Synthetic Polymers for Organ 3D Printing
Abstract
:1. Introduction
2. Synthetic Polymers for 3D Printing
2.1. Inkjet-Based 3D Printing
2.2. Fused Deposition Modeling (FDM)
2.3. Extrusion-Based Printing
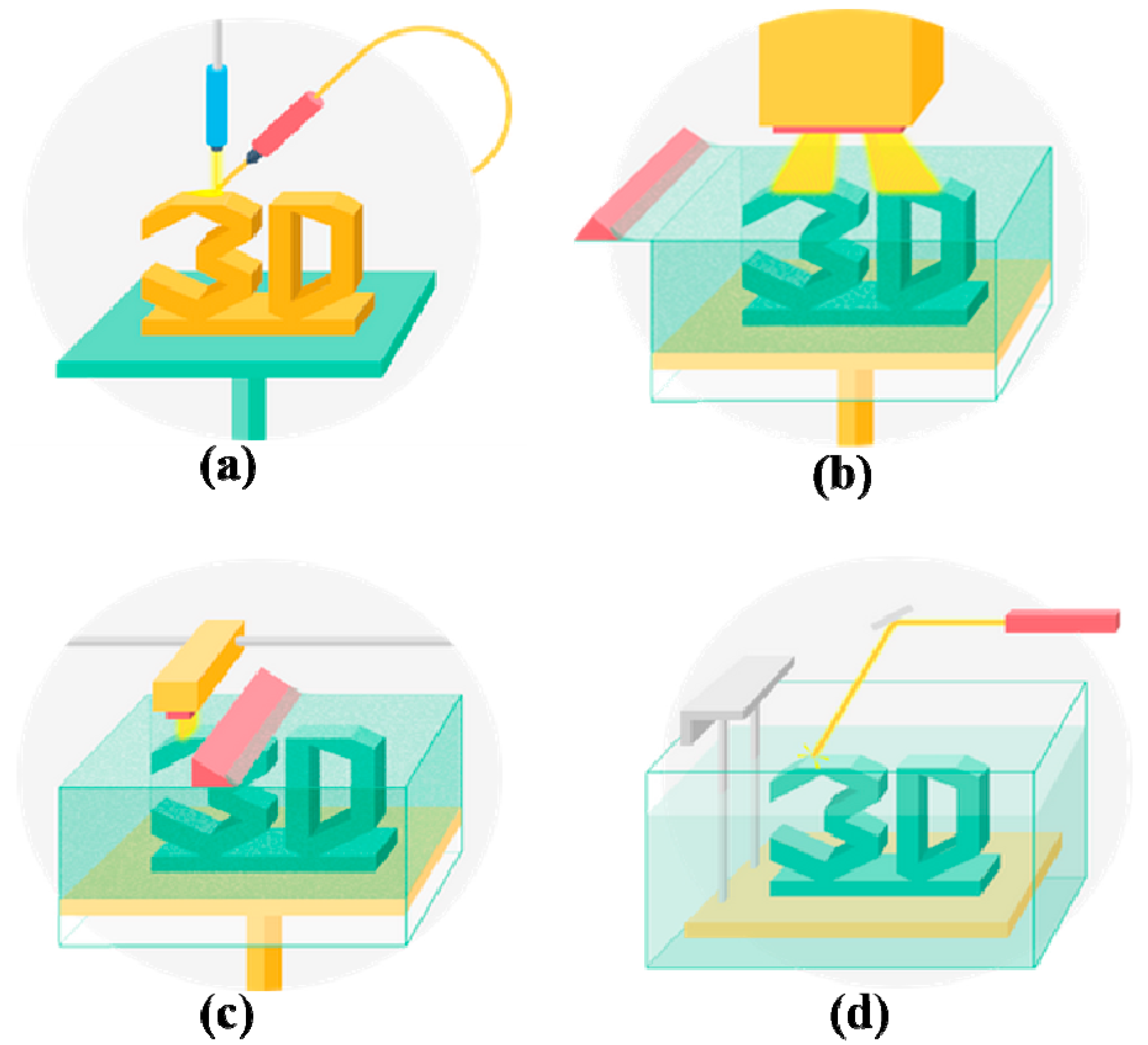
2.4. Stereolithography (SLA)
2.5. Digital Light Processing (DLP)
2.6. Aerosol Jet 3D Printing
3. Synthetic Polymers for 3D Bioprinting
3.1. Properties of Synthetic Polymers
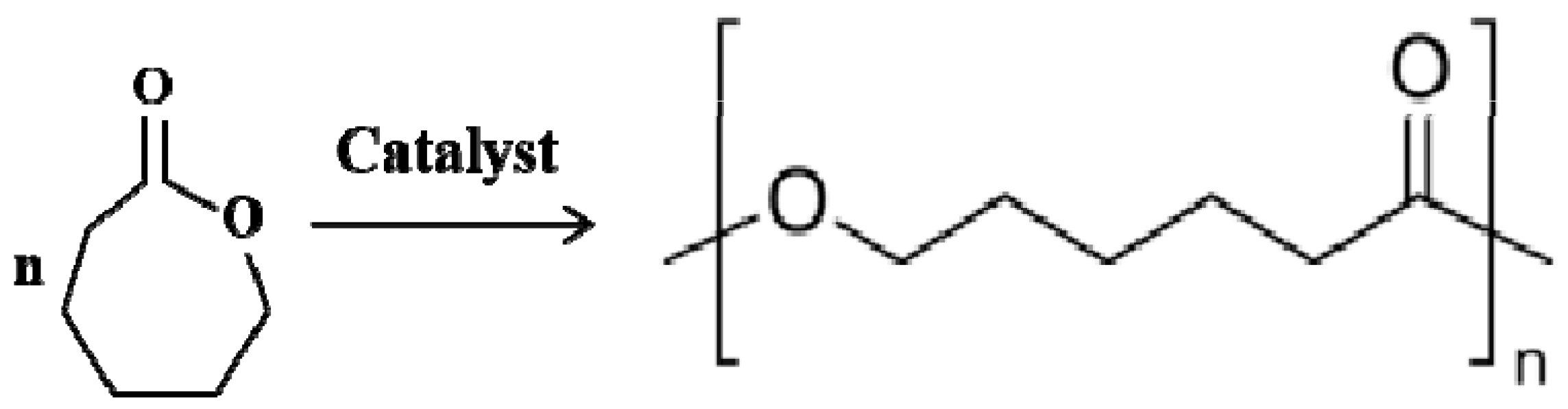
3.2. Polycaprolactone (PCL)
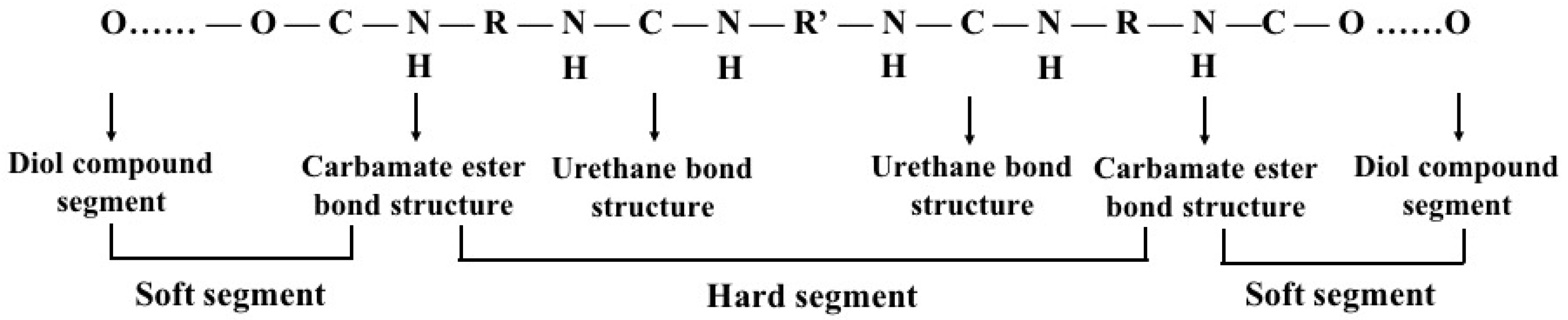
3.3. Polyurethane (PU)
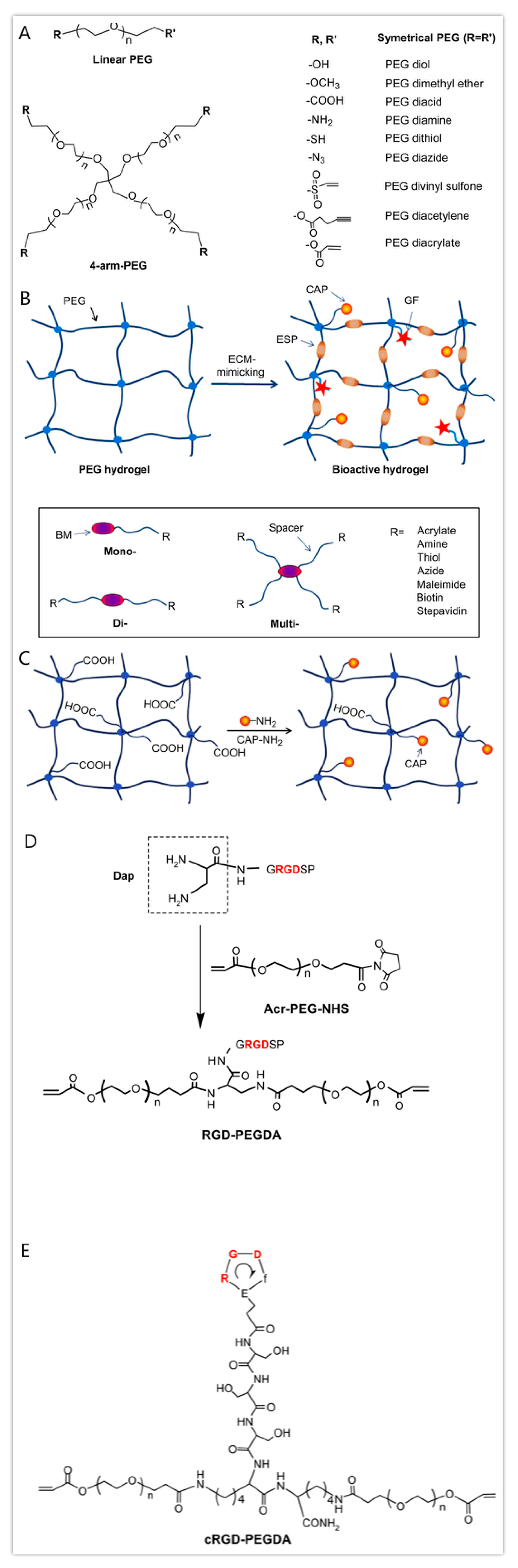
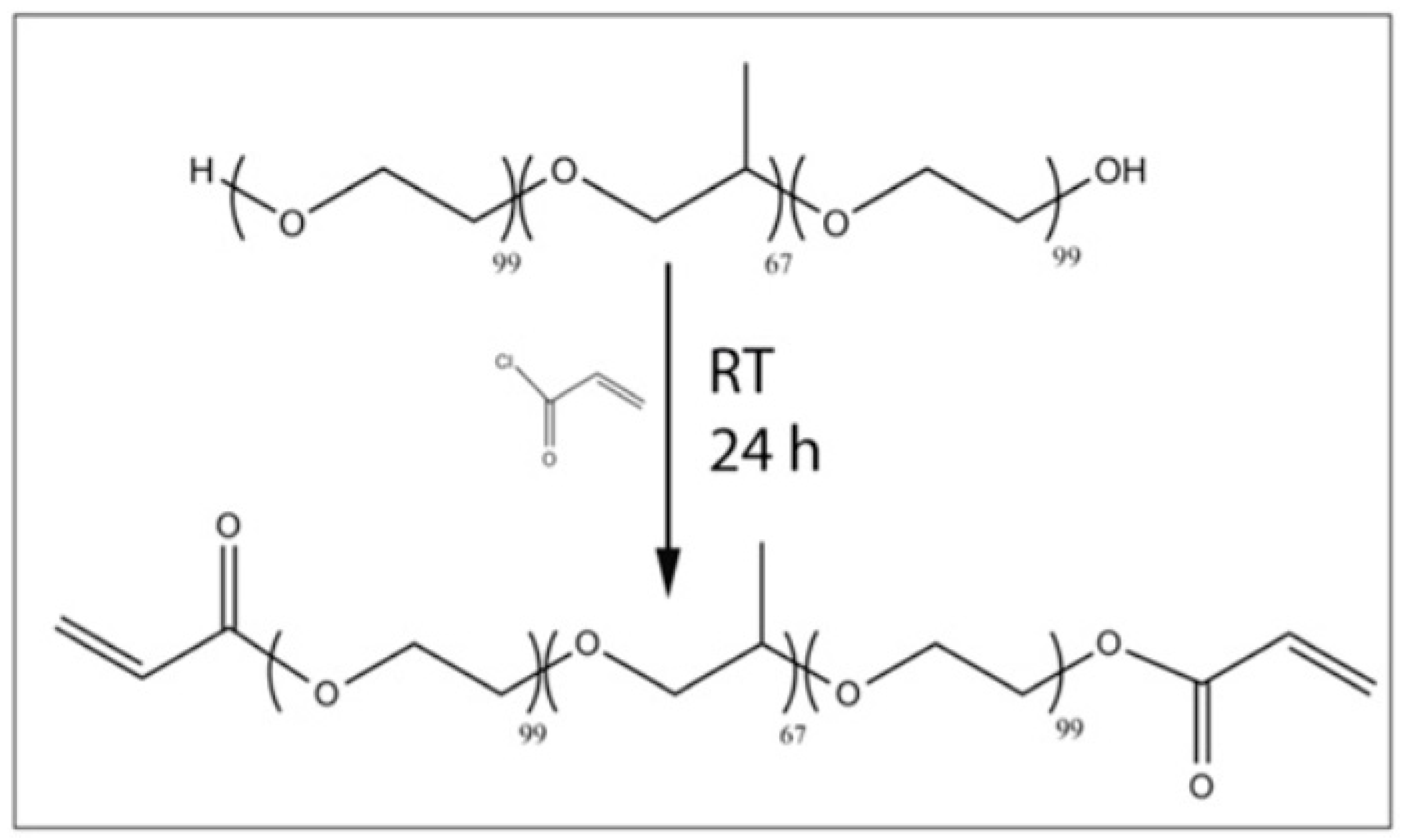
3.4. Polyethylene Glycol (PEG)
3.5. Polylactic-co-glycolic Acid (PLGA)
3.6. Pluronic Acid (or Poloxamer)
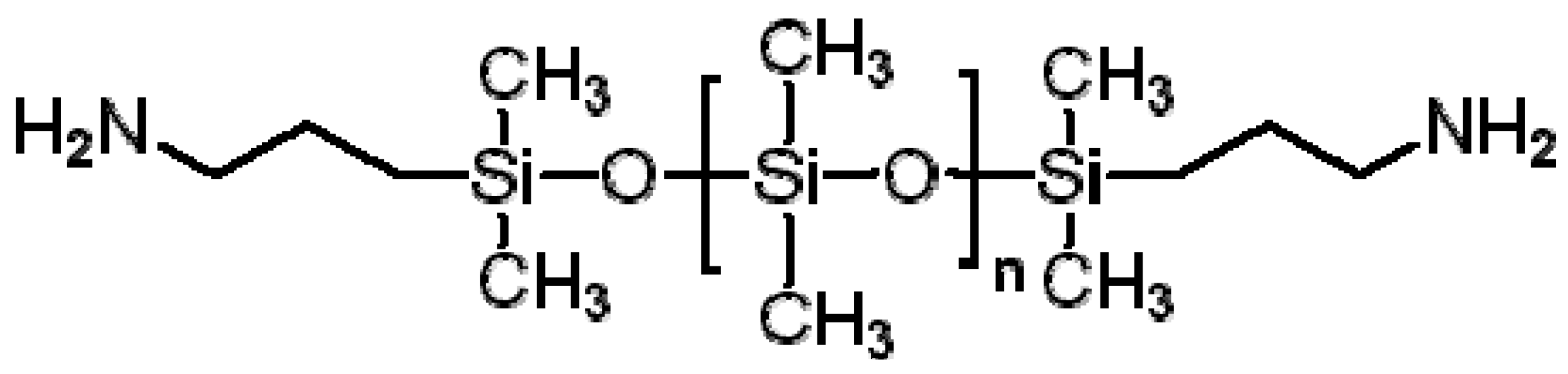
3.7. Polydimethylsiloxane (PDMS)
4. Typical Organ 3D Printing Technologies
4.1. Typical Organ 3D Printing Steps
4.2. Double-Nozzle Low-Temperature Organ 3D Printing
4.3. Combined Multi-Nozzle Organ 3D Printing Technologies
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schubert, C.; Van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Intelligent freeform manufacturing of complex organs. Artif. Organs 2012, 36, 951–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, C.W., Jr. Adipose tissue engineering: The future of breast and soft tissue reconstruction following tumor resection. Semin. Surg. Oncol. 2000, 19, 302–311. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.L.; Clark, E.A.; Murriky, A.; Smay, J.E. Three-dimensional printing of bone repair and replacement materials: Impact on craniofacial surgery. J. Craniofacial Surg. 2012, 23, 304–308. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. Recent trends and challenges in complex organ manufacturing. Tissue Eng. Part B 2010, 16, 189–197. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.F.; Zhang, W.M.; Khang, G.; Wang, X. In vitro vascularization of a combined system based on a 3D bioprinting technique. J. Tissue Eng. Regen. Med. 2016, 10, 833–842. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. Gelatin-based hydrogels for controlled cell assembly. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Ed.; Springer: New York, NY, USA, 2010; pp. 269–284. [Google Scholar]
- Lei, M.; Wang, X. Biodegradable polymers and stem cells for bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef]
- Aijaz, A.; Perera, D.; Olabisi, R.M. Polymeric materials for cell microencapsulation. Methods Mol. Biol. 2017, 1479, 79–93. [Google Scholar]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2004, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, T.; Yan, Y.; Zhang, R. Peroneal nerve regeneration along a new polyurethane-collagen guide conduit. J. Bioact. Compat. Polym. 2009, 24, 109–127. [Google Scholar] [CrossRef]
- Li, S.; Yan, Y.; Xiong, Z.; Weng, C.; Zhang, R.; Wang, X. Gradient hydrogel construct based on an improved cell assembling system. J. Bioact. Compat. Polym. 2009, 24, 84–99. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Liu, P.; Yan, Y.; Zhang, R. Cryopreservation of cells in 3D constructs based on controlled cell assembly processes. J. Bioact. Compat. Polym. 2009, 24, 473–487. [Google Scholar] [CrossRef]
- Wang, X.; Paloheimo, K.-S.; Xu, H.; Liu, C. Cryopreservation of cell/hydrogel constructs based on a new cell-assembling technique. J. Bioact. Compat. Polym. 2010, 25, 634–653. [Google Scholar] [CrossRef] [Green Version]
- Yao, R.; Zhang, R.; Yan, Y.; Wang, X. In vitro angiogenesis of 3D tissue engineered adipose tissue. J. Bioact. Compat. Polym. 2009, 24, 5–24. [Google Scholar]
- Xu, M.; Yan, Y.; Liu, H.; Yao, Y.; Wang, X. Control adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3-D structure established by cell-assembly technique. J. Bioact. Compat. Polym. 2009, 24, 31–47. [Google Scholar] [CrossRef]
- Wang, J. Vascularization and adipogenesis of a spindle hierarchical adipose-derived stem cell/collagen/alginate-PLGA construct for breast manufacturing. IJITEE 2015, 4, 1–8. [Google Scholar]
- Wang, X.; Huang, Y.W.; Liu, C. A combined rotational mold for manufacturing a functional liver system. J. Bioact. Compat. Polym. 2015, 39, 436–451. [Google Scholar] [CrossRef]
- Huang, Y.; He, K.; Wang, X. Rapid Prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater. Sci. Eng. C 2013, 33, 3220–3229. [Google Scholar] [CrossRef]
- Wang, X.; Sui, S.; Yan, Y.; Zhang, R. Design and fabrication of PLGA sandwiched cell/fibrin constructs for complex organ regeneration. J. Bioact. Compat. Polym. 2010, 25, 229–240. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Yan, Y.; Yao, R.; Ge, Y. A cell-assembly derived physiological 3D model of the metabolic syndrome, based on adipose-derived stromal cells and a gelatin/alginate/fibrinogen matrix. Biomaterials 2010, 31, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Poloheimo, K.-S.; Partanen, J.; Yliperttula, M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In Advances in Biomaterials Science and Applications in Biomedicine; Lazinica, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 437–463. [Google Scholar]
- Zhao, X.; Du, S.; Chai, L.; Xu, Y.; Liu, L.; Zhou, X.; Wang, J.; Zhang, W.; Liu, C.-H.; Wang, X. Anti-cancer drug screening based on an adipose-derived stem cell/hepatocyte 3D printing technique. J. Stem Cell Res. Ther. 2015, 5, 273. [Google Scholar]
- Zhou, X.; Liu, C.; Zhao, X.; Wang, X. A 3D bioprinting liver tumor model for drug screening. World J. Pharm. Pharm. Sci. 2016, 5, 196–213. [Google Scholar]
- Wang, X. Editorial: Drug delivery design for regenerative medicine. Curr. Pharm. Des. 2015, 21, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Overview on biocompatibilities of implantable biomaterials. In Advances in Biomaterials Science and Biomedical Applications in Biomedicine; Lazinica, R., Ed.; In Tech: Rijeka, Croatia, 2013; pp. 111–155. [Google Scholar]
- Wang, X.; Ma, J.; Wang, Y.; He, B. Bone repair in radii and tibias of rabbits with phosphorylated chitosan reinforced calcium phosphate cements. Biomaterials 2002, 23, 4167–4176. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Feng, Q.; Cui, F. Skeletal repair in of rabbits with calcium phosphate cements incorporated phosphorylated chitin reinforced. Biomaterials 2002, 23, 4591–4600. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 11, 1278. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, C. 3D bioprinting of adipose-derived stem cells for organ manufacturing. In Enabling Cutting Edge Technology for Regenerative Medicine; Khang, G., Ed.; Springer, SBM Singapore Pte Ltd.: Singapore, 2018; pp. 3–14. [Google Scholar]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.A.; Elisseeff, J.H.; Dorafshar, A.H.; Grayson, W.L. Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef]
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mott, E.J.; Busso, M.; Luo, X.; Dolder, C.; Wang, M.O.; Fisher, J.P.; Dean, D. Digital micromirror device (DMD)-based 3D printing of poly (propylene fumarate) scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Rizvi, G.M.; Bellehumeur, C.T.; Gu, P. Effect of processing conditions on the bonding quality of FDM polymer filaments. Rapid Prototyp. J. 2008, 15, 72–80. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Mercelis, P.; Vaerenbergh, J.; van Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2013, 11, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Ifkovits, J.L.; Burdick, J.A. Review: Photopolymerizable and degradable. Tissue Eng. 2007, 13, 2369–2385. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.J.; Saksena, R.; Kolesky, D.B.; Vericella, J.J.; Kranz, S.J.; Muldowney, G.P.; Christensen, K.T.; Lewis, J.A. High-throughput printing via microvascular multinozzle arrays. Adv. Mater. 2013, 25, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Choi, Y.C.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Nikzad, S. The evolution of rapid prototyping in dentistry: A review. Rapid Prototyp. J. 2009, 15, 216–225. [Google Scholar] [CrossRef]
- Elkaseer, A.; Schneider, S.; Scholz, S.G. Experiment-based process modeling and optimization for high-quality and resource-efficient FFF 3D printing. Appl. Sci. 2020, 10, 2899. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D Bioprinting technologies for hard tissue and organ engineering. Materials 2016, 27, 802. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X. Creation of a vascular system for complex organ manufacturing. Int. J. Bioprint. 2015, 1, 77–86. [Google Scholar]
- Kim, J.E.; Kim, S.H.; Jung, Y. Current status of three-dimensional printing inks for soft tissue regeneration. Tissue Eng. Regen. Med. 2016, 13, 636–646. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell. Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, D.-W. Blended PCL/PLGA scaffold fabrication using multi-head deposition system. Microelectron. Eng. 2009, 86, 1447–1450. [Google Scholar] [CrossRef]
- Uchida, T.; Onoe, H. 4D printing of multi-hydrogels using direct ink writing in a supporting viscous liquid. Micromachines 2019, 10, 433. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, J.J.; Boydston, A.J. Multimaterial actinic spatial control 3D and 4D printing. Nat. Commun. 2019, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Leberfinger, A.N.; Dinda, S.; Wu, Y.; Koduru, S.V.; Ozbolat, V.; Ravnic, D.J.; Ozbolat, I.T. Bioprinting functional tissues. Acta Biomater. 2019, 95, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.; Rosen, D.; Stucker, B. The impact of low-cost AM systems. In Additive Manufacturing Technologies, 2nd ed.; Springer: New York, NY, USA, 2015; pp. 293–301. [Google Scholar]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panwar, A.; Tan, L.P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Dalela, M.; Sybil, D.; Patra, P.; Mohanty, S. Advances in 3D bioprinting of bone: Progress and challenges. J. Tissue Eng. Regen. Med. 2019, 13, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, H. Incorporation of DMSO and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryopreservation. Cryobiology 2010, 61, 345–351. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping of polyurethane for the creation of vascular systems. J. Bioact. Compat. Polym. 2008, 23, 103–114. [Google Scholar] [CrossRef]
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-dimensional photopatterning of hydrogels using stereolithography for long- term cell encapsulation. Lab Chip 2010, 10, 2062–2070. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef]
- Karaiskou, A.; Zergioti, I.; Fotakis, C.; Kapsetaki, M.; Kafetzopoulos, D. Microfabrication of biomaterials by the sub-ps laser-induced forward transfer process. Appl. Surf. Sci. 2003, 208, 245–249. [Google Scholar] [CrossRef]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Catros, S.; Guillotin, B.; Bacakova, M.; Fricain, J.C.; Guillemot, F. Effect of laser energy, substrate film thickness and bioink viscosity on viability of endothelial cells printed by laser-assisted bioprinting. Appl. Surf. Sci. 2011, 257, 5142–5147. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Mironi-Harpaz, I.; Wang, D.Y.; Venkatraman, S.; Seliktar, D. Photopolymerization of cell-encapsulating hydrogels: Crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012, 8, 1838–1848. [Google Scholar] [CrossRef]
- Wang, X. 3D printing of tissue/organ analogues for regenerative medicine. In Handbook of Intelligent Scaffolds for Regenerative Medicine, 2nd ed.; Pan Stanford Publishing: Palo Alto, CA, USA, 2016; pp. 557–570. [Google Scholar]
- Censi, R.; Schuurman, W.; Malda, J.; di Dato, G.; Burgisser, P.E.; Dhert, W.J.A.; van Nostrum, C.F.; di Martino, P.; Vermonden, T.; Hennink, W.E. A printable photopolymerizable thermosensitive p(HPMAm-lactate)-PEG hydrogel for tissue engineering. Adv. Funct. Mater. 2011, 21, 1833–1842. [Google Scholar] [CrossRef]
- Lee, S.-J.; Nowicki, M.; Harris, B.; Zhang, L.G. Fabrication of a highly aligned neural scaffold via a table top stereolithography 3D printing and electrospinning. Tissue Eng. Part A 2017, 23, 491–502. [Google Scholar] [CrossRef]
- Revilla-León, M.; Özcan, M. Additive manufacturing technologies used for processing polymers: Current status and potential application in prosthetic dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, B.Y.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.-I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Felzmann, R.; Gruber, S.; Mitteramskogler, G.; Tesavibul, P.; Boccaccini, A.R.; Liska, R.; Stampfl, J. Lithography-based additive manufacturing of cellular ceramic structures. Adv. Eng. Mater. 2012, 14, 1052–1058. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, E.; Miyanishi, S.; Hashimoto, K.; Tajima, K. Preparation of active layers in polymer solar cells by aerosol jet printing. ACS Appl. Mater. Interfaces 2011, 3, 4053–4058. [Google Scholar] [CrossRef] [PubMed]
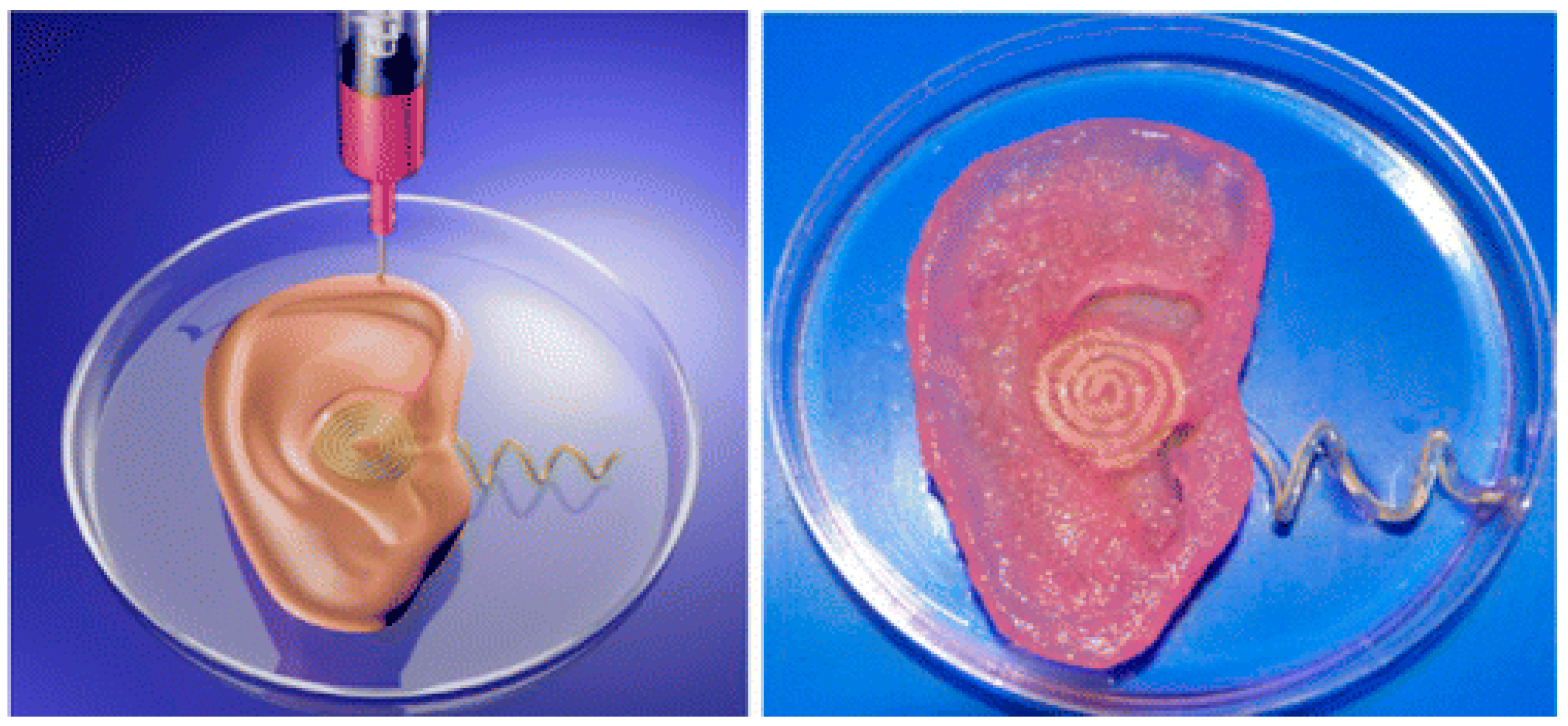
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D printed bionic ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, A.; Frisbie, C.D.; Francis, L.F. Optimization of aerosol jet printing for high-resolution, high-aspect ratio silver lines. ACS Appl. Mater. Interfaces 2013, 5, 4856–4864. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, Y. Colloidal nanoparticle inks for printing functional devices: Emerging trends and future prospects. J. Mater. Chem. A 2019, 7, 23301–23336. [Google Scholar] [CrossRef]
- Jing, L.; Li, K.; Yang, H.; Chen, P.Y. Recent advances in integration of 2D materials with soft matter for multifunctional robotic materials. Mater. Horiz. 2020, 7, 54–70. [Google Scholar] [CrossRef]
- Saunders, R.E.; Gough, J.E.; Derby, B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008, 29, 193–203. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Schrepfer, I.; Wang, X. Progress in 3D printing technology in health care. In Organ Manufacturing; Wang, X.H., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 29–74. [Google Scholar]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Prakash, B. 3D printing and its applications. Int. J. Sci. Res. 2016, 5, 1532–1535. [Google Scholar]
- Luo, Y.X.; Luo, G.L.; Gelinsky, M.; Huang, P.; Ruan, C.S. 3D bioprinting scaffold using alginate/polyvinyl alcohol bioinks. Mater. Lett. 2017, 189, 295–298. [Google Scholar] [CrossRef]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X. Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Biotechnol. Bioeng. 2015, 112, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Yang, S. Extrusion-based additive manufacturing of PEEK for biomedical applications. Virtual Phys. Prototyp. 2015, 10, 123–135. [Google Scholar] [CrossRef]
- Yan, Y.; Cui, F.; Zhang, R.; Hu, Y. Rapid prototyping manufacturing for artificial human bone. Mater. Rev. 2000, 14, 11–13. [Google Scholar]
- Puebla, K.; Arcaute, K.; Quintana, R.; Wicker, R.B. Effects of environmental conditions, aging, and build orientations on the mechanical properties of ASTM type I specimens manufactured via stereolithography. Rapid Prototyp. J. 2012, 18, 374–388. [Google Scholar] [CrossRef]
- Hendriks, J.; Willem Visser, C.; Henke, S. Optimizing cell viability in droplet-based cell deposition. Sci. Rep. 2015, 11, 11304. [Google Scholar] [CrossRef]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Henzler, T.; Chai, L.; Wang, X.H. Integrated model for organ manufacturing: A systematic approach from medical imaging to rapid prototyping. In Organ Manufacturing; Wang, X.H., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2015; pp. 171–200. [Google Scholar]
- Odde, D.J.; Renn, M.J. Laser-guided direct writing of living cells. Biotechnol. Bioeng. 2000, 67, 312–318. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.; Huang, Y. Modeling of bubble expansion-induced cell mechanical profile in laser-assisted cell direct writing. J. Manuf. Sci. Eng. 2009, 131, 051013. [Google Scholar] [CrossRef]
- Hopp, B.; Smausz, T.; Szabó, G.; Kolozsvári, L.; Kafetzopoulos, D.; Fotakis, C.; Nógrádi, A. Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt. Eng. 2012, 51, 014312. [Google Scholar] [CrossRef]
- Hung, K.-C.; Tseng, C.-S.; Hsu, S.-H. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv. Healthc. Mater. 2014, 3, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; De Coninck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 2018, 7, e1700939. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Tasoglu, S.; Demirci, U. Bioprinting for stem cell research. Trends Biotechnol. 2013, 31, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Leigh, S.J.; Bradley, R.J.; Purssell, C.P.; Billson, D.R.; Hutchins, D.A. A simple, low-cost conductive composite material for 3D printing of electronic sensors. PLoS ONE 2012, 7, e49365. [Google Scholar] [CrossRef]
- Phillippi, J.A.; Miller, E.; Weiss, L. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells 2008, 26, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Kolessky, D.B.; Homan, K.A.; SkylarScott, M.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [Green Version]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused Deposition Modeling of Novel Scaffold Architectures for Tissue Engineering Applications. Biomaterials 2001, 23, 1169–1185. [Google Scholar] [CrossRef]
- Johnson, N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D printed anatomical nerve regeneration pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef]
- Temple, J.P.; Hutton, D.L.; Hung, B.P.; Huri, P.Y.; Cook, C.; Kondragunta, R.; Jia, X.; Grayson, W.L. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J. Biomed. Mater. Res. A 2014, 102, 4317–4325. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.H.; Jang, J.; Kim, S.W.; Cho, D.W. An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef]
- Boere, K.W.; Visser, J.; Seyednejad, H.; Rahimian, S.; Gawlitta, D.; van Steenbergen, M.J.; Dhert, W.J.; Hennink, W.E.; Vermonden, T.; Malda, J. Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 2014, 10, 2602–2611. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, N. Preparation and characterization of poly(ε-caprolactone) scaffolds modified with cell-loaded fibrin gel. Int. J. Biol. Macromol. 2019, 15, 683–689. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 2008, 29, 3781–3791. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Li, S.; Hu, S.-G.; Chang, W.-C.; Jeng, U.-S.; Hsu, S.-H. Synthesis of thermoresponsive amphiphilic polyurethane gel as a new cell printing material near body temperature. ACS Appl. Mater. Interfaces 2015, 7, 27613–27623. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsu, S.-H. Synthesis and characterization of dual stimuli-sensitive biodegradable polyurethane soft hydrogels for 3D cell-laden bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 29273–29287. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Hsu, S.-H. 3D bioprinting: A new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis 2015, 11, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S.-H. 3D bioprinting of neual stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Ho, L.; Hsu, S.-H. Cell reprogramming by 3D bioprinting of human fibroblasts in polyurethane hydrogel for fabrication of neural-like constructs. Acta Biomater. 2018, 70, 50–70. [Google Scholar] [CrossRef]
- Hung, K.-C.; Tseng, C.-S.; Dai, L.-G.; Hsu, S.-H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016, 83, 156–168. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Yin, D.; Zhang, R. A new polyurethane/heparin vascular graft for small-caliber vein repair. J. Bioact. Compat. Polym. 2007, 22, 323–341. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Yan, Y.; Zhang, R. Preliminary studies on peripheral nerve regeneration along a new polyurethane conduit. J. Bioact. Compat. Polym. 2007, 22, 143–159. [Google Scholar]
- Cui, T.; Yan, Y.; Zhang, R.; Liu, L.; Xu, W.; Wang, X. Rapid prototyping of a double layer polyurethane-collagen conduit for peripheral nerve regeneration. Tissue Eng. Part C 2009, 15, 1–9. [Google Scholar] [CrossRef]
- Cui, T.; Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping a new polyurethane-collagen conduit and its Schwann cell compatibilities. J. Bioact. Compat. Polym. 2009, 24, 5–17. [Google Scholar] [CrossRef]
- He, K.; Wang, X. Rapid prototyping of tubular polyurethane and cell/hydrogel construct. J. Bioact. Compat. Polym. 2011, 26, 363–374. [Google Scholar]
- Wang, X.; He, K.; Zhang, W. Optimizing the fabrication processes for manufacturing a hybrid hierarchical polyurethane–cell/hydrogel construct. J. Bioact. Compat. Polym. 2013, 28, 303–319. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. A polyurethane-gelatin hybrid construct for the manufacturing of implantable bioartificial livers. J. Bioact. Compat. Polym. 2008, 23, 409–422. [Google Scholar] [CrossRef]
- Clapper, J.D.; Skeie, J.M.; Mullins, R.F.; Guymon, C.A. Development and characterization of photopolymerizable biodegradable materials from PEG-PLA-PEG block macromonomers. Polymers 2007, 48, 6554. [Google Scholar] [CrossRef]
- Jiang, Z.; Hao, J.; You, Y.; Liu, Y.; Wang, Z.; Deng, X. Biodegradable and thermosensitive hydrogels of poly(ethylene glycol)-poly(ε-caprolactone-co-glycolide)-poly(ethylene glycol) aqueous solutions. J. Biomed. Mater. Res. A 2008, 87, 45–51. [Google Scholar] [CrossRef]
- Yee, S.W.; Chor, Y.T.; Feng, W.; Subbu, S.V.; Lay, P.T. Engineered polymeric biomaterials for tissue engineering. Curr. Tissue Eng. 2012, 1, 41–53. [Google Scholar]
- Choe, G.; Park, J.; Park, H.; Lee, J.Y. Hydrogel biomaterials for stem cell microencapsulation. Polymers 2018, 10, 997. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, I.; Weigel, C.A.; Bryant, S.J. Cell-matrix interactions and dynamic mechanical loading influence chondrocyte gene expression and bioactivity in PEG-RGD hydrogels. Acta Biomater. 2009, 5, 2832–2846. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef]
- Gao, G.; Schilling, A.F.; Hubbell, K. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, D.M.; Stone, R.; Wrice, N.; Laborde, A.; Becerra, S.C.; Natesan, S.; Christy, R.J. Delivery of allogeneic adipose stem cells in polyethylene glycol-fibrin hydrogels as an adjunct to meshed autografts after sharp debridement of deep partial thickness burns. Stem Cells Transl. Med. 2018, 7, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Samadi, N.; Abbadessa, A.; Di Stefano, A.; van Nostrum, C.F.; Vermonden, T.; Rahimian, S.; Teunissen, E.A.; van Steenbergen, M.J.; Amidi, M.; Hennink, W.E. The effect of lauryl capping group on protein release and degradation of poly (D, L-lactic-co-glycolic acid) particles. J. Control. Release 2013, 172, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yan, Y.; Xiong, Z.; Zhang, R.; Wang, X. A novel poly(lactic-co-glycolic acid)-collagen hybrid scaffold fabricated via multi-nozzle low-temperature deposition. In Proceedings of the 3rd International Conference on Advanced Research in Virtual and Rapid Prototyping (VRAP 2007)Virtual Rapid Manufacturing (September 24–28, 2007 in Leiria, Portugal); Bártolo, P., Ed.; © Taylor & Francis Group: London, UK, 2008; ISBN 978-0-415-41602-3. [Google Scholar]
- Wang, X.; Sui, S. Pulsatile culture of a poly (DL-lactic-co-glycolic acid) sandwiched cell/hydrogel construct fabricated using a step-by-step mold/extraction method. Artif. Organs 2011, 35, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mäkitie, A.A.; Poloheimo, K.-S.; Tuomi, J.; Paloheimo, M.; Sui, S.; Zhang, Q. Characterization of a PLGA sandwiched cell/fibrin tubular construct and induction of the adipose derived stem cells into smooth muscle cells. Mater. Sci. Eng. C 2011, 31, 801–808. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Yan, Y.; Zhang, R. Liver tissue responses to gelatin and gelatin/chitosan gels. J. Biomed. Mater. Res. A 2008, 87A, 62–68. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X. Preparation of an adipose-derived stem cell/fibrin-poly(dl-lactic-co-glycolic acid) construct based on a rapid prototyping technique. J. Bioact. Compat. Polym. 2013, 28, 191–203. [Google Scholar] [CrossRef]
- Wang, X.; Rijff, B.L.; Khang, G. A building block approach into 3D printing a multi-channel organ regenerative scaffold. J. Tissue Eng. Regen. Med. 2017, 11, 1403–1411. [Google Scholar] [CrossRef]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef]
- Matthew, J.E.; Nazario, Y.L.; Roberts, S.C.; Bhatia, S.R. Effect of mammalian cell culture medium on the gelation properties of Pluronic (R) F127. Biomaterials 2002, 23, 4615–4619. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Chen, Q.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Progress in organ 3D bioprinting. Int. J. Bioprint. 2017, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Di Biase, M.; de Leonardis, P.; Castelletto, V.; Hamley, I.W.; Derby, B.; Tirelli, N. Photopolymerization of Pluronic F127 diacrylate: A colloid-templated polymerization. Soft Matter 2011, 7, 4928–4937. [Google Scholar] [CrossRef]
- Frisman, I.; Seliktar, D.; Bianco-Peled, H. Nanostructuring PEG-fibrinogen hydrogels to control cellular morphogenesis. Biomaterials 2011, 32, 7839–7846. [Google Scholar] [CrossRef]
- Frisman, I.; Seliktar, D.; Bianco-Peled, H. Nanostructuring biosynthetic hydrogels for tissue engineering: A cellular and structural analysis. Acta Biomater. 2012, 8, 51–60. [Google Scholar] [CrossRef]
- Tendulkar, S.; Mirmalek-Sani, S.H.; Childers, C.; Saul, J.; Opara, E.C.; Ramasubramanian, M.K. A three-dimensional microfluidic approach to scaling up microencapsulation of cells. Biomed. Microdevices 2012, 14, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Hinton, T.J.; Hudson, A.; Pusch, K.; Lee, A.; Feinberg, A.W. 3D Printing PDMS elastomer in a hydrophilic support bath via freeform reversible embedding. ACS Biomater. Sci. Eng. 2016, 2, 1781–1786. [Google Scholar] [CrossRef]
- Mu, X.; Zheng, W.; Sun, J.; Zhang, W.; Jiang, X. Microfluidics for manipulating cells. Small 2013, 9, 9–21. [Google Scholar] [CrossRef]
- Huang, P.; Xia, Z.; Cui, S. 3D printing of carbon fiber-filled conductive silicon rubber. Mater. Des. 2018, 142, 11–21. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.H.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Ozbolat, I.T. Direct bioprinting of vessel-like tubular microfluidic channels. J. Nanotechnol. Eng. Med. 2013, 4, 0210011–0210017. [Google Scholar] [CrossRef]
- Pateman, C.J.; Harding, A.J.; Glen, A.; Taylor, C.S.; Christmas, C.R.; Robinson, P.P.; Rimmer, S.; Boissonade, F.M.; Claeyssens, F.; Haycock, J.W. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials 2015, 49, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X. Advanced polymers for organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J. Development of a Combined 3D Printer and Its Application in Complex Organ Construction. Master’s Thesis, Tsinghua University, Beijing, China, 2014. [Google Scholar]
- Wang, X. Bioartificial organ manufacturing technologies. Cell Transplant. 2018, 27, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X. Spatial effects of stem cell engagement in 3D printing constructs. J. Stem Cells Res. Rev. Rep. 2014, 1, 5–9. [Google Scholar]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [Green Version]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Nie, J.; Gao, Q.; Xie, C.; Lv, S.; Qiu, J.; Liu, Y.; Guo, M.; Guo, R.; Fu, J.; He, Y. Construction of multi-scale vascular chips and modelling of the interaction between tumours and blood vessels. Mater. Horiz. 2020, 7, 82–92. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, G.M. Development of an air-knife system for highly reproducible fabrication of polydimethylsiloxane microstencils. J. Micromech. Microeng. 2015, 25, 085014. [Google Scholar] [CrossRef]
- Pirlo, R.K.; Wu, P.; Liu, J.; Ringeisen, B. PLGA/hydrogel biopapers as a stackable substrate for printing HUVEC networks via BioLP. Biotechnol. Bioeng. 2012, 109, 262–273. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, K.; Muzaffar, A.; Kovářík, T.; Křenek, T.; Ahamed, M.B.; Pasha, S.K.K. Fundamentals and applications of 3D and 4D printing of polymers: Challenges in polymer processing and prospects of future research (source: Nielsen Book Data). In 3D and 4D Printing of Polymer Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 5271–5560. [Google Scholar]
- Deshmukh, K.; Muzaffar, A.; Kovářík, T.; Křenek, T.; Ahamed, M.B.; Pasha, S.K.K.; Chakraborty, P.; Zhou, C.; Chung, D.D.L. Piezoelectric behavior of three-dimensionally printed acrylate polymer without filler or poling. J. Mater. Sci. 2018, 53, 6819–6830. [Google Scholar]
- Gundrati, N.B.; Chakraborty, P.; Zhou, C.; Chung, D.D.L. Effects of printing conditions on the molecular alignment of three-dimensionally printed polymer. Compos. Part B Eng. 2018, 134, 164–168. [Google Scholar] [CrossRef]
- Zhang, R.; Larsen, N.B. Stereolithographic hydrogel printing of 3D culture chips with biofunctionalized complex 3D perfusion networks. Lab Chip 2017, 17, 4273–4282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, R.J.; Coulombe, K.L.K. Integrated approaches to spatiotemporally directing angiogenesis in host and engineered tissues. Acta Biomater. 2018, 69, 42–62. [Google Scholar] [CrossRef]
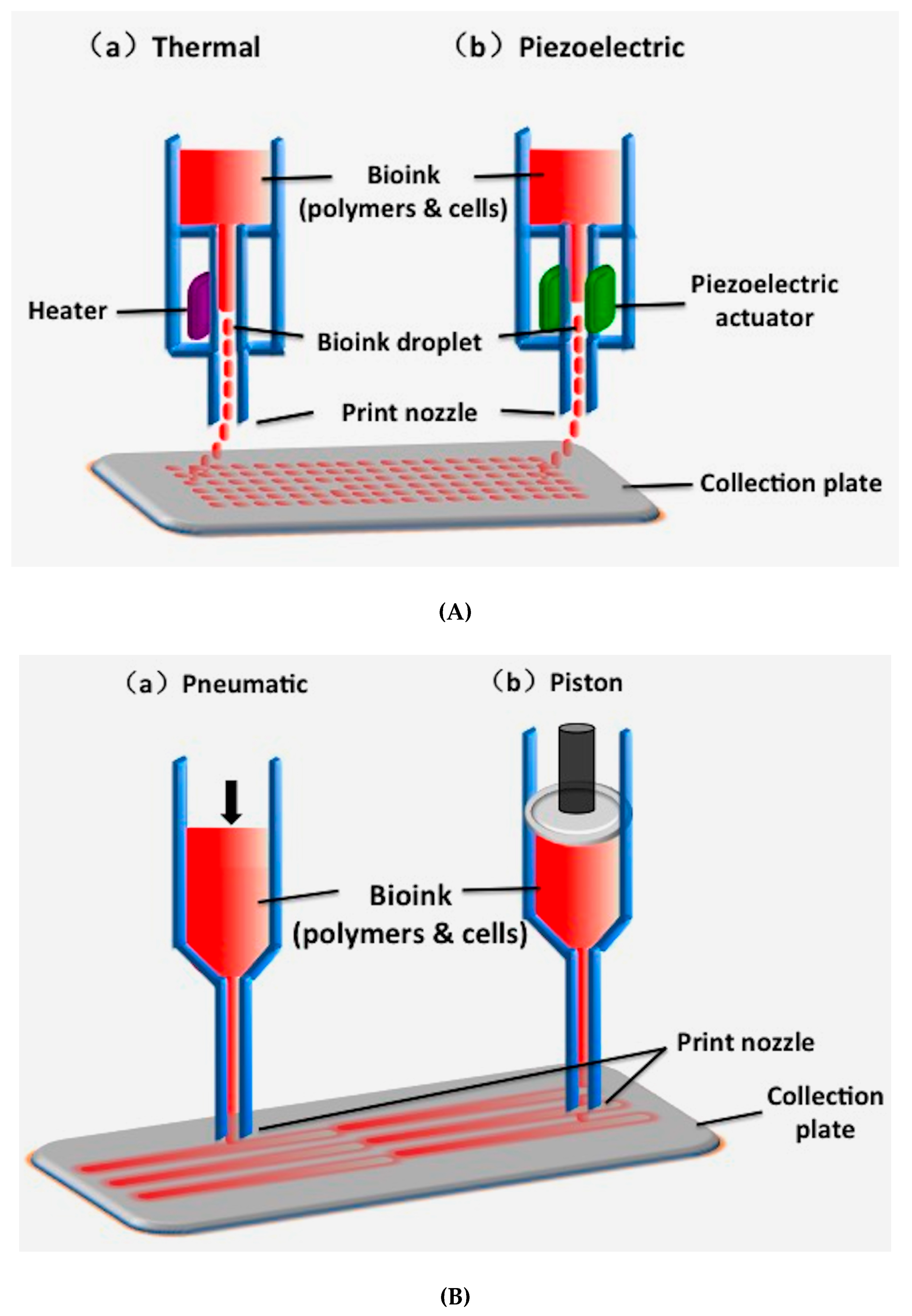


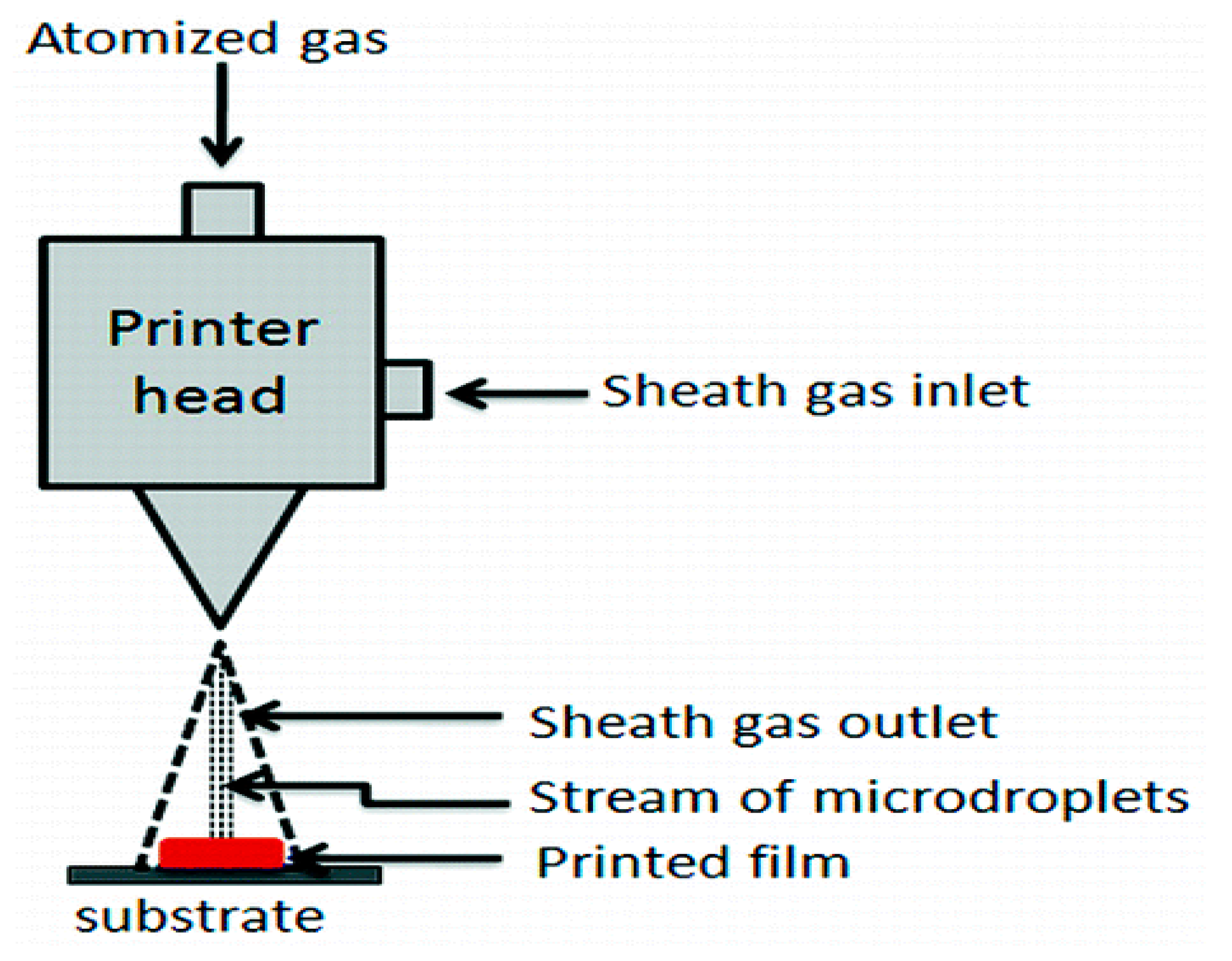

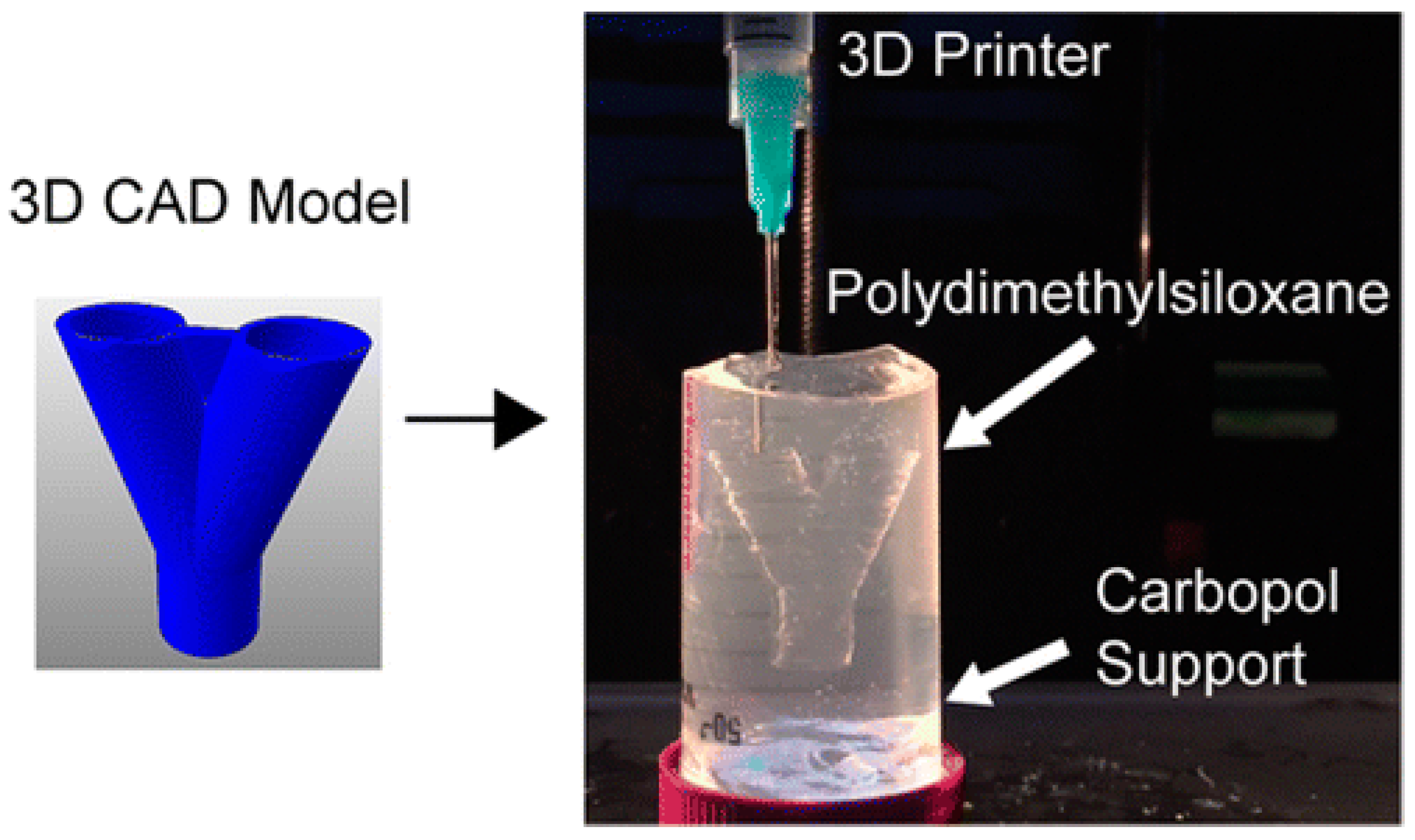


| 3D Printing Technique | Working Principle | Bioinks | Cell Density | Cell Viability | Printing Speed | Resolution | Cost | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Inkjet-based 3D printing | Using thermal or acoustic force to eject very small size ‘bioink’ drops onto a substrate | Thermosensitive hydrogels (e.g., PEG) and some nature polymers (e.g., alginate collagen, fibrinogen) with viscosity of 3.5–12 mPa/s | Low (<1 × 106 cells/mL) | 85% | Fast (1–10,000 droplets/s) | High (≈50 μm) | Low | Low cost; high printing resolution; low viscosity; fast printing speed | Poor mechanical properties; poor cell sedimentation effects; low cell densities | [84,85,86] |
| Fusion deposition modeling (FDM) | Molten thermoplastic materials through one or more heated extrusion heads with a small orifice in a specific lay-down pattern | Thermoplastic materials (e.g., PCL, PLA, PVA, ABS, TPU) with viscosity of 30 mPa/s to >6 × 107 mPa/s | None | None | Slow (200 μm–10 mm/s) | Low (100 μm to 1 mm | Medium | Low cost; a wide range of materials; excellent mechanical properties | Only applicable for thermoplastic materials; high temperature; cannot incorporate cells, growth factors, and other bioactive agents | [87,88] |
| Extrusion-based 3D printing | Biomaterials are extruded though one or more nozzles under controlled pressure in a layer-by-layer pattern | Most nature polymers and some synthetic polymers (e.g., alginate, gelatin, collagen, PEG, PLGA, PU) with viscosity of 30 mPa/s to >6 × 107 mPa/s | High (>1 × 108 cells/mL) | 40%–100% | Medium (5–20 mm/s) | Medium (10–100 μm) | Low | High cell densities; high cell viability; various printing materials; flexible geometric shapes | Only applicable for viscous hydrogels; moderate resolution | [89,90,91,92,93] |
| Stereolithography (SLA) | A solid freeform, nozzle-free technology based on photosensitive polymer formulation under laser beam | Photopolymers | Medium (<1 × 108 cells/mL) | 90% | Fast (normally 30–45 min) | High (100 μm) | Low | High printing resolution; fast printing speed; difficult to print multiple cell types | Cytotoxicity of the laser beam and photoinitiators; additional post-curing process may be necessary to remove the unpolymerized liquid resin; poor cell deposition effects | [46,94,95] |
| Digital light processing (DLP) | A solid freeform, nozzle-free technology based on photosensitive polymer formulation under laser beam | Photopolymers | Medium (<1 × 108 cells/mL) | 90% | Higher than SLA (10–50 μm) | Higher than SLA (10–50 μm) | Low | High printing resolution; fast printing speed; difficult to print multiple cell types | Cytotoxicity of the laser beam and photoinitiators; additional post-curing process may be necessary to remove the unpolymerized liquid resin; poor cell deposition effects | [96,97] |
| Laser-based 3D printing | Laser pulse generates a high-pressure bubble towards the collector substrate | Nature polymers (e.g., alginate, gelatin, fibrinogen) and some synthetic polymers (e.g., PCL, PLGA) with viscosity of 1–300 mPa/s | Medium (≈1 × 108 cells/mL) | 90%–95% | High (10–40 μm) | High (10–40 μm) | High | High printing resolution; wide range of printable viscosity; moderate cell viability | High printing resolution; wide range of printable viscosity; moderate cell viability | [98,99,100] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. https://doi.org/10.3390/polym12081765
Liu F, Wang X. Synthetic Polymers for Organ 3D Printing. Polymers. 2020; 12(8):1765. https://doi.org/10.3390/polym12081765
Chicago/Turabian StyleLiu, Fan, and Xiaohong Wang. 2020. "Synthetic Polymers for Organ 3D Printing" Polymers 12, no. 8: 1765. https://doi.org/10.3390/polym12081765
APA StyleLiu, F., & Wang, X. (2020). Synthetic Polymers for Organ 3D Printing. Polymers, 12(8), 1765. https://doi.org/10.3390/polym12081765







