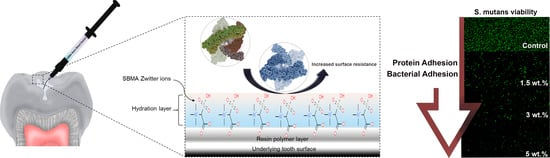
Improvement in the Microbial Resistance of Resin-Based Dental Sealant by Sulfobetaine Methacrylate Incorporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Incorporation of SB into Sealant
2.2. Mechanical Properties
2.3. Wettability
2.4. Depth of Cure
2.5. Protein Adsorption
2.6. Colony-Forming Units
2.7. Bacterial Viability
2.8. Cell Cytotoxicity
2.9. Statistical Analysis
3. Results and Discussion
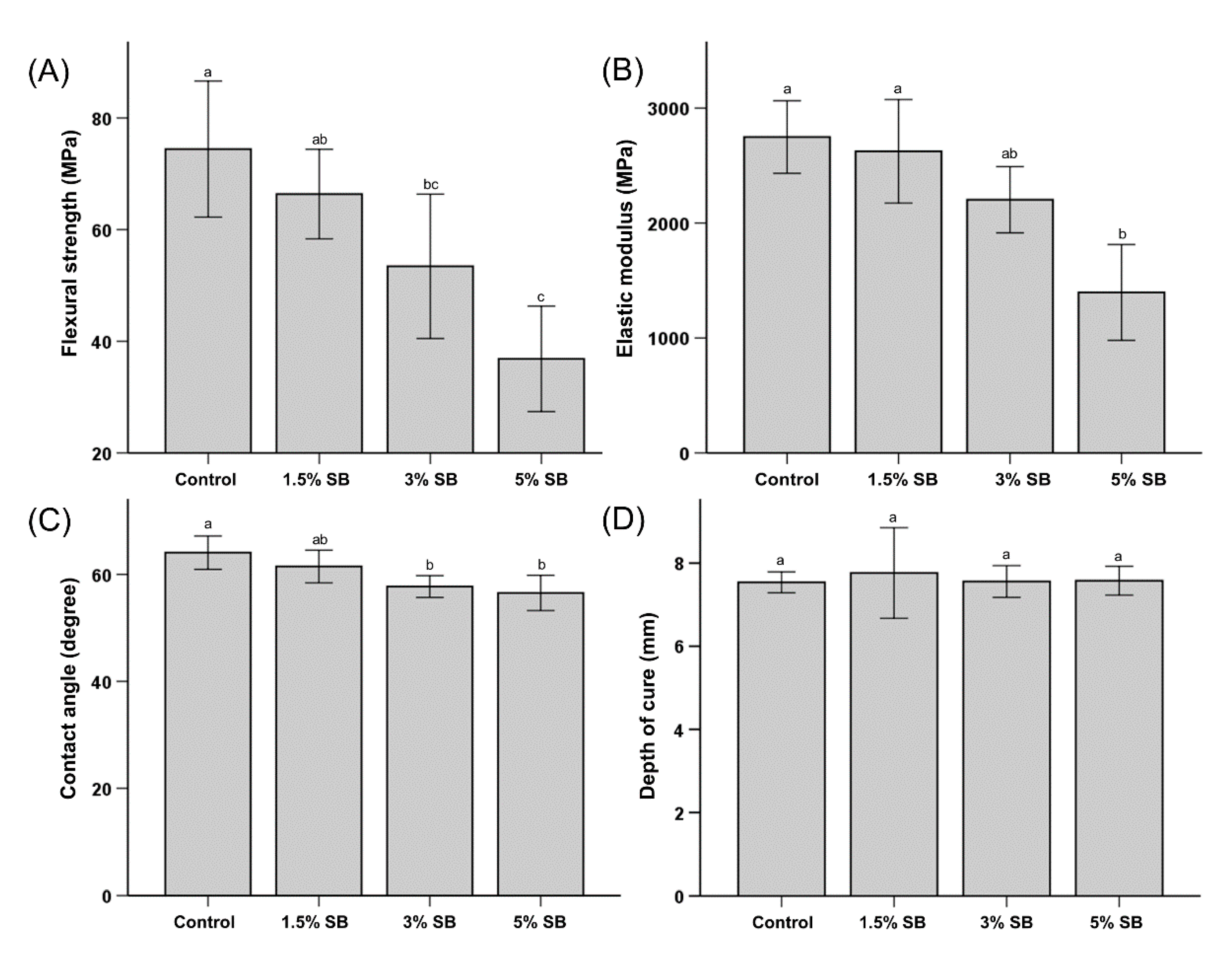
3.1. Mechanical Properties
3.2. Wettability
3.3. Depth of Cure
3.4. Protein Adsorption
3.5. Bacterial Attachment
3.6. Cell Cytotoxicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petersen, P.E. Sociobehavioural risk factors in dental caries-international perspectives. Community Dent. Oral Epidemiol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Ahovuo-Saloranta, A.; Forss, H.; Walsh, T.; Nordblad, A.; Mäkelä, M.; Worthington, V.H. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rugg-Gunn, A.J.; Welbury, R.R.; Toumba, J. British society of paediatric dentistry: A policy document on the use of amalgam in paediatric dentistry. Int. J. Paediatr. Dent. 2001, 11, 233–238. [Google Scholar] [PubMed]
- Ekstrand, K.; Martignon, S.; Bakhshandeh, A.; Ricketts, D.N.J. The non-operative resin treatment of proximal caries lesions. Dent. Update 2012, 39, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Kritika, S.; Jothimani, B.; Vidhya, S.; Sanjeev, K.; Mahalaxmi, S.; Venkatachalapathy, B.; Sureshkumar, S. Incorporation of hydrophobic nanochitosan improves wear resistance of dental sealants. Int. J. Polym. Mater. Polym. Biomater. 2020. [Google Scholar] [CrossRef]
- Naaman, R.; El-Housseiny, A.; Alamoudi, N. The use of pit and fissure sealants—A literature review. Dent. J. 2017, 5, 34. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Kwon, J.S.; Lee, M.J.; Kim, J.Y.; Kim, D.; Ryu, J.H.; Jang, S.; Kim, K.M.; Hwang, C.J.; Choi, S.H. Novel anti-biofouling bioactive calcium silicate-based cement containing 2-methacryloyloxyethyl phosphorylcholine. PLoS ONE 2019. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Kim, D.; Lee, M.J.; Kim, J.Y.; Lee, D.; Kwon, J.S.; Choi, S.H. Incorporation of zwitterionic materials into light-curable fluoride varnish for biofilm inhibition and caries prevention. Sci. Rep. 2019, 9, 19550. [Google Scholar] [CrossRef]
- Lee, M.-J.; Kwon, J.-S.; Kim, J.-Y.; Ryu, J.-H.; Seo, J.-Y.; Jang, S.; Kim, K.-M.; Hwang, C.-J.; Choi, S.-H. Bioactive resin-based composite with surface pre-reacted glass-ionomer filler and zwitterionic material to prevent the formation of multi-species biofilm. Dent. Mater. 2019, 35, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-N.; Venault, A.; Cho, C.-H.; Sin, M.-C.; Yeh, L.-C.; Jhong, J.-F.; Chinnathambi, A.; Chang, Y.; Chang, Y. Epoxylated zwitterionic triblock copolymers grafted onto metallic surfaces for general biofouling mitigation. Langmuir 2017, 33, 9822–9835. [Google Scholar] [CrossRef]
- Iqbal, Z.; Kim, S.; Moyer, J.; Moses, W.; Abada, E.; Wright, N.; Kim, E.J.; Park, J.; Fissell, W.H.; Vartanian, S.; et al. In vitro and in vivo hemocompatibility assessment of ultrathin sulfobetaine polymer coatings for silicon-based implants. J. Biomater. Appl. 2019, 34, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Venault, A.; Yang, H.S.; Chiang, Y.C.; Lee, B.S.; Ruaan, R.C.; Chang, Y. Bacterial resistance control on mineral surfaces of hydroxyapatite and human teeth via surface charge-driven antifouling coatings. Acs Appl. Mater. Interfaces 2014, 6, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wu, J.; Zhang, Q.; Baras, B.; Bhadila, G.; Li, Y.; Melo, M.A.S.; Weir, M.D.; Bai, Y.; Zhang, N.; et al. Novel protein-repellent and antibacterial resins and cements to inhibit lesions and protect teeth. Int. J. Polym. Sci. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, N.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Novel multifunctional dental cement to prevent enamel demineralization near orthodontic brackets. J. Dent. 2017, 64, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, M.J.; Oh, S.H.; Kwon, J.S. Novel dental poly(methyl methacrylate) containing phytoncide for antifungal effect and inhibition of oral multispecies biofilm. Materials 2020, 13, 371. [Google Scholar] [CrossRef]
- Kuo, W.H.; Wang, M.J.; Chien, H.W.; Wei, T.C.; Lee, C.; Tsai, W.B. Surface modification with poly(sulfobetaine methacrylate-co-acrylic acid) to reduce fibrinogen adsorption, platelet adhesion, and plasma coagulation. Biomacromolecules 2011, 12, 4348–4356. [Google Scholar] [CrossRef]
- Compagnoni, M.A.; Pero, A.C.; Ramos, S.M.M.; Marra, J.; Paleari, A.G.; Rodriguez, L.S. Antimicrobial activity and surface properties of an acrylic resin containing a biocide polymer. Gerodontology 2014, 31, 220–226. [Google Scholar] [CrossRef]
- Gong, S.q.; Epasinghe, J.; Rueggeberg, F.A.; Niu, L.n.; Mettenberg, D.; Yiu, C.K.Y.; Blizzard, J.D.; Wu, C.D.; Mao, J.; Drisko, C.L.; et al. An ormosil-containing orthodontic acrylic resin with concomitant improvements in antimicrobial and fracture toughness properties. PLoS ONE 2012, 7, e42355. [Google Scholar] [CrossRef]
- Imazato, S.; Chen, J.-h.; Ma, S.; Izutani, N.; Li, F. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn. Dent. Sci. Rev. 2012, 48, 115–125. [Google Scholar] [CrossRef]
- Vidal, M.L.; Rego, G.F.; Viana, G.M.; Cabral, L.M.; Souza, J.P.B.; Silikas, N.; Schneider, L.F.; Cavalcante, L.M. Physical and chemical properties of model composites containing quaternary ammonium methacrylates. Dent. Mater. 2018, 34, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.d.P.L.; Viana, G.M.; Cabral, L.M.; Portela, M.B.; Hirata Junior, R.; Cavalcante, L.M.; Lourenço, E.J.V.; Telles, D.d.M. Self-cured resin modified by quaternary ammonium methacrylates and chlorhexidine: Cytotoxicity, antimicrobial, physical, and mechanical properties. Dent. Mater. 2020, 36, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Terayama, Y.; Yamaguchi, H.; Terada, M.; Murakami, D.; Ishihara, K.; Takahara, A. Wettability and antifouling behavior on the surfaces of superhydrophilic polymer brushes. Langmuir 2012, 28, 7212–7222. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.B. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000. [Google Scholar] [CrossRef] [PubMed]
- Arya Rajendran, B.S. Shafer’s Textbook of Oral Pathology.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Amdjadi, P.; Nojehdehian, H.; Najafi, F.; Ghasemi, A.; Seifi, M.; Dashtimoghadam, E.; Fahimipour, F.; Tayebi, L. Ultraviolet-induced surface grafting of octafluoropentyl methacrylate on polyether ether ketone for inducing antibiofilm properties. J. Biomater. Appl. 2017, 32, 3–11. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, S.; Zhang, Z.; Jiang, S. Highly protein-resistant coatings from well-defined diblock copolymers containing sulfobetaines. Langmuir 2006, 22, 2222–2226. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Chang, Y.; Higuchi, A.; Chen, W.Y.; Ruaan, R.C. Sulfobetaine-grafted poly(vinylidene fluoride) ultrafiltration membranes exhibit excellent antifouling property. J. Membr. Sci. 2009, 339, 151–159. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, J.; Kim, J. Streptococcus mutans extracellular DNA levels depend on the number of bacteria in a biofilm. Sci. Rep. 2018, 8, 1–6. [Google Scholar]
- Yu, F.; Dong, Y.; Yu, H.-h.; Lin, P.-t.; Zhang, L.; Sun, X.; Liu, Y.; Xia, Y.-n.; Huang, L.; Chen, J.-h. Antibacterial activity and bonding ability of an orthodontic adhesive containing the antibacterial monomer 2-methacryloxylethyl hexadecyl methyl ammonium bromide. Sci. Rep. 2017, 7, 41787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Melo, M.A.S.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Bai, Y.; Xu, H.H.K. Novel dental cement to combat biofilms and reduce acids for orthodontic applications to avoid enamel demineralization. Materials 2016, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Regis, R.R.; Vecchia, D.M.P.; Pizzolitto, A.C.; Compagnoni, M.A.; Souza, P.P.C.; de Souza, R.F. Antimicrobial properties and cytotoxicity of an antimicrobial monomer for application in prosthodontics. J. Prosthodont. 2012. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Kim, J.Y.; Choi, W.; Lee, M.J.; Seo, J.Y.; Yu, J.; Kwon, J.S.; Choi, S.H. Incorporation of carboxybetaine methacrylate into poly(Methyl methacrylate) to prevent multi-species biofilm formation. J. Ind. Eng. Chem. 2020, 86, 194–204. [Google Scholar] [CrossRef]



| Groups | Group Code | Sealant (wt%) | SB (wt%) |
|---|---|---|---|
| 1 | Control | 100 | 0 |
| 2 | 1.5% SB | 98.5 | 1.5 |
| 3 | 3% SB | 97 | 3 |
| 4 | 5% SB | 95 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-J.; Mangal, U.; Kim, S.-J.; Yoon, Y.-P.; Ahn, E.-S.; Jang, E.-S.; Kwon, J.-S.; Choi, S.-H. Improvement in the Microbial Resistance of Resin-Based Dental Sealant by Sulfobetaine Methacrylate Incorporation. Polymers 2020, 12, 1716. https://doi.org/10.3390/polym12081716
Lee M-J, Mangal U, Kim S-J, Yoon Y-P, Ahn E-S, Jang E-S, Kwon J-S, Choi S-H. Improvement in the Microbial Resistance of Resin-Based Dental Sealant by Sulfobetaine Methacrylate Incorporation. Polymers. 2020; 12(8):1716. https://doi.org/10.3390/polym12081716
Chicago/Turabian StyleLee, Myung-Jin, Utkarsh Mangal, Se-Jin Kim, Yeo-Phil Yoon, Eun-So Ahn, Ee-Seul Jang, Jae-Sung Kwon, and Sung-Hwan Choi. 2020. "Improvement in the Microbial Resistance of Resin-Based Dental Sealant by Sulfobetaine Methacrylate Incorporation" Polymers 12, no. 8: 1716. https://doi.org/10.3390/polym12081716
APA StyleLee, M.-J., Mangal, U., Kim, S.-J., Yoon, Y.-P., Ahn, E.-S., Jang, E.-S., Kwon, J.-S., & Choi, S.-H. (2020). Improvement in the Microbial Resistance of Resin-Based Dental Sealant by Sulfobetaine Methacrylate Incorporation. Polymers, 12(8), 1716. https://doi.org/10.3390/polym12081716