Evaluation of the Physicochemical and Antibacterial Properties of Experimental Adhesives Doped with Lithium Niobate
Abstract
1. Introduction
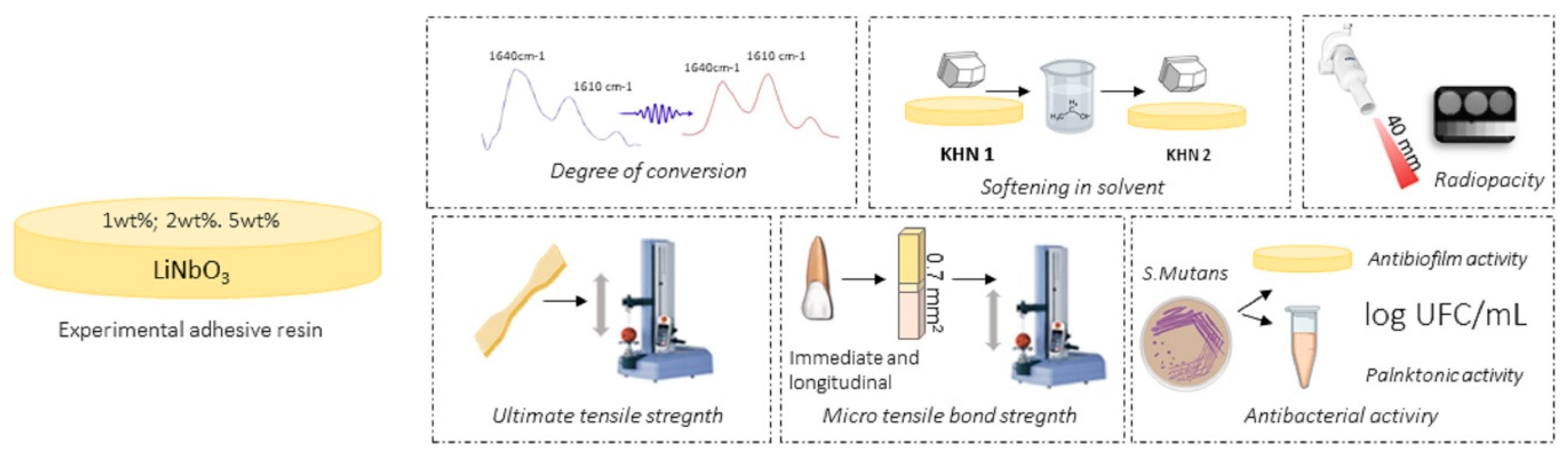
2. Materials and Methods
2.1. Preparation of Experimental Adhesives
2.2. Degree of Conversion (DC)
2.3. Radiopacity
2.4. Softening in Solvent (ΔKHN%)
2.5. Ultimate Tensile Strength
2.6. Microtensile Bond Strength to Dentin (μ-TBS)
2.7. Evaluation of Antibacterial Activity against Biofilm Formation and Planktonic Bacteria
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leitune, V.C.B.; Collares, F.M.; Takimi, A.; de Lima, G.B.; Petzhold, C.L.; Bergmann, C.P.; Samuel, S.M. Niobium pentoxide as a novel filler for dental adhesive resin. J. Dent. 2013, 41, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Leitune, V.C.; Kist, T.L.; Takimi, A.; Samuel, S.M.; Collares, F.M. Quantum dots as nonagglomerated nanofillers for adhesive resins. J. Dent. Res. 2016, 95, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Esterase from a cariogenic bacterium hydrolyzes dental resins. Acta Biomater. 2018, 71, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, e41–e53. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjaderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Models of caries formation around dental composite restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef]
- Reis, A.; Carrilho, M.; Breschi, L.; Loguercio, A.D. Overview of clinical alternatives to minimize the degradation of the resin-dentin bonds. Oper. Dent. 2013, 38, 1–25. [Google Scholar] [CrossRef]
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.D.; Grabow, J.; Müller, F.A. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010, 6, 4539–4546. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Garcia, I.M.; Kensara, A.; Balhaddad, A.; Collares, F.M.; Williams, M.A.; Ibrahim, A.S.; Lin, N.J.; Weir, M.D.; Xu, H.H.K.; et al. How we are assessing the developing antibacterial resin-based dental materials? A scoping review. J. Dent. 2020, 103369. [Google Scholar] [CrossRef]
- Hafshejani, T.M.; Zamanian, A.; Venugopal, J.R.; Rezvani, Z.; Sefat, F.; Saeb, M.R.; Vahabi, H.; Zarrintaj, P.; Mozafari, M. Antibacterial glass-ionomer cement restorative materials: A critical review on the current status of extended release formulations. J. Control Release 2017, 262, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent. Mater. 2010, 26, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Leitune, V.C.; Collares, F.M.; Trommer, R.M.; Andrioli, D.G.; Bergmann, C.P.; Samuel, S.M. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013, 41, 321–327. [Google Scholar] [CrossRef]
- Schulz, H.; Schimmoeller, B.; Pratsinis, S.E.; Salz, U.; Bock, T. Radiopaque dental adhesives: Dispersion of flame-made Ta2O5/SiO2 nanoparticles in methacrylic matrices. J. Dent. 2008, 36, 579–587. [Google Scholar] [CrossRef]
- Provenzi, C.; Collares, F.M.; Cuppini, M.; Samuel, S.M.W.; Alvez, A.K.; Bergmann, C.P.; Leitune, V.C.B. Effect of nanostructured zirconium dioxide incorporation in an experimental adhesive resin. Clin. Oral. Investig. 2018, 22, 2209–2218. [Google Scholar] [CrossRef]
- Falanga, A.; Laheurte, P.; Vahabi, H.; Tran, N.; Khamseh, S.; Saeidi, H.; Khodadadi, M.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Niobium-Treated Titanium Implants with Improved Cellular and Molecular Activities at the Tissue–Implant Interface. Materials 2019, 12, 3861. [Google Scholar] [CrossRef]
- Karlinse, R.L.; Yi, K. Self-assembly and bioactive response of a crystalline metal oxide in a simulated blood fluid. J. Mater. Sci. Mater. Med. 2008, 19, 1349–1354. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Breme, J. Biomimetic implant coatings. Biomol. Eng. 2007, 24, 27–32. [Google Scholar] [CrossRef]
- Xu, Y. Lithium Niobate and Lithium Tantalate. In Ferroelectric Materials and Their Applications; Xu, Y., Ed.; Elsevier Science Publishers: Amsterdan, The Netherlands, 1991; pp. 217–236. [Google Scholar]
- Gutmann, E.; Benke, A.; Gerth, K.; Böttcher, H.; Mehner, E.; Klein, C.; Krause-Buchholz, U.; Bergmann, U.; Pompe, W.; Meyer, D.C. Pyroelectrocatalytic Disinfection Using the Pyroelectric Effect of Nano- and Microcrystalline LiNbO3 and LiTaO3 Particles. J. Phys. Chem. 2012, 116, 5383–5393. [Google Scholar] [CrossRef]
- Palmer, D.S.; Barco, M.T.; Billy, E.J. Temperature extremes produced orally by hot and cold liquids. J. Prosthet. Dent. 1992, 67, 325–327. [Google Scholar] [CrossRef]
- Collares, F.M.; Portella, F.F.; Leitune, V.C.; Samuel, S.M. Discrepancies in degree of conversion measurements by FTIR. Braz. Oral Res. 2013, 27, 453–454. [Google Scholar] [PubMed]
- Garcia, I.M.; Souza, V.S.; Hellriegel, C.; Scholten, J.D.; Collares, F.M. Ionic liquid-stabilized titania quantum dots applied in adhesive resin. J. Dent. Res. 2019, 98, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Kalachandra, S. Influence of fillers on the water sorption of composites. Dent. Mater. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Mehdawi, I.M.; Pratten, J.; Spratt, D.A.; Knowle, J.C.; Young, A.M. High strength re-mineralizing, antibacterial dental composites with reactive calcium phosphates. Dent. Mater. 2013, 29, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Kanehira, M.; FingeR, W.J.; Hoffmann, M.; Endo, T.; Komatsu, M. Relationship between degree of polymerization and enamel bonding strength with self-etching adhesives. J. Adhes. Dent. 2006, 4, 211–216. [Google Scholar]
- Shortall, A.C.; Palin, W.M.; Burtscher, P. Refractive index mismatch and monomer reactivity influence composite curing depth. J. Dent. Res. 2008, 87, 84–88. [Google Scholar] [CrossRef]
- Condon, J.R.; Ferracane, J.L. Assessing the effect of composite formulation on polymerization stress. J. Am. Dent. Assoc. 2000, 131, 497–503. [Google Scholar] [CrossRef]
- Gaglianone, L.A.; Lima, A.F.; Goncalves, L.S.; Cavalcanti, A.N.; Aguiar, F.H.; Marchi, G.M. Mechanical properties and degree of conversion of etch-and-rinse and self-etch adhesive systems cured by a quartz tungsten halogen lamp and a light-emitting diode. J. Mech. Behav. Biomed. Mater. 2012, 12, 139–143. [Google Scholar] [CrossRef]
- Belli, R.; Kreppel, S.; Petschelt, A.; Hornberger, H.; Boccaccini, A.R.; Lohbauer, U. Strengthening of dental adhesives via particle reinforcement. J. Mech. Behav. Biomed. Mater. 2014, 37, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, E.; Simon, D.A.; Schrekker, H.S.; Lavorgna, M.; Ambrosio, L.; Liberman, S.A.; Mauler, R.S. Ionic liquid tailored interfaces in halloysite nanotubes/heterophasic ethylene-propylene copolymer nanocomposites with enhanced mechanical properties. Eur. Polym. J. 2016, 82, 82–92. [Google Scholar] [CrossRef]
- Elshereksi, N.W.; Ghazali, M.; Muchtar, A.; Azhari, C.H. Review of titanate coupling agents and their application for dental composite fabrication. Dent. Mater. J. 2017, 36, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Collares, F.M.; Leitune, V.C.B.; Ogliari, F.A.; Piva, E.; Fontanella, V.R.C.; Samuel, S.M.W. Influence of the composition of an experimental adhesive on conversion kinetics, flexural strength and radiodensity. J. Dent. Sci. 2009, 24, 414–419. [Google Scholar]
- Oztas, B.; Kursun, S.; Dinc, G.; Kamburoglu, K. Radiopacity evaluation of composite restorative resins and bonding agents using digital and film x-ray systems. Eur. J. Dent. 2012, 6, 115–122. [Google Scholar] [CrossRef]
- Bae, J.H.; Cho, B.H.; Kim, J.S.; Kim, M.S.; Lee, I.B.; Son, H.H.; Um, C.K.; Kim, C.K.; Kim, O.Y. Adhesive layer properties as a determinant of dentin bond strength. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 822–828. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between bond-strength tests and clinical outcomes. Dent. Mater. 2010, 26, 100–112. [Google Scholar] [CrossRef]
- Toledano, M.; Yamauti, M.; Ruiz-Requena, M.E.; Osorio, R. A ZnO-doped adhesive reduced collagen degradation favouring dentine remineralization. J. Dent. 2012, 40, 756–765. [Google Scholar] [CrossRef]


| Groups | DC (%) | Radiopacity (Pixel Density) | UTS (MPa) |
|---|---|---|---|
| 0% LiNbO3 | 62.61 (±0.40) A | 29.45 (±2.56) B | 52.81 (±10.11) B |
| 1% LiNbO3 | 61.22 (±2.61) AB | 31.37 (±2.70) B | 58.90 (±4.14) AB |
| 2% LiNbO3 | 57.99 (±0.45) B | 32.27 (±4.92) B | 62.04 (±6.90) A |
| 5% LiNbO3 | 53.55 (±3.83) C | 38.40 (±3.68) A | 57.89 (±4.44) AB |
| Groups | Immediate µ-TBS 24 (MPa) | Longitudinal µ-TBS (MPa) |
|---|---|---|
| 0% LiNbO3 | 31.85 (±13.55) Ba | 30.80 (±11.45) Ba |
| 1% LiNbO3 | 38.42 (±9.22) ABa | 24.38 (±11.49) Bb |
| 2% LiNbO3 | 38.80 (±12.91) ABa | 28.11 (±9.48) Bb |
| 5% LiNbO3 | 45.03 (±8.58) Aa | 38.95 (±13.63) Aa |
| Groups | KHN1 | KHN2 | ΔKHN% |
|---|---|---|---|
| 0% LiNbO3 | 19.64 (±1.05) Aa | 11.68 (±1.82) b | 40.52 (±8.84) A |
| 1% LiNbO3 | 18.73 (±1.24) Aa | 13.68 (±0.84) b | 25.59 (±8.14) B |
| 2% LiNbO3 | 19.19 (±0.63) Aa | 10.34 (±1.35) b | 45.86 (±8.22) A |
| 5% LiNbO3 | 17.48 (±1.94) Aa | 8.4 (±2.36) b | 51.79 (±10.41) A |
| Groups | Biofilm | Planktonic |
|---|---|---|
| Log UFC/mL | Log UFC/mL | |
| 0% LiNbO3 | 4.74 (±0.78) A | 7.94 (±0.04) A |
| LiNb1% | 5.14 (±0.17) A | 8.18 (±0.04) A |
| LiNb2% | 5.54 (±0.43) A | 8.06 (±0.19) A |
| LiNb5% | 4.27 (±0.51) A | 8.18 (±0.02) A |
| Negative Control | - | 8.00 (±0.03) A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruzetta, L.; Garcia, I.M.; de Souza Balbinot, G.; Motta, A.S.; Collares, F.M.; Sauro, S.; C. B. Leitune, V. Evaluation of the Physicochemical and Antibacterial Properties of Experimental Adhesives Doped with Lithium Niobate. Polymers 2020, 12, 1330. https://doi.org/10.3390/polym12061330
Cruzetta L, Garcia IM, de Souza Balbinot G, Motta AS, Collares FM, Sauro S, C. B. Leitune V. Evaluation of the Physicochemical and Antibacterial Properties of Experimental Adhesives Doped with Lithium Niobate. Polymers. 2020; 12(6):1330. https://doi.org/10.3390/polym12061330
Chicago/Turabian StyleCruzetta, Laisa, Isadora M. Garcia, Gabriela de Souza Balbinot, Amanda S. Motta, Fabrício M. Collares, Salvatore Sauro, and Vicente C. B. Leitune. 2020. "Evaluation of the Physicochemical and Antibacterial Properties of Experimental Adhesives Doped with Lithium Niobate" Polymers 12, no. 6: 1330. https://doi.org/10.3390/polym12061330
APA StyleCruzetta, L., Garcia, I. M., de Souza Balbinot, G., Motta, A. S., Collares, F. M., Sauro, S., & C. B. Leitune, V. (2020). Evaluation of the Physicochemical and Antibacterial Properties of Experimental Adhesives Doped with Lithium Niobate. Polymers, 12(6), 1330. https://doi.org/10.3390/polym12061330






