime Llanos, and Iván Brito. 2020. "Crystallization Induced Enhanced Emission in Two New Zn(II) and Cd(II) Supramolecular Coordination Complexes with the 1-(3,4-Dimethylphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carboxylate Ligand" Polymers 12, no. 8: 1756. https://doi.org/10.3390/polym12081756
APA StyleNarea, P., Cisterna, J., Cárdenas, A., Amo-Ochoa, P., Zamora, F., Climent, C., Alemany, P., Conejeros, S., Llanos, J., & Brito, I. (2020). Crystallization Induced Enhanced Emission in Two New Zn(II) and Cd(II) Supramolecular Coordination Complexes with the 1-(3,4-Dimethylphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carboxylate Ligand. Polymers, 12(8), 1756. https://doi.org/10.3390/polym12081756









