Photoresponsive Photoacid-Macroion Nano-Assemblies
Abstract
1. Introduction
2. Materials and Methods
3. Results
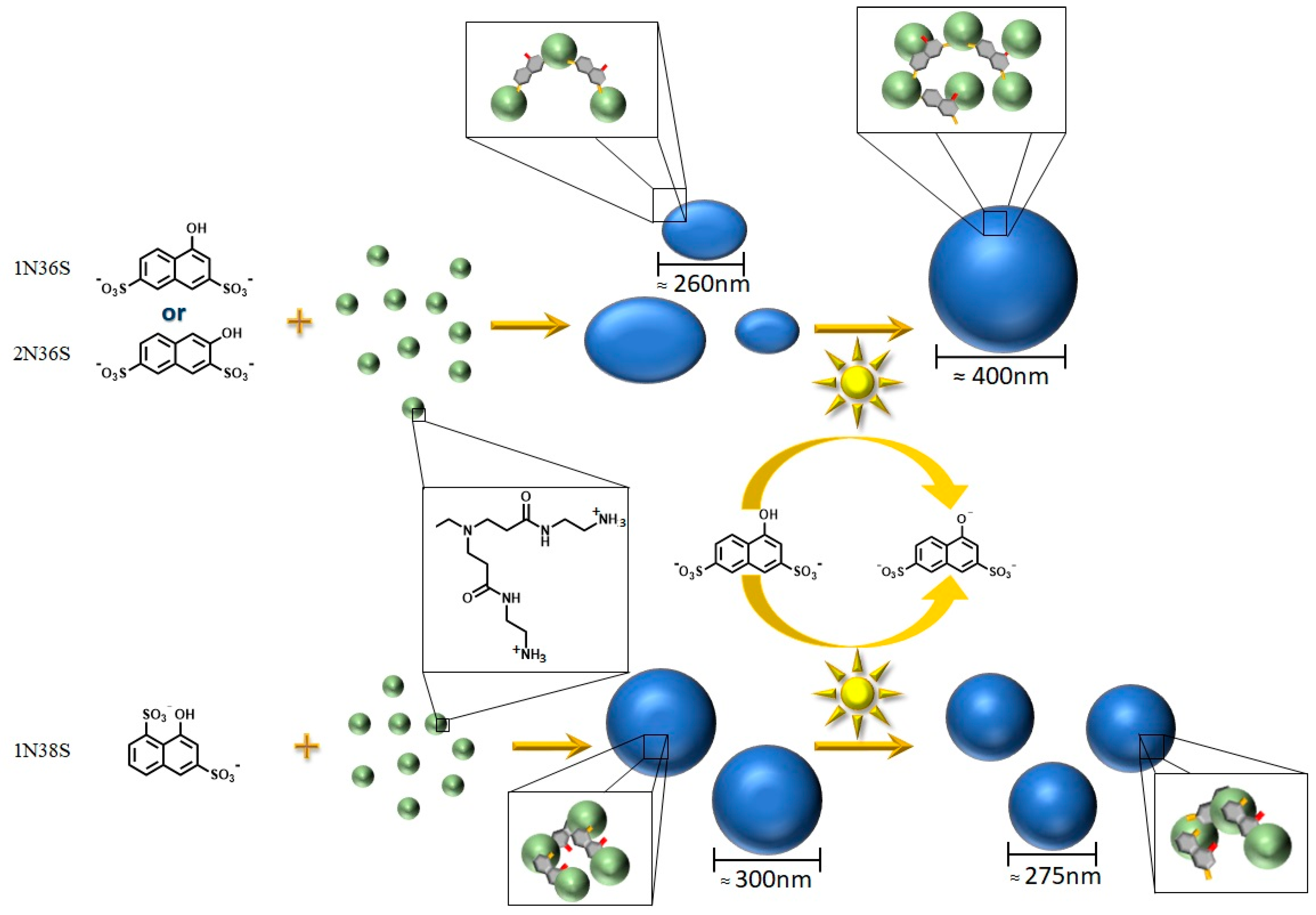
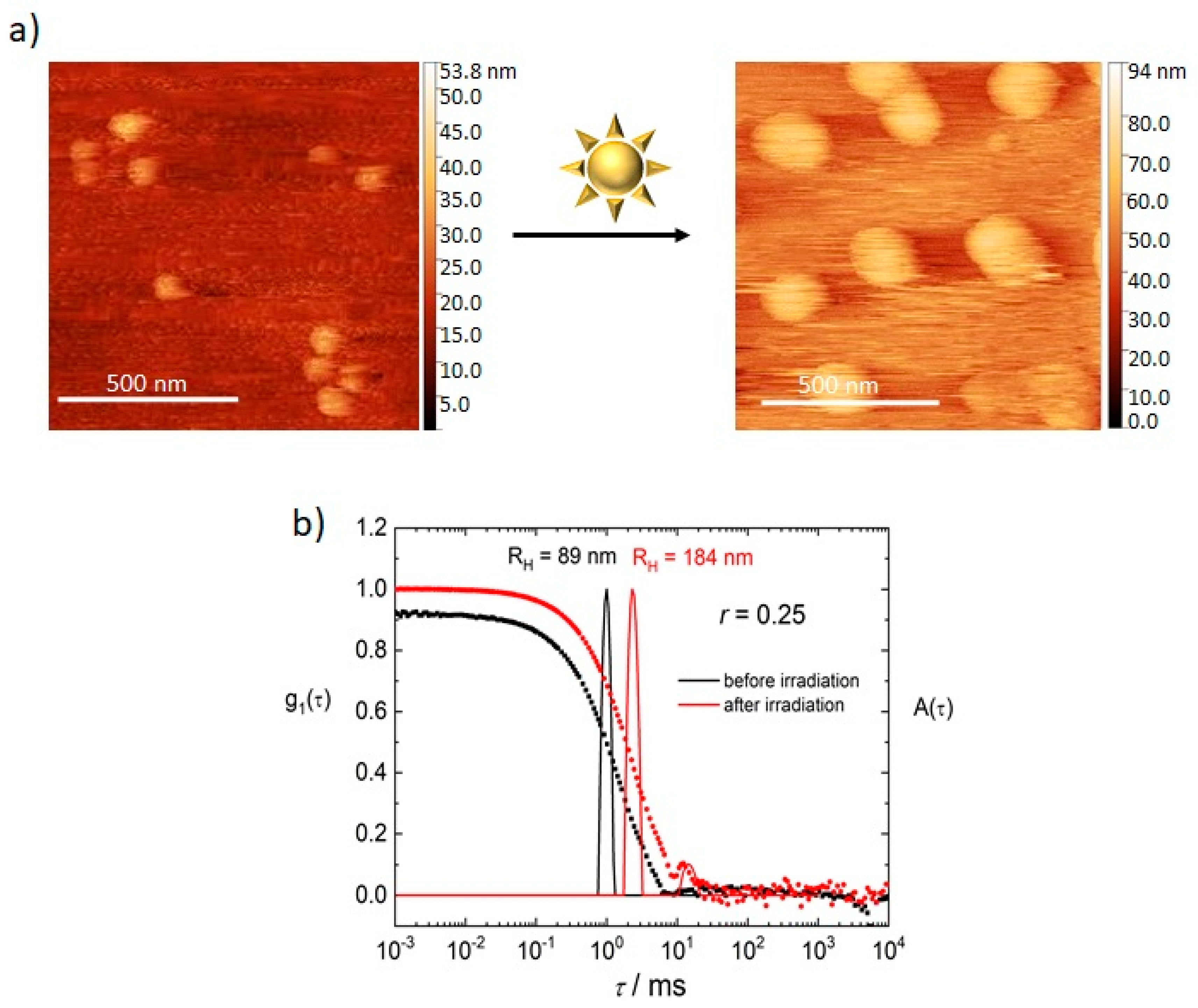
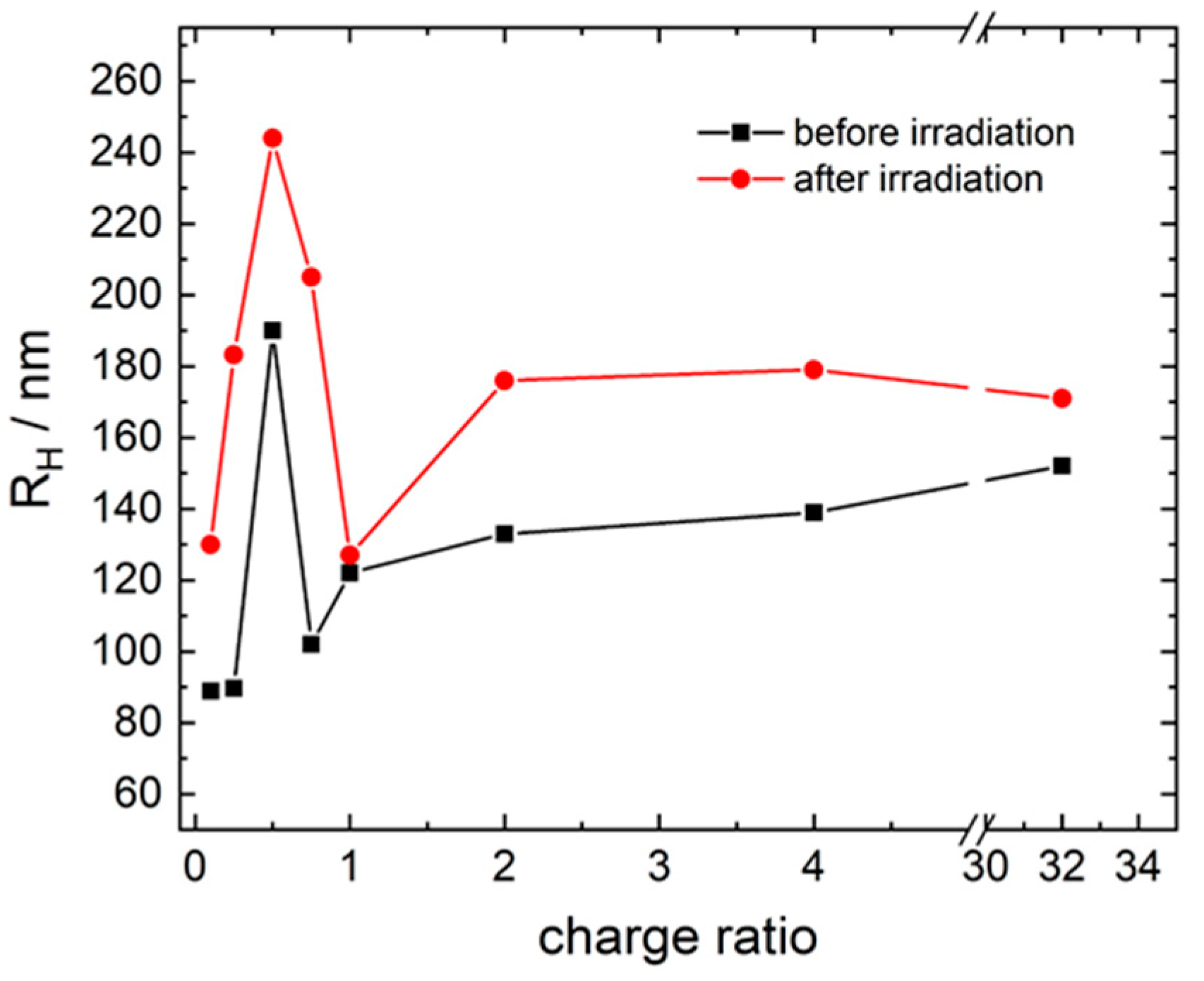
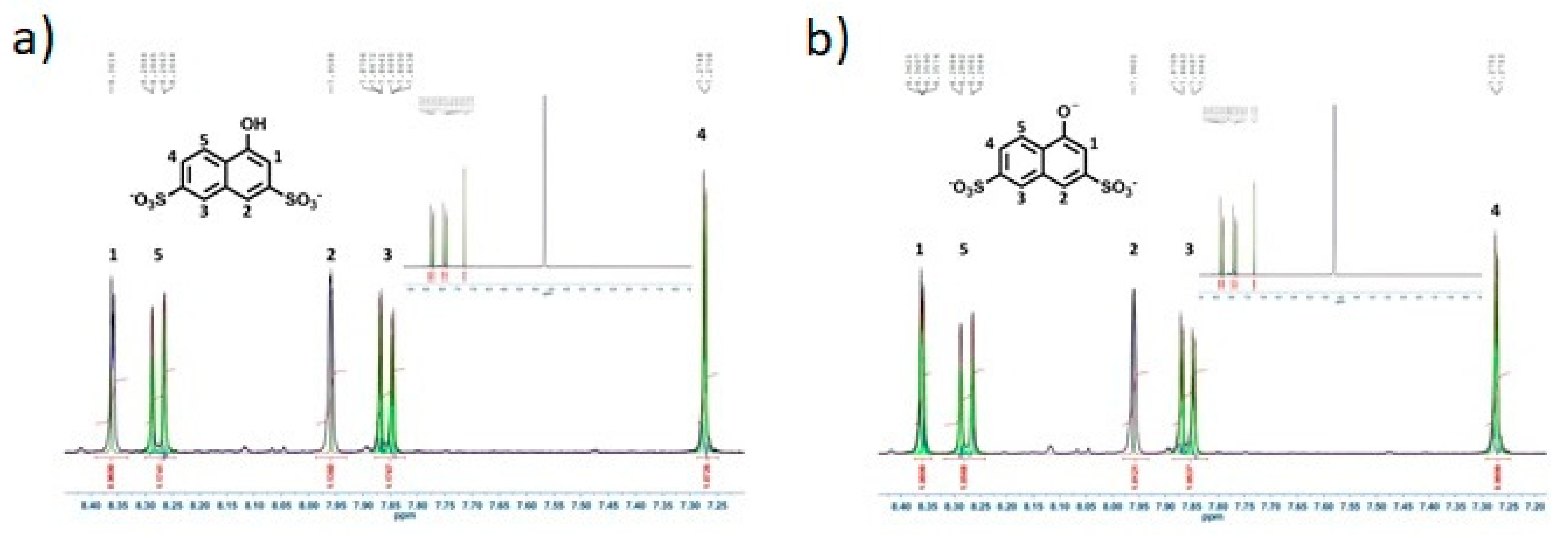
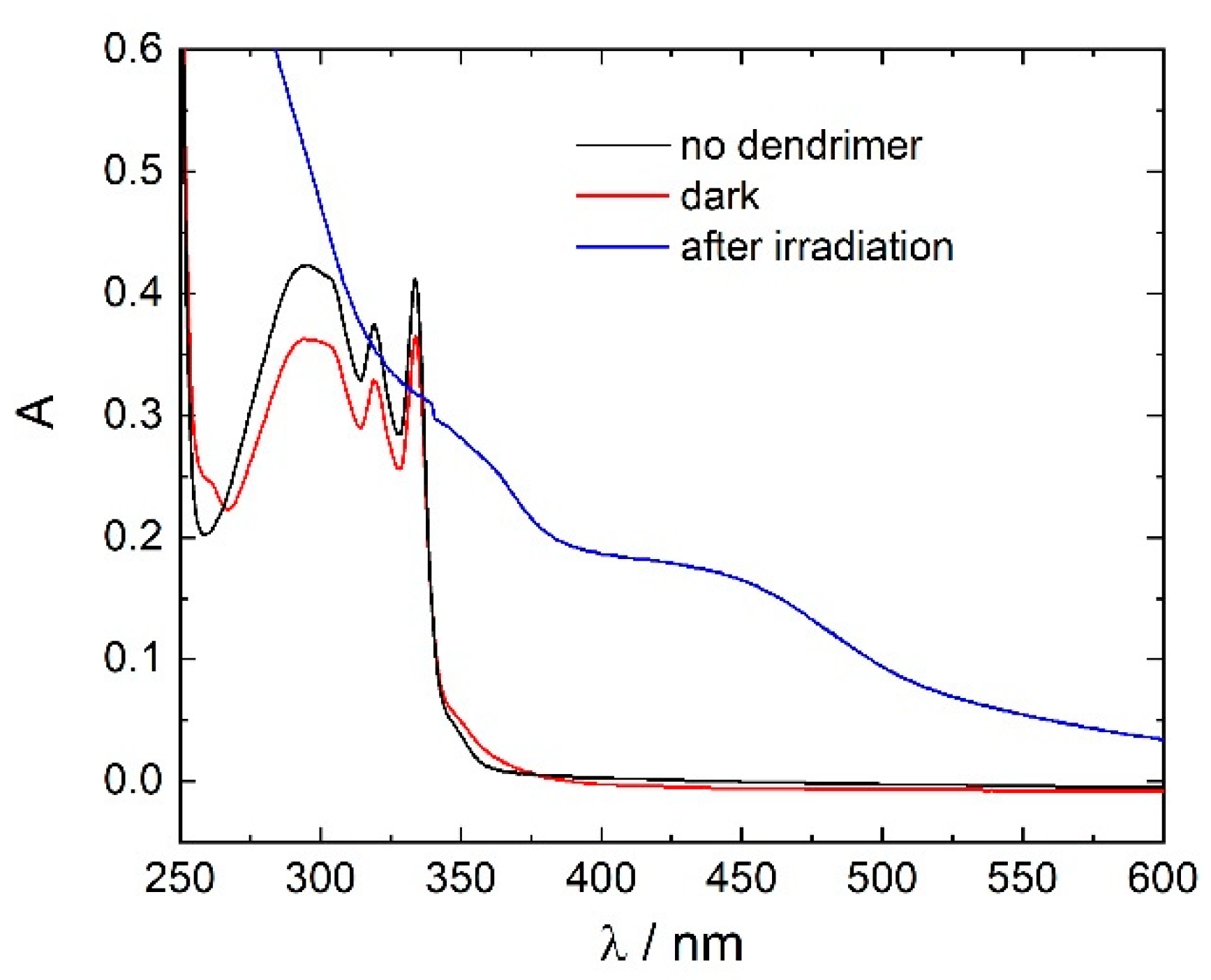
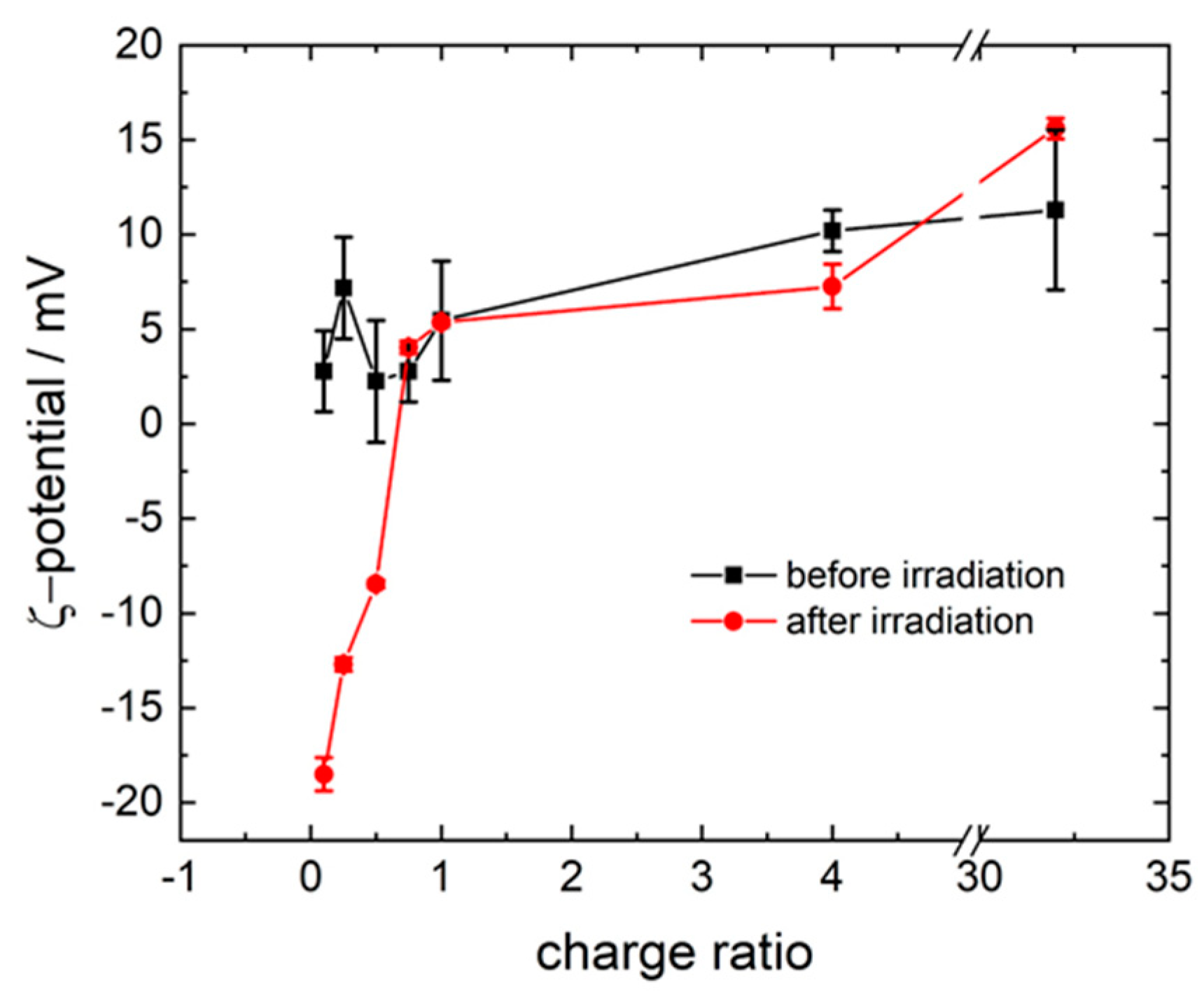
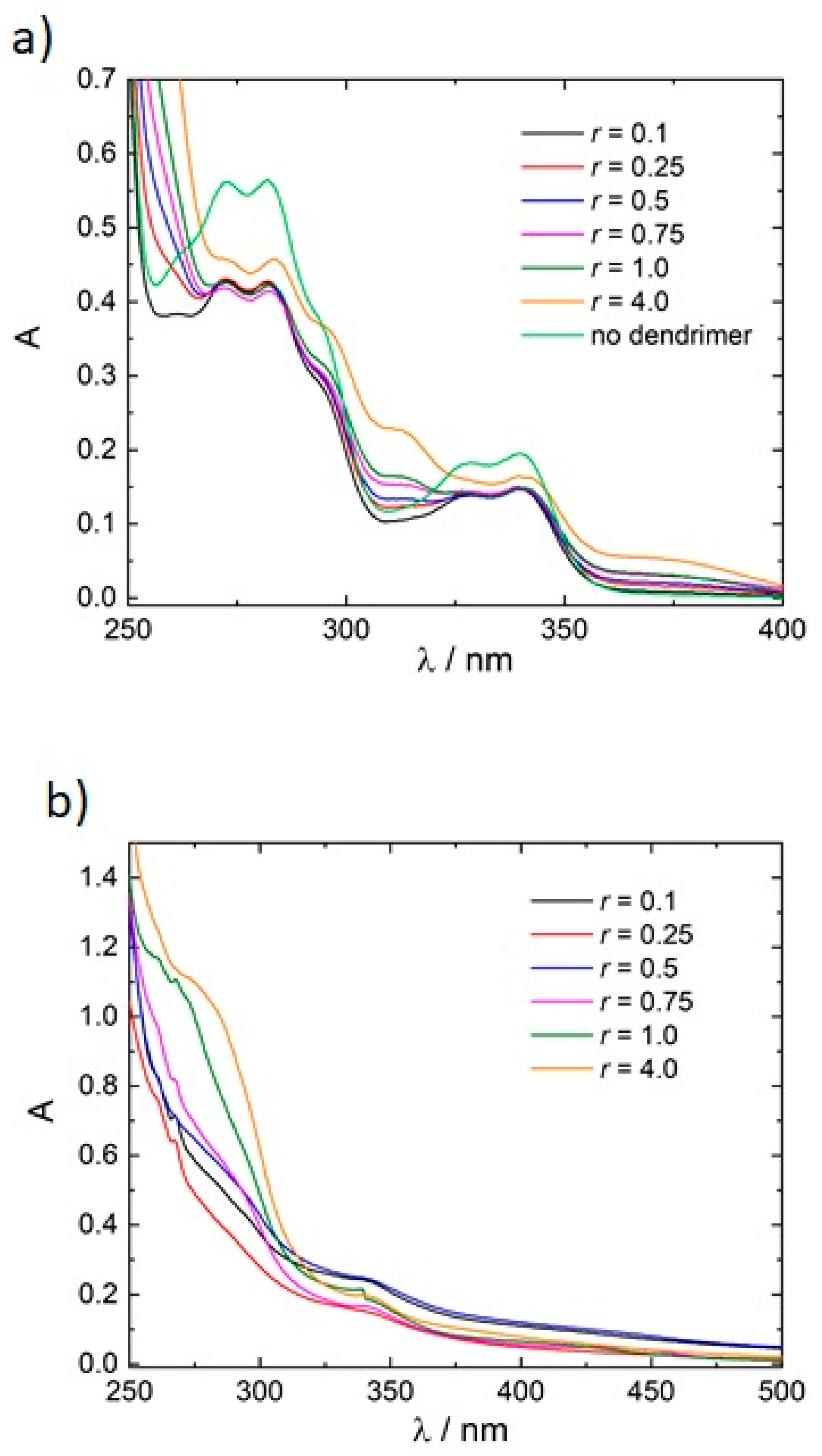
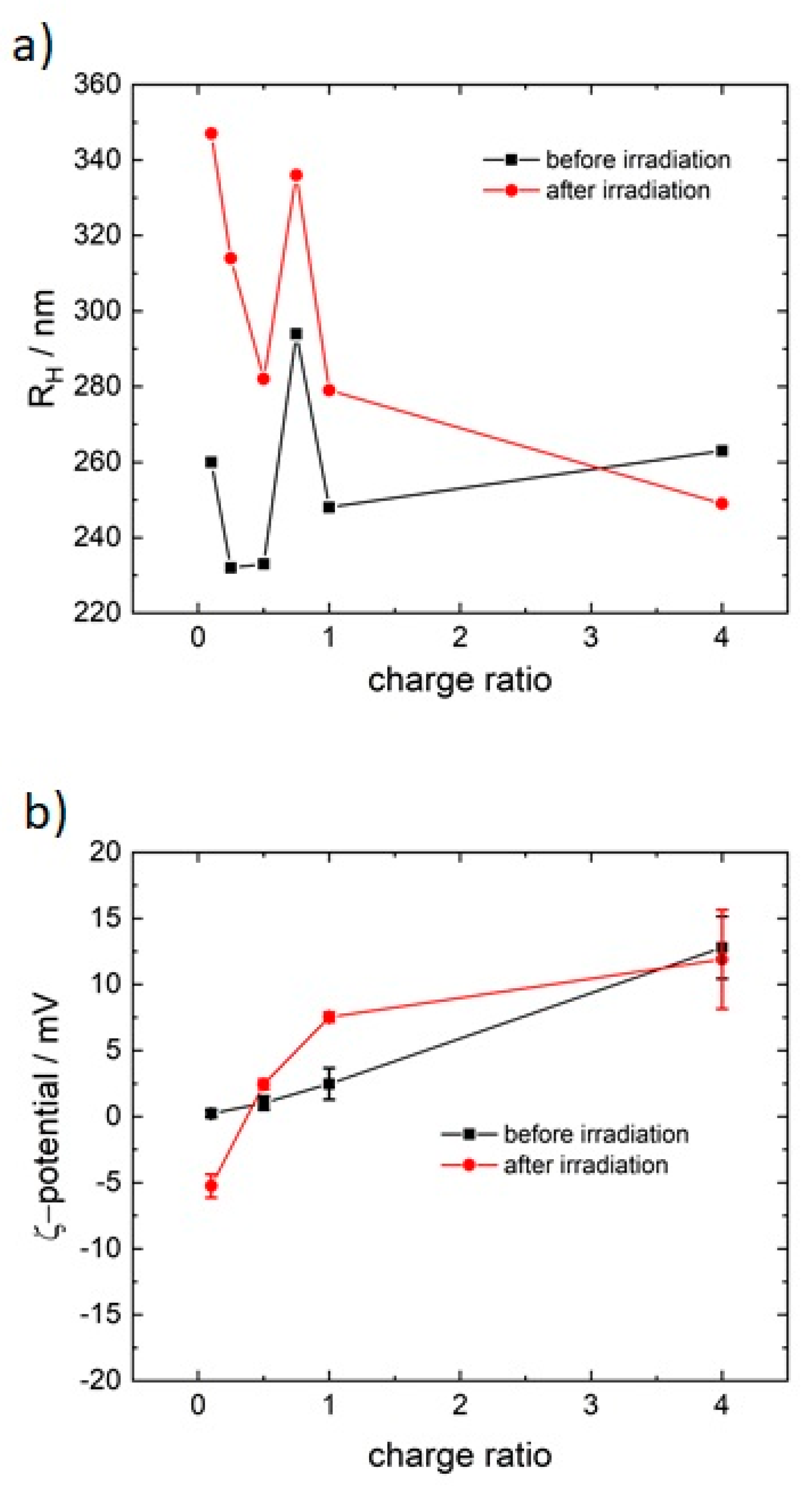
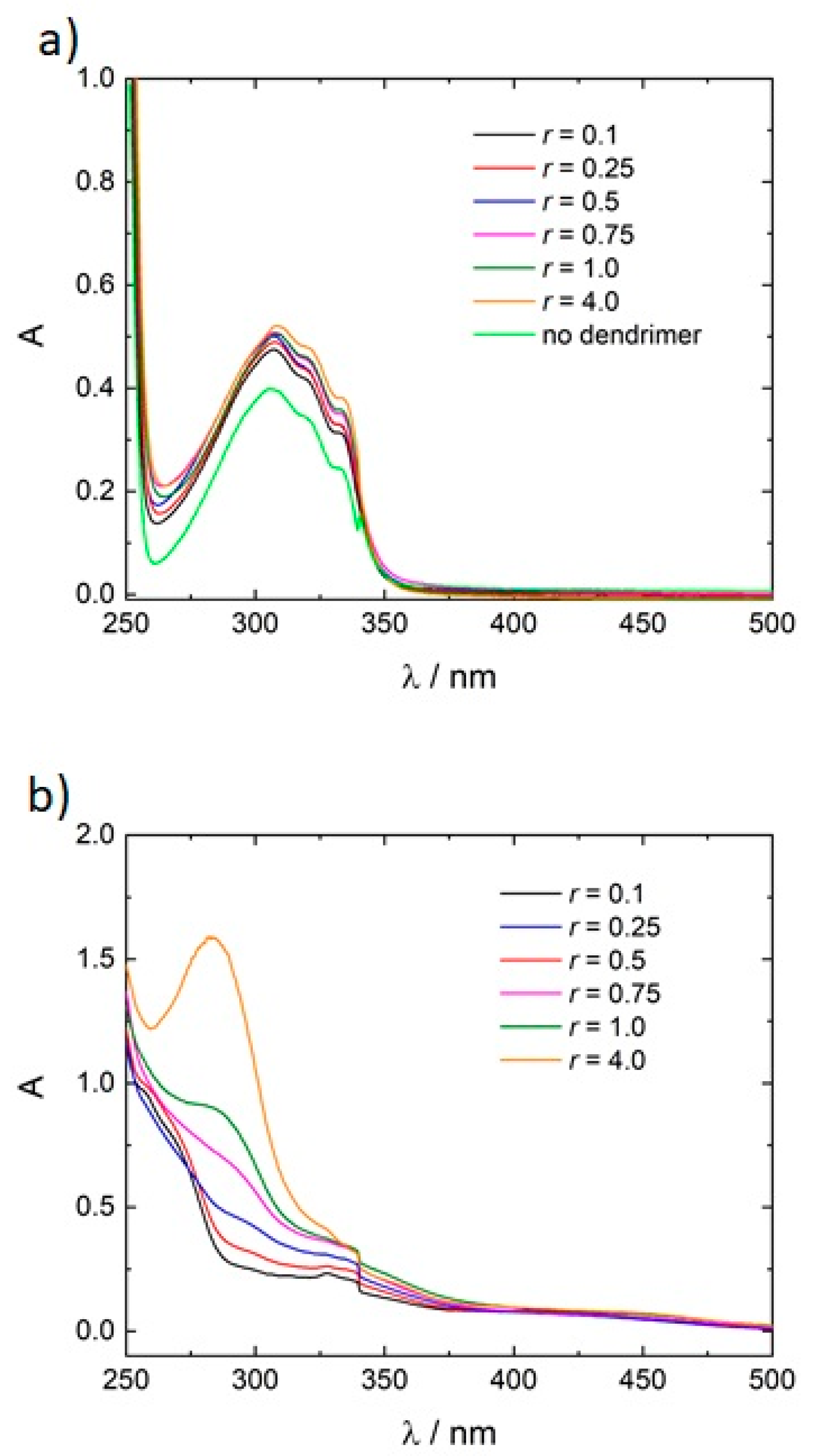
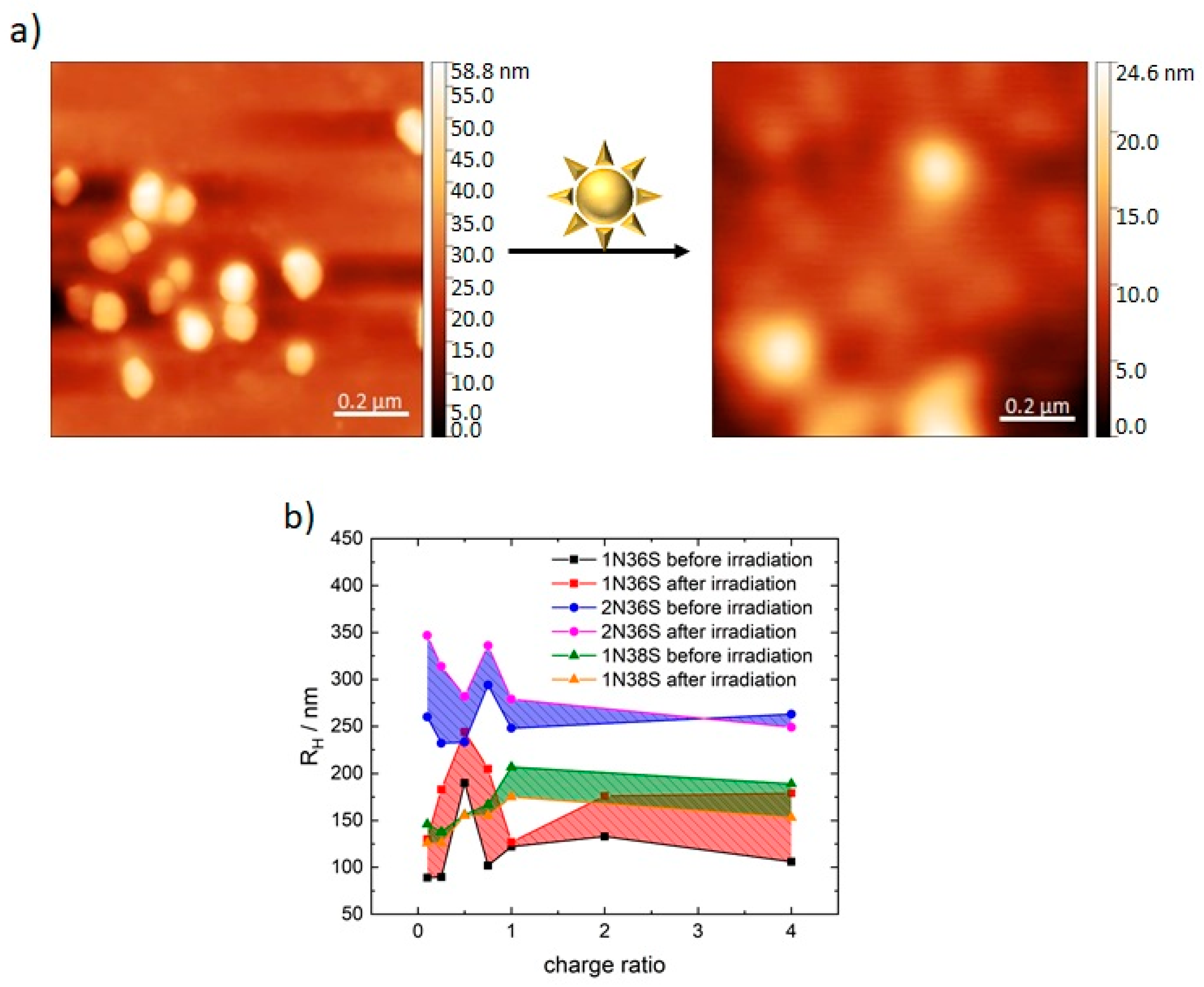
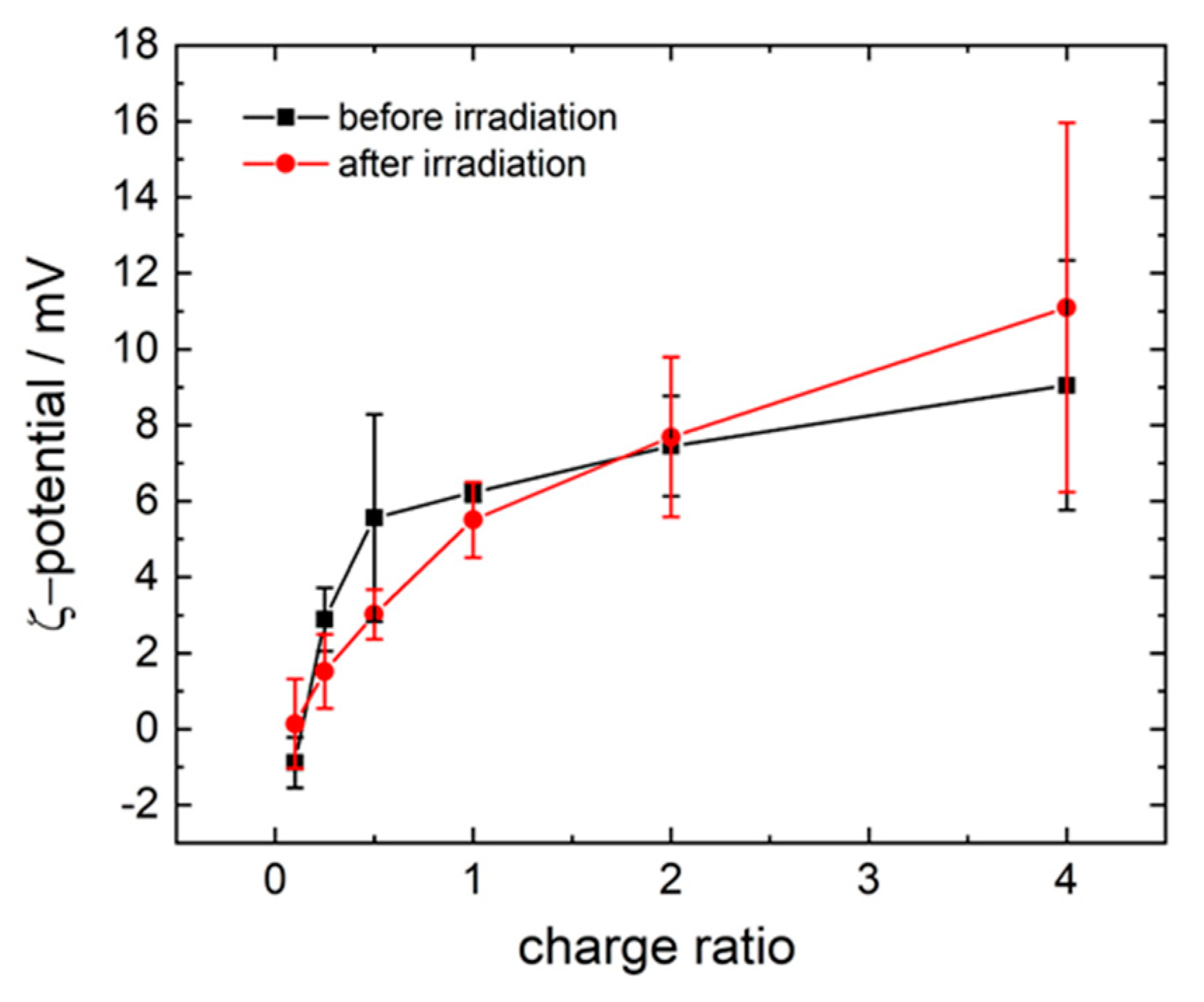
3.1. Model System: Electrostatic Self-Assembly with 1-Napthol-3,6-Disulfonate (1N36S)
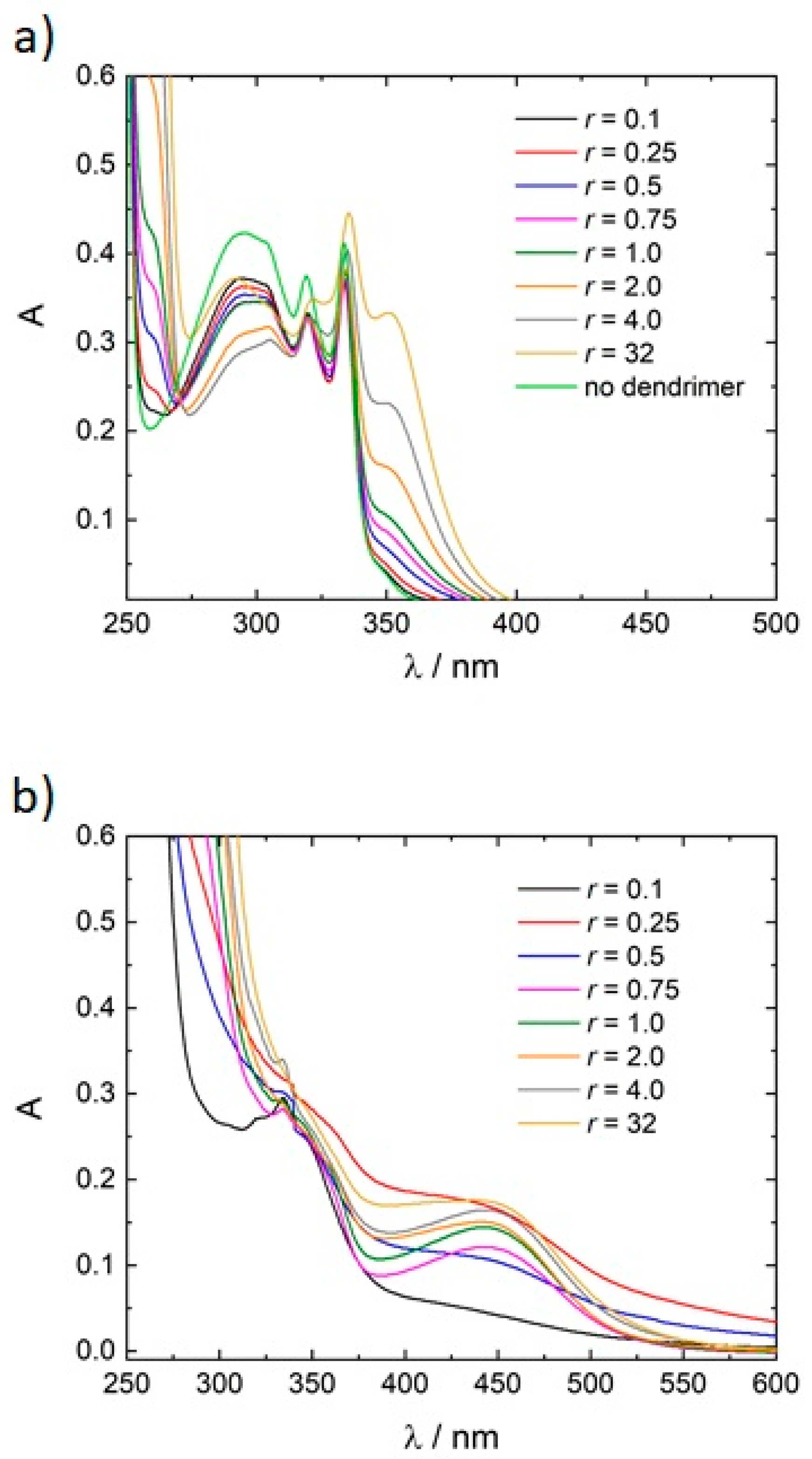
3.2. Variation of the Photoacid
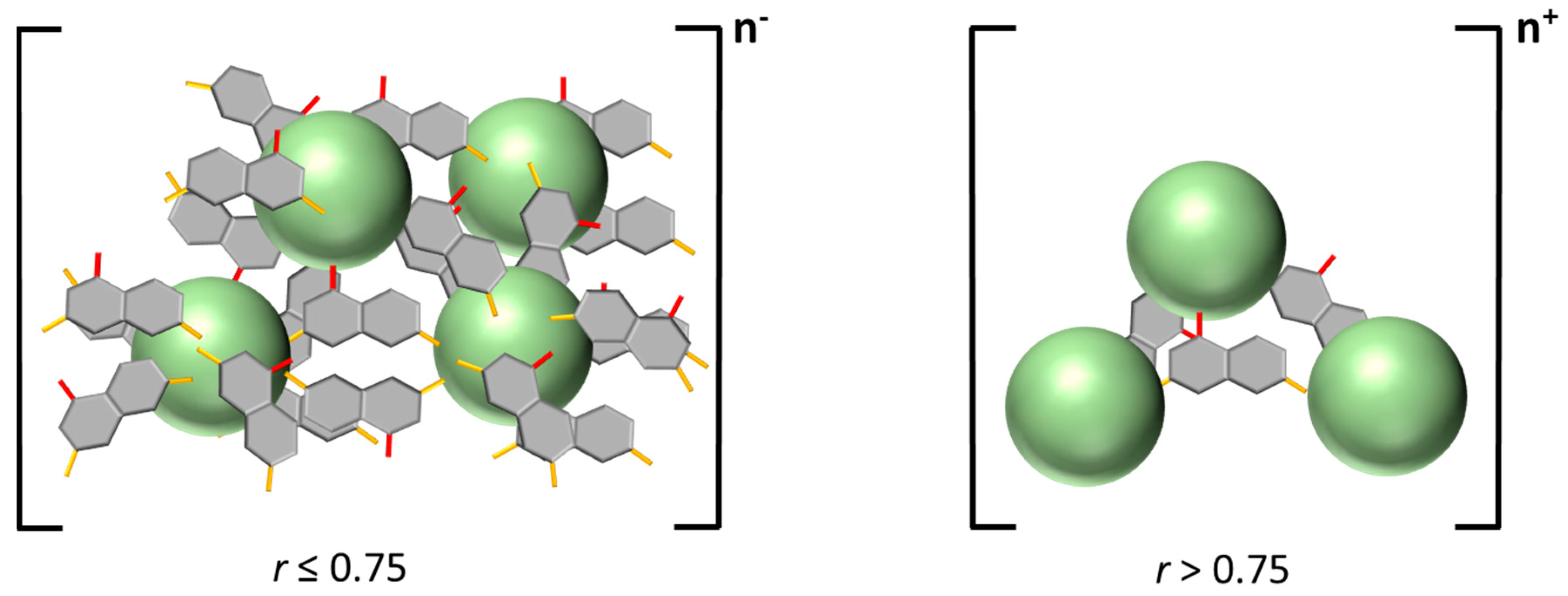
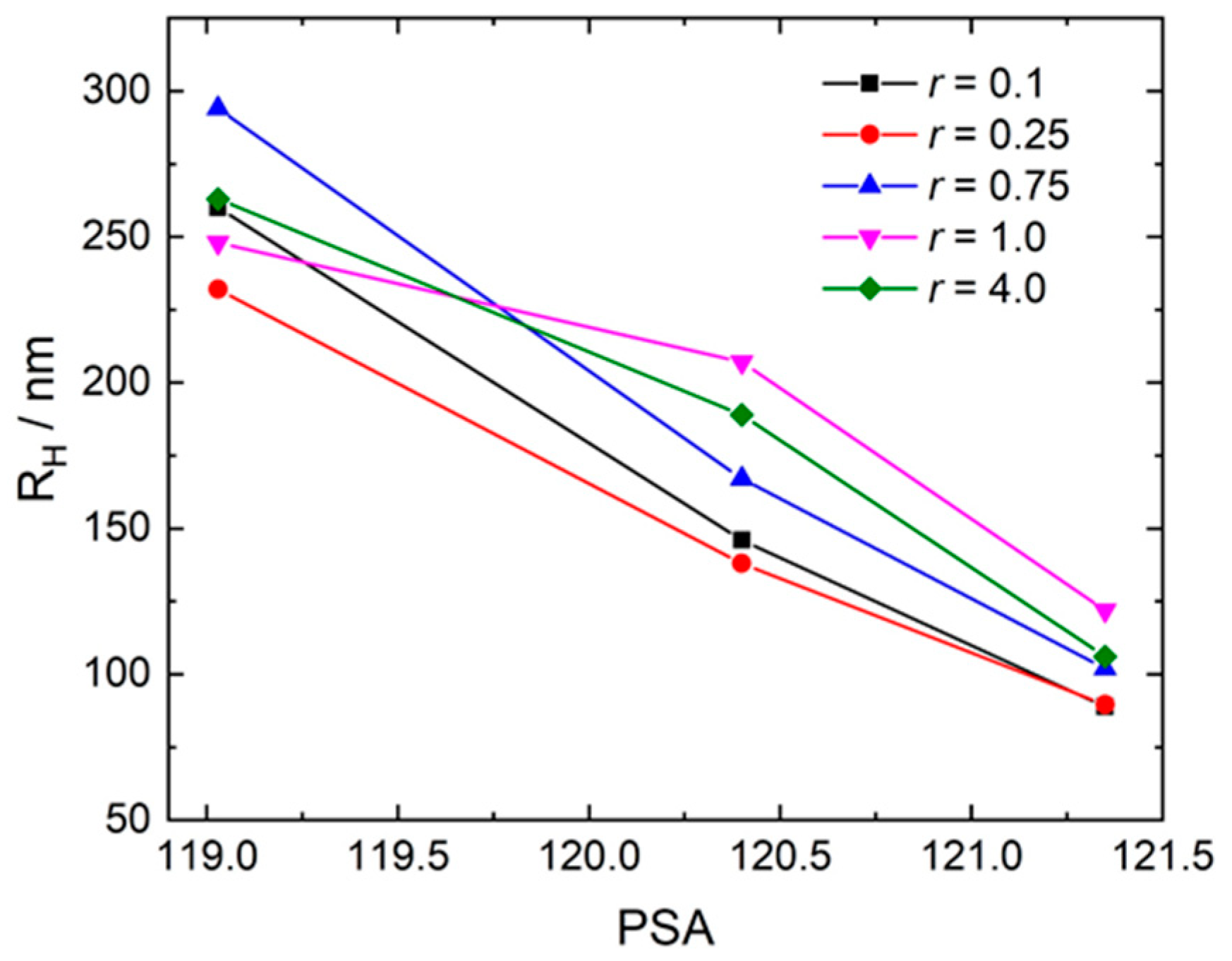
3.3. Polar Surface Area of the Photoacid Molecules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendes, A.C.; Baran, E.T.; Reis, R.L.; Azevedo, H.S. Self-Assembly in Nature: Using the Principles of Nature to Create Complex Nanobiomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 582–612. [Google Scholar] [CrossRef]
- Gröger, G.; Meyer-Zaika, W.; Böttcher, C.; Gröhn, F.; Ruthard, C.; Schmuck, C. Switchable Supramolecular Polymers from the Self-Assembly of a Small Monomer with Two Orthogonal Binding Interactions. J. Am. Chem. Soc. 2011, 133, 8961–8971. [Google Scholar] [CrossRef]
- Akcora, P.; Liu, H.; Kumar, S.K.; Moll, J.; Li, Y.; Benicewicz, B.C.; Schadler, L.S.; Acehan, D.; Panagiotopoulos, A.Z.; Pryamitsyn, V.; et al. Anisotropic self-assembly of spherical polymer-grafted nanoparticles. Nat. Mater. 2009, 8, 354. [Google Scholar] [CrossRef]
- Reuther, F.J.; Siriwardane, D.A.; Campos, R.; Novak, B.M. Solvent Tunable Self-Assembly of Amphiphilic Rod–Coil Block Copolymers with Chiral, Helical Polycarbodiimide Segments: Polymeric Nanostructures with Variable Shapes and Sizes. Macromolecules 2015, 48, 6890–6899. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, J.; Zhang, F.; Elsabahy, M.; Felder, S.E.; Zhu, J.; Pochan, D.J.; Wooley, K.L. Rapid and Versatile Construction of Diverse and Functional Nanostructures Derived from a Polyphosphoester-Based Biomimetic Block Copolymer System. J. Am. Chem. Soc. 2012, 134, 18467–18474. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.S.; Behzad, A.R.; Hooghan, B.; Sougrat, R.; Karunakaran, M.; Pradeep, N.; Vainio, U.; Peinemann, K.V. Switchable pH-Responsive Polymeric Membranes Prepared via Block Copolymer Micelle Assembly. ACS Nano 2011, 5, 3516–3522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, L.; Wang, B.; He, J.; Li, Y.; Deng, Z.; Mao, C. Universal pH-Responsive and Metal-Ion-Free Self-Assembly of DNA Nanostructures. Angew. Chem. Int. Ed. 2018, 130, 7008–7011. [Google Scholar] [CrossRef]
- Martin-Fabiani, I.; Fortini, A.; Lesage de la Haye, J.; Koh, M.L.; Taylor, S.E.; Bourgeat-Lami, E.; Lansalot, M.; D’Agosto, F.; Sear, R.P.; Keddie, J.L. pH-Switchable Stratification of Colloidal Coatings: Surfaces “On Demand”. ACS Appl. Mater. Interfaces 2016, 8, 34755–34761. [Google Scholar] [CrossRef] [PubMed]
- Yuwei, H.; Cecconello, A.; Idili, A.; Ricci, F.; Willner, I. Triplex DNA Nanostructures: From Basic Properties to Applications. Angew. Chem. Int. Ed. 2017, 56, 15210–15233. [Google Scholar]
- Yao, X.; Li, T.; Wang, J.; Ma, X.; Tian, H. Recent Progress in Photoswitchable Supramolecular Self-Assembling Systems. Adv. Opt. Mater. 2016, 4, 1322–1349. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liu, G.; Tian, J.; Wang, H.; Gong, M.; Liu, S. Reversibly Switching Bilayer Permeability and Release Modules of Photochromic Polymersomes Stabilized by Cooperative Noncovalent Interactions. J. Am. Chem. Soc. 2015, 137, 15262–15275. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, C.; Lee, S.; Lee, D.; Chung, H.M.; Kim, G.; Heo, S.H.; Kim, C.; Hong, K.S.; Yoon, J. Nanostructured Phthalocyanine Assemblies with Protein-Driven Switchable Photoactivities for Biophotonic Imaging and Therapy. J. Am. Chem. Soc. 2016, 139, 10880–10886. [Google Scholar] [CrossRef] [PubMed]
- Yuming, C.; Dong, J.; Li, X. Light-Switchable Self-Assembly of Non-Photoresponsive Gold Nanoparticles. Langmuir 2018, 34, 6117–6124. [Google Scholar]
- Hejin, J.; Jiang, Y.; Han, J.; Zhang, L.; Liu, M. Helical nanostructures: Chirality transfer and a photodriven transformation from superhelix to nanokebab. Angew. Chem. Int. Ed. 2019, 58, 785–790. [Google Scholar]
- Simnick, J.A.; Valencia, C.A.; Liu, R.; Chilkoti, A. Morphing Low-Affinity Ligands into High-Avidity Nanoparticles by Thermally Triggered Self-Assembly of a Genetically Encoded Polymer. ACS Nano 2010, 4, 2217–2227. [Google Scholar] [CrossRef] [PubMed]
- Lewandowksi, W.; Fruhnert, M.; Mieczkowski, J.; Rockstuhl, C.; Górecka, E. Dynamically self-assembled silver nanoparticles as a thermally tunable metamaterial. Nat. Commun. 2015, 6, 1–9. [Google Scholar]
- Xie, F.; Qina, L.; Liu, M. A dual thermal and photo-switchable shrinking–swelling supramolecular peptide dendron gel. Chem. Commun. 2016, 52, 930–933. [Google Scholar] [CrossRef]
- Michailova, I.V.; Momekova, D.B.; Velichkova, H.A.; Ivanov, E.H.; Kotsilkova, R.K.; Karashanova, D.B.; Mileva, E.D.; Dimitrov, I.V.; Rangelov, S.M. Self-Assembly of a Thermally Responsive Double-Hydrophilic Copolymer in Ethanol–Water Mixtures: The Effect of Preferential Adsorption and Co-Nonsolvency. J. Phys. Chem. B 2018, 122, 6072–6078. [Google Scholar] [CrossRef]
- Snezhko, A.; Aranson, S.I. Magnetic manipulation of self-assembled colloidal asters. Nat. Mater. 2011, 10, 698–703. [Google Scholar] [CrossRef]
- Zhang, M.; Magagnosc, D.J.; Liberal, I.; Yu, Y.; Yun, H.; Yang, H.; Wu, Y.; Guo, J.; Chen, W.; Shin, Y.J.; et al. High-strength magnetically switchable plasmonic nanorods assembled from a binary nanocrystal mixture. Nat. Nanotechnol. 2017, 12, 228–232. [Google Scholar]
- Kumar, S. Microtubule assembly Switched on with magnets. Nat. Nanotechnol. 2013, 8, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Shi, W.; Huang, J.; Yan, Y. Adaptive soft molecular self-assemblies. Soft Matter 2016, 12, 337–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kaifer, A.E. Electrochemical switching and size selection in cucurbit[8]uril-mediated dendrimer self-assembly. Angew. Chem. Int. Ed. 2006, 45, 7042–7046. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Chang, J.; Leem, S.; Park, J.S.; Thordarson, P.; Sessler, J.L. Redox- and pH-Responsive Orthogonal Supramolecular Self-Assembly: An Ensemble Displaying Molecular Switching Characteristics. J. Am. Chem. Soc. 2016, 137, 16038–16042. [Google Scholar] [CrossRef]
- Chong, D.; Tan, J.; Zhang, J.; Zhou, Y.; Wan, X.; Zhang, J. Dual electrical switching permeability of vesicles via redox-responsive self-assembly of amphiphilic block copolymers and polyoxometalates. Chem. Commun. 2018, 54, 7838–7841. [Google Scholar] [CrossRef]
- Wani, M.O.; Zeng, H.; Priimagi, A. A light-driven artificial flytraNat. Communications 2017, 8, 1–7. [Google Scholar]
- Pengcheng, Y.; Jianga, D.; Tiana, Y.; Xua, L.; Qian, J.; Lia, H.; Xia, J.; Lia, H. A sensitive signal-on photoelectrochemical sensor for tetracycline determination using visible-light-driven flower-like CN/BiOBr composites. Biosens. Bioelectron. 2018, 111, 74–81. [Google Scholar]
- Ningyan, C.; Liu, Q.; Xing, W.; Sun, X. Cobalt Phosphide Nanowires: Efficient Nanostructures for Fluorescence Sensing of Biomolecules and Photocatalytic Evolution of Dihydrogen from Water under Visible Light. Angew. Chem. Int. Ed. 2015, 54, 5493–5497. [Google Scholar]
- Liu, K.; Xing, R.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Simple Peptide-Tuned Self-Assembly of Photosensitizers towards Anticancer Photodynamic Therapy. Angew. Chem. Int. Ed. 2016, 55, 3036–3039. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S.; et al. A Light-Driven Therapy of Pancreatic Adenocarcinoma Using Gold Nanorods-Based Nanocarriers for Co-Delivery of Doxorubicin and siRNA. Theranostics 2015, 5, 813–833. [Google Scholar]
- Xiaju, C.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-Triggered Assembly of Gold Nanoparticles for Photothermal Therapy and Photoacoustic Imaging of Tumors in Vivo. Adv. Mat. 2017, 29, 1604894. [Google Scholar]
- Blasco, E.; Piñol, M.; Oriol, L.; Schmidt, B.V.K.J.; Welle, A.; Trouillet, V.; Bruns, M.; Barner-Kowollik, C. Photochemical Generation of Light Responsive Surfaces. Adv. Funct. Mater. 2013, 23, 4011–4019. [Google Scholar] [CrossRef]
- Kamiya, Y.; Asanuma, H. Light-driven DNA nanomachine with a photoresponsive molecular engine. Acc. Chem. Res. 2014, 47, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.K.; Das, D.; Ahrensa, J.; Klajn, R. Controlling the lifetimes of dynamic nanoparticle aggregates by spiropyran functionalization. Nanoscale 2016, 8, 19280–19286. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, F.; Cao, Z.; Wang, G.; Dang, Z.M. A photo, temperature, and pH responsive spiropyran-functionalized polymer: Synthesis, self-assembly and controlled release. Polymer 2016, 83, 85–91. [Google Scholar]
- Agmon, N.; Rettig, W.; Groth, C. Electronic Determinants of Photoacidity in Cyanonaphthols. J. Am. Chem. Soc. 2002, 124, 1089–1096. [Google Scholar] [CrossRef]
- Cardenas-Daw, C.; Gröhn, F. Photo-Induced Assembly of Nanostructures Triggered by Short-Lived Proton Transfers in the Excited-State. J. Am. Chem. Soc. 2015, 137, 8660–8663. [Google Scholar] [CrossRef]
- Krieger, A.; Werner, J.P.F.; Mariani, G.; Gröhn, F. Functional Supramolecular Porphyrin–Dendrimer Assemblies for Light Harvesting and Photocatalysis. Macromolecules 2017, 50, 3464–3475. [Google Scholar] [CrossRef]
- Düring, J.; Hölzer, A.; Kolb, U.; Branscheid, R.; Gröhn, F. Supramolecular Organic–Inorganic Hybrid Assemblies with Tunable Particle Size: Interplay of Three Noncovalent Interactions. Angew. Chem. Int. Ed. 2015, 52, 8742–8745. [Google Scholar] [CrossRef]
- Reinhold, F.; Kolb, U.; Lieberwirth, I.; Gröhn, F. Assemblies of Double Hydrophilic Block Copolymers and Oppositely Charged Dendrimers. Langmuir 2009, 25, 1345–1351. [Google Scholar] [CrossRef]
- Willerich, I.; Gröhn, F. Molecular Structure Encodes Nanoscale Assemblies: Understanding Driving Forces in Electrostatic Self-Assembly. J. Am. Chem. Soc. 2011, 133, 20341–20356. [Google Scholar] [CrossRef] [PubMed]
- Mariani, G.; Moldenhauer, D.; Schweins, R.; Gröhn, F. Elucidating Electrostatic Self-Assembly: Molecular Parameters as Key to Thermodynamics and Nanoparticle Shape. J. Am. Chem. Soc. 2016, 138, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Willerich, I.; Gröhn, F. Structure and Thermodynamics of Ionic Dendrimer−Dye Assemblies. J. Phys. Chem. B 2009, 113, 3339–3354. [Google Scholar] [CrossRef]
- Mariani, G.; Schweins, R.; Gröhn, F. Electrostatic Self-Assembly of Dendrimer Macroions and Multivalent Dye Counterions: The Role of Solution Ionic Strength. Macromolecules 2016, 49, 8661–8671. [Google Scholar] [CrossRef]
- Düring, J.; Gröhn, F. Filamentous supramolecular structures with polyelectrolyte and cadmium sulfide. Soft Matter 2016, 12, 1868–1875. [Google Scholar] [CrossRef]
- Mariani, G.; Krieger, A.; Moldenhauer, D.; Schweins, R.; Gröhn, F. Light-Responsive Shape: From Micrometer-Long Nanocylinders to Compact Particles in Electrostatic Self-Assembly. Macromol. Rapid Commun. 2018, 39, 17800860. [Google Scholar] [CrossRef]
- Moldenhauer, D.; Gröhn, F. Water-Soluble Spiropyrans with Inverse Photochromism and Their Photoresponsive Electrostatic Self-Assembly. Chem. Eur. J. 2017, 23, 3966–3978. [Google Scholar] [CrossRef]
- Weller, A. Quantitative Untersuchungen der Fluoreszenz- umwandlung bei Naphtholen. Z. Elektrochem. Ber. Bunsenges. Physik. Chem. 1952, 56, 662. [Google Scholar]
- Förster, T. Elektrolytische Dissoziation angeregter Moleküle. Z. Elektrochem. Ber. Bunsenges. Physik. Chem. 1950, 54, 42–46. [Google Scholar]
- Prémont-Schwarz, M.; Barak, T.; Nibbering, E.T.; Pines, E. Ultrafast Excited-State Proton-Transfer Reaction of 1-Naphthol-3,6-Disulfonate and Several 5-Substituted 1-Naphthol Derivatives. J. Phys. Chem. B 2013, 117, 4594–4603. [Google Scholar] [CrossRef]
- Maiti, P.K.; Cagin, T.; Lin, S.T.; Goddard, W.A. Effect of Solvent and pH on the Structure of PAMAM Dendrimers. Macromolecules 2005, 38, 979–991. [Google Scholar] [CrossRef]
- Dictionary of Organic Compounds, 5th ed.; Chapman and Hall: New York, NY, USA, 1982.
- Schulmann, S.G.; Rosenberg, L.S.; Vincent, W.R., Jr. Proton exchange in the lowest excited singlet state of 2-naphthol-6,8-disulfonate. Demonstration of the establishment of prototropic equilibrium in the excited state. J. Am. Chem. Soc. 1979, 101, 139–142. [Google Scholar] [CrossRef]
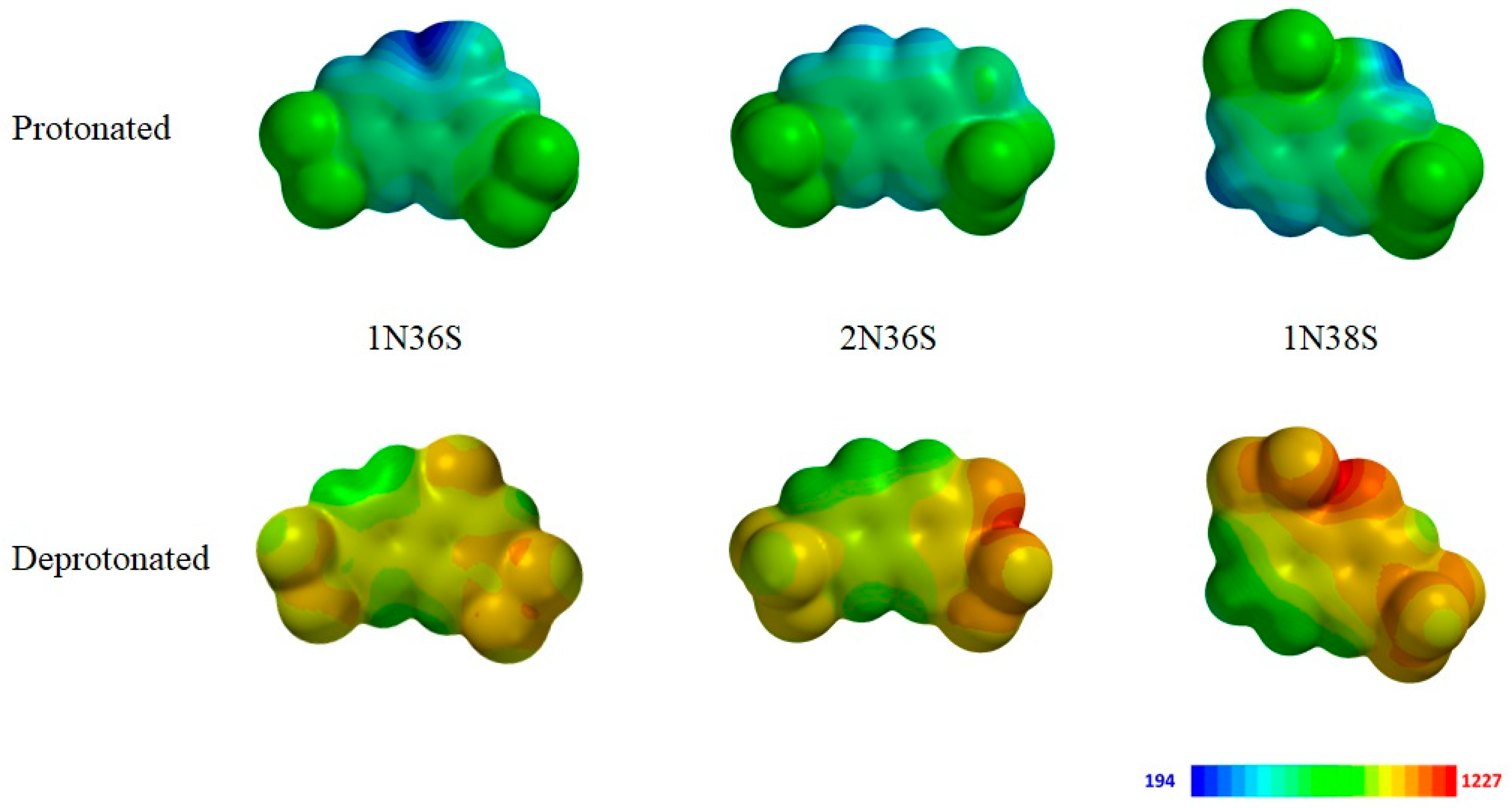
| Photoacid | Charge | PSA [Å2] |
|---|---|---|
| 1N36S | Protonated | 121.4 |
| Deprotonated | 123.6 | |
| 2N36S | Protonated | 119.0 |
| Deprotonated | 121.7 | |
| 1N38S | Protonated | 120.4 |
| Deprotonated | 120.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zika, A.; Bernhardt, S.; Gröhn, F. Photoresponsive Photoacid-Macroion Nano-Assemblies. Polymers 2020, 12, 1746. https://doi.org/10.3390/polym12081746
Zika A, Bernhardt S, Gröhn F. Photoresponsive Photoacid-Macroion Nano-Assemblies. Polymers. 2020; 12(8):1746. https://doi.org/10.3390/polym12081746
Chicago/Turabian StyleZika, Alexander, Sarah Bernhardt, and Franziska Gröhn. 2020. "Photoresponsive Photoacid-Macroion Nano-Assemblies" Polymers 12, no. 8: 1746. https://doi.org/10.3390/polym12081746
APA StyleZika, A., Bernhardt, S., & Gröhn, F. (2020). Photoresponsive Photoacid-Macroion Nano-Assemblies. Polymers, 12(8), 1746. https://doi.org/10.3390/polym12081746






