Poly(3-aminobenzoic acid) Decorated with Cobalt Zeolitic Benzimidazolate Framework for Electrochemical Production of Clean Hydrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
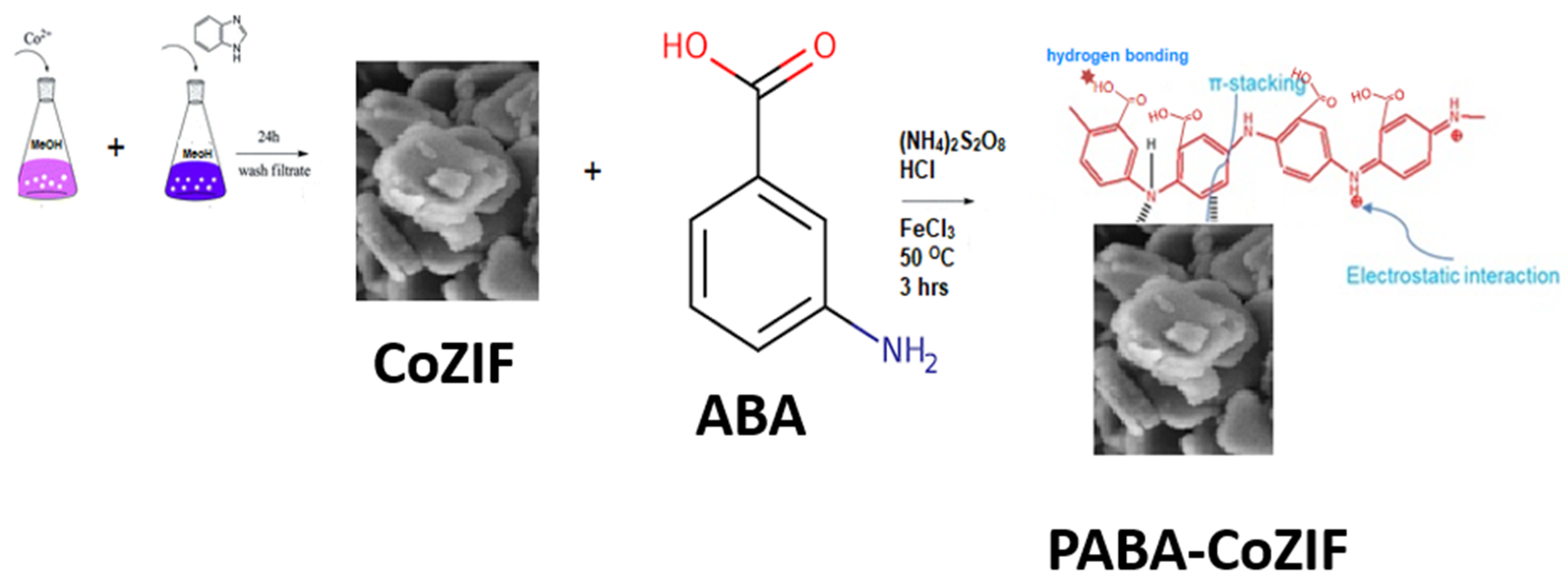
2.2. Synthesis of PABA, CoZIF and PABA/CoZIF Composite
2.2.1. Fabrication of CoZIF
2.2.2. Preparation of PABA/CoZIF
2.3. Characterization Methods
2.4. Hydrogen Studies
3. Results and Discussion
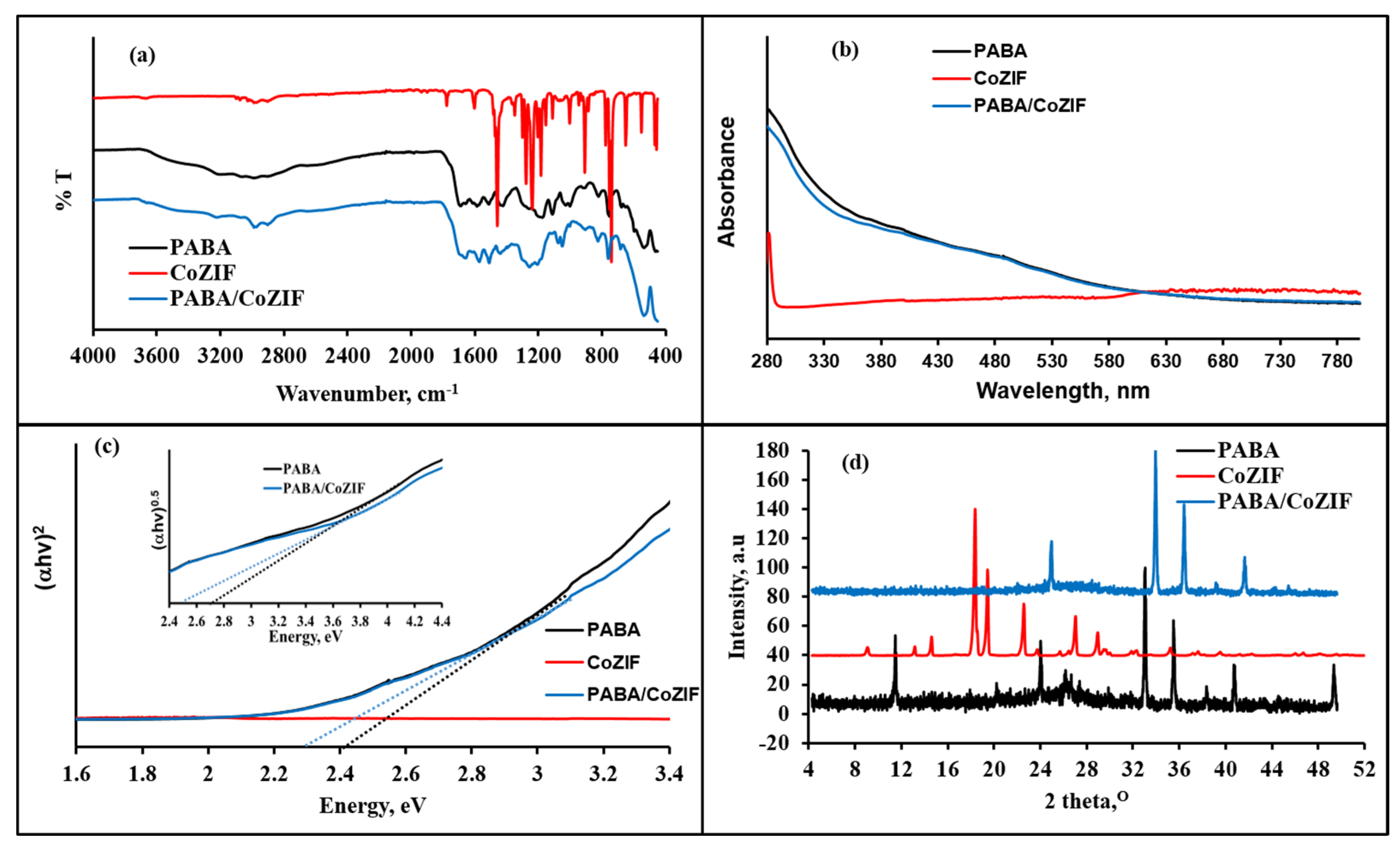
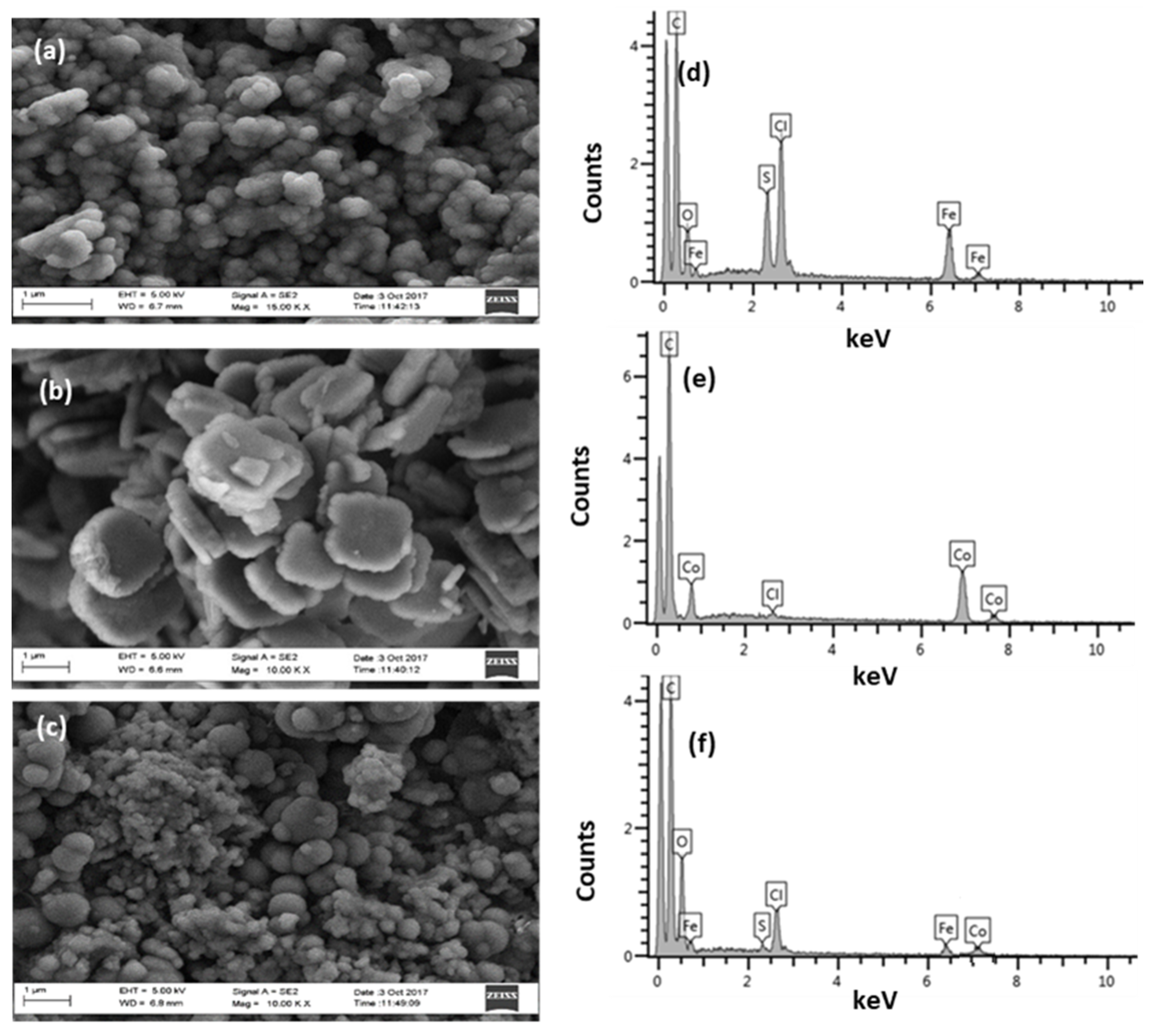
3.1. Characterization of Synthesized Materials
3.2. Electrochemical Studies
3.3. Hydrogen Evolution Reaction Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramohlola, K.E.; Hato, M.J.; Monama, G.R.; Makhado, E.; Iwuoha, E.I.; Modibane, K.D. State-of-the-Art Advances and Perspectives for Electrocatalysis. In Methods for Electrocatalysis; Springer: Cham, Switzerland, 2020; pp. 311–352. [Google Scholar]
- Luo, W.; Gan, J.; Huang, Z.; Chen, W.; Qian, G.; Zhou, X.; Duan, X. Boosting HER performance of Pt-based catalysts immobilized on functionalized vulcan carbon by atomic layer deposition. Front. Mater. 2019, 6, 251. [Google Scholar] [CrossRef]
- Railey, P.; Song, Y.; Liu, T.; Li, Y. Metal organic frameworks with immobilized nanoparticles: Synthesis and applications in photocatalytic hydrogen generation and energy storage. Mater. Res. Bull. 2017, 96, 385–394. [Google Scholar] [CrossRef]
- Langmi, H.W.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen storage in metal-organic frameworks: A review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Monama, G.R.; Mdluli, S.B.; Mashao, G.; Makhafola, M.D.; Ramohlola, K.E.; Molapo, K.M.; Hato, M.J.; Makgopa, K.; Iwuoha, E.I.; Modibane, K.D. Palladium deposition on copper(II) phthalocyanine/metal organic framework composite and electrocatalytic activityof the modified electrode towards the hydrogen evolution reaction. Renew. Energy 2018, 119, 62–72. [Google Scholar] [CrossRef]
- Song, D.; Wang, H.; Wang, X.; Yu, B.; Chen, Y. NiSe2 nanoparticles embedded in carbon nanowires as highly efficient and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2017, 254, 230–237. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Gan, J.; Chen, W.; Zhou, F.; Zhou, X.; Yu, Z.; Zhu, J.; Duan, X.; Wu, Y. Electrochemical conversion of bulk platinum into platinum single-atom sites for hydrogen evolution reaction. J. Mater. Chem. A 2020. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Corte, D.A.D.; Torres, C.; Correa, P.S.; Reider, E.S.; Malfatti, C.F. The hydrogen evolution reaction on nickel-polyaniline composite electrodes. Int. J. Hydrog. Energy 2012, 37, 3025–3032. [Google Scholar] [CrossRef]
- Attia, N.F.; Geckeler, K.E. Polyaniline as a material for hydrogen storage applications. Macromol. Rapid Commun. 2013, 34, 1043–1055. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Wang, G. Synthesis of a super-hydrophilic conducting polyaniline/titanium oxide hybrid with a narrow pore size distribution. Appl. Surf. Sci. 2012, 258, 4788–4793. [Google Scholar] [CrossRef]
- Heydrai, M.H.; Zebhi, H.; Farhadi, K.; Moghadam, P.N. Electrochemical synthesis of nanostructure poly(3-aminobenzoic acid), polyaniline and their bilayers on 430SS and their corrosion protection performances. Synth. Met. 2016, 220, 78–85. [Google Scholar] [CrossRef]
- Aydın, R.; Köleli, F. Hydrogen evolution on conducting polymer electrodes in acidic media. Prog. Org. Coat. 2006, 56, 76–80. [Google Scholar] [CrossRef]
- El-Deeb, M.M.; Alenezi, K.; El Moll, H.; El-Masry, M.; Matarneh, Z. Preparation and characterization of polyaniline/glassy carbon modified electrode as an electrocatalyst for the production of hydrogen from Et3NHCl/[Bu4N][BF4]-CH3CN solution. Int. J. Electrochem. Sci. 2017, 12, 10140–10151. [Google Scholar] [CrossRef]
- Ramohlola, K.E.; Masikini, M.; Mdluli, S.B.; Monama, G.R.; Hato, M.J.; Molapo, K.M.; Iwuoha, E.I.; Modibane, K.D. Electrcatalytic Hydrogen Production Properties of Poly(3-Aminobenzoic acid) doped with Metal Organic frameworks. Int. J. Electrochem. Sci. 2017, 12, 4392–4405. [Google Scholar] [CrossRef]
- Ramohlola, K.E.; Monana, G.R.; Hato, M.J.; Modibane, K.D.; Molapo, K.M.; Masikini, M.; Mdlili, S.B.; Iwuoha, E.I. Polyaniline-metalorganic framework nanocomposite as an efficient electrocatalyst for hydrogen evolution reaction. Compos. Part B 2018, 137, 129–139. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Coudert, F.X. Molecular mechanismof swing effect in zeolitic imidazolate framework ZIF-8. ChemPhysChem 2017, 18, 2732–2738. [Google Scholar] [CrossRef]
- Chang, N.; Gu, Z.Y.; Yan, X.P. Zeolitic imidazolate framework−8 coated capillary for molecular sieving of branched alkanes form linear alkanes along with high−resolution chromatographic separation of linear alkanes. J. Am. Chem. Soc. 2010, 132, 13645–13647. [Google Scholar] [CrossRef]
- Mashao, G.; Modibane, K.D.; Mdluli, S.B.; Iwuoha, E.I.; Hato, M.J.; Makgopa, K.; Molapo, K.M. Polyaniline-cobalt benzimidazolate zeolitic metal-organic framework composite material for electrochemical hydrogen gas sensing. Electrocatalysis 2019, 10, 406–419. [Google Scholar] [CrossRef]
- Chen, X.P.; Jiang, J.K.; Liang, Q.H.; Yang, N.; Ye, H.Y.; Cai, M.; Shen, L.; Yang, D.G.; Ren, T.L. First principles study of the effect of functional groups on polymer backbone. Sci. Rep. 2015, 12, 16907. [Google Scholar]
- Chen, B.; Zhu, Y.; Xia, Y. Controlled in situ synthesis of graphene oxide/zeolitic imidazolate framework composites with enhanced CO2 uptake capacity. RCS Adv. 2015, 5, 30464–30471. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of metal-organic frameworks with rod secondary building units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Bai, H.; Zhang, R.; Shi, H.; Yuan, B. CoZIF with enhanced supercapacitor and electrocatalytic for oxygen evolution reaction performances in alkaline electrolyte. Int. J. Electrochem. Sci. 2016, 11, 7519–7526. [Google Scholar] [CrossRef]
- Slimane, A.B.; Al-Hossainy, A.F.; Zoromba, M.S. Synthesis and optoelectronic properties of conductive nanostructured poly (aniline-co-o-aminophenol) thin film. J. Mater. Sci. Mater. Electron. 2018, 29, 8431–8445. [Google Scholar] [CrossRef]
- Mazur, M.; Michota-Kaminska, A.; Bukpwska, J. Surface-catalyzrd growth of poly(2-methoxyaniline) on gold. Electrochim. Acta 2007, 52, 5669–5676. [Google Scholar] [CrossRef]
- Cordeiro, M.A.M.; Goncalvel, D.; Bulhoes, L.O.S.; Cordeiro, J.M.M. Synthesis and characterization of poly-o-toluidine: Kinetics and structural aspects. Med. Res. 2005, 8, 5–10. [Google Scholar] [CrossRef]
- Saourina, I.Y.; Stejskal, J. The effect of pH on the oxidative polymerization of aniline and the morphology and properties of products. Russ. Chem. Rev. 2010, 79, 1123–1143. [Google Scholar] [CrossRef]
- Molapo, K.M.; Ndaglili, P.M.; Ajayi, R.F.; Mbambisa, G.; Mailu, S.M.; Njomo, N.; Masikini, M.; Baker, P.; Iwuoha, E.I. Electronics of conjugated polymers (I): Polyaniline. Int. J. Electrochem. Sci. 2012, 7, 11859–11875. [Google Scholar]
- Al-Thani, N.; Hassan, M.K.; Bhadra, J. Polyaniline/polystyrene blends: In-depth analysis of the effect of sulfonic acid dopant concentration on ac conductivity using broadband dielectric spectroscopy. Int. J. Polym. Sci. 2018, 9. [Google Scholar] [CrossRef]
- Li, Q.; Kim, H. Hydrogen production from NaBH4 hydrolysis via CoZIF catalyst. Fuel Process. Technol. 2012, 100, 43–48. [Google Scholar] [CrossRef]
- Mashao, G.; Ramohlola, K.E.; Mdluli, S.B.; Monama, G.R.; Hato, M.J.; Makgopa, K.; Molapo, K.M.; Ramoroka, M.E.; Iwuoha, E.I.; Modibane, K.D. Zinc-based zeolitic benzimidazolate framework/polyaniline nanocomposite for electrochemical sensing of hydrogen gas. Mater. Chem. Phys. 2019, 230, 287–298. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Le, K.A.; Truong, X.H.; Phan, T.S. Metal-organic frameworks for catalysis: The knoevenagel reaction using zeolite imidazolate framework CoZIF as an efficient heterogeneous catalyst. Catal. Sci. Technol. 2012, 2, 521–528. [Google Scholar] [CrossRef]
- Sharifirad, M.; Kiani, F.; Koohyar, F. Glassy carbon electrode modified by poly(m-aminobenzoic acid)/nano SiO2 film and electrical and electrochemical properties. Eur. Online J. Nat. Soc. Sci. 2013, 2, 366–378. [Google Scholar]
- Zare, E.N.; Lakouraj, M.M.; Moosavi, E. Poly(3-aminobenzoic acid)@MWCNTs hybrid conducting nanocomposite: Preparation, characterization and application as a coating for copper corrosion protection. Compos. Interfaces 2016. [Google Scholar] [CrossRef]
- Patra, S.; Munichandraiah, N. Insoluble poly(anthranilic acid) confined in Nafion membrabe by chemical and electrochemical polymerization of anthranilic acid. Synth. Met. 2005, 150, 285–290. [Google Scholar] [CrossRef]
- Yang, S.; Pattengale, B.; Kovrigin, E.L.; Huang, J. Photoactive zeolitic imidazolate framework as intrinsic heterogeneous catalysts for light-driven hydrogen generation. Acs Energy Lett. 2017, 2, 75–80. [Google Scholar] [CrossRef]
- Wu, C.; Li, C.; Yang, B.; Zhou, S.; Shi, D.; Wang, Y.; Yang, G.; He, J.; Shan, Y. Electrospun MnCo2O4 nanofibers for efficient hydrogen evolution reaction. Mater. Res. Express 2016, 3, 095018. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, J.; Lu, J.; Yang, L.; Hou, D.; Li, G.; Chen, S. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 2016, 28, 29–43. [Google Scholar] [CrossRef]
- Gao, M.R.; Liang, J.X.; Zheng, Y.R.; Xu, Y.F.; Jiang, J.; Gao, Q.; Li, J.; Yu, S.H. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 2015, 6, 5982–5988. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L. Recent developments in electrochemical Hydrogen Evolution Reaction. Curr. Opin. Electrochem. 2018, 7, 7–14. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Rouhaghdam, S. Nickel nanocones as efficient and stable catalyst for electrochemical hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 14560–14565. [Google Scholar] [CrossRef]
- Torres, C.; Moreno, B.; Chirrano, E.; Malfatti, C.F. Nickel-polyaniline composite electrodes for hydrogen evolution reaction in alkaline media. Int. J. Hydrog. Energy 2017, 32, 20410–20419. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, W.; Lin, T. Graphene/graphene nanoribbon aerogels as tunable three-dimensional framework for efficient hydrogen evolution reaction. Electrochim. Acta 2017, 250, 91–98. [Google Scholar] [CrossRef]
- Jinlong, L.; Wang, Z.; Miura, H. The effects of ball milling on microstructures of graphene/Ni composites and their catalytic activity for hydrogen evolution reaction. Mater. Lett. 2017, 206, 124–127. [Google Scholar] [CrossRef]



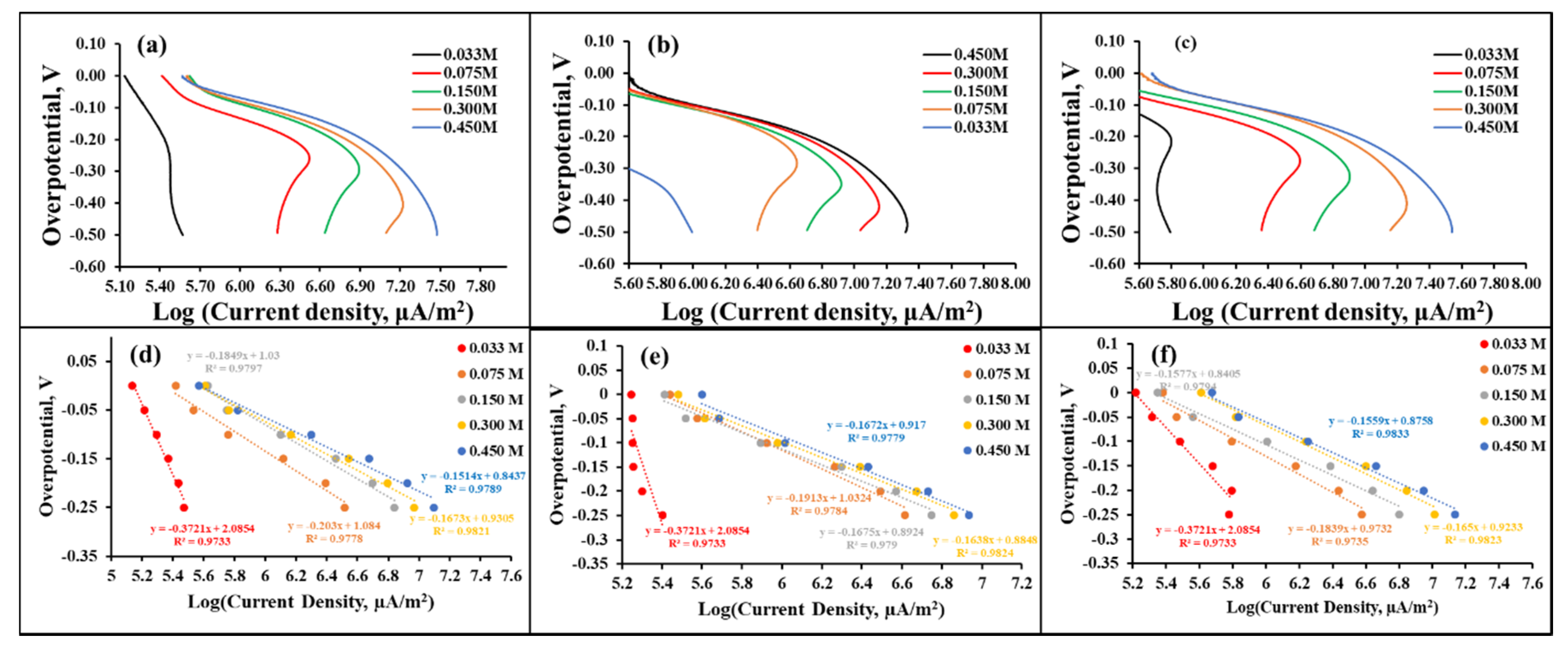
| Material | H2SO4 (mol L−1) | Slope (b) (V dec−1) | −b (mV dec−1) | 1−α | Log (j0/μA m−2) | j0 (A m−2) |
|---|---|---|---|---|---|---|
| Blank | 0.033 | −0.4593 | 459.3 | 0.1288 | 5.88 | 0.76 |
| 0.15 | −0.4714 | 471.4 | 0.1255 | 5.88 | 0.76 | |
| PABA | 0.033 | −0.3721 | 372.1 | 0.1589 | 5.93 | 0.85 |
| 0.075 | −0.203 | 203.0 | 0.2913 | 6.22 | 1.66 | |
| 0.15 | −0.1849 | 184.9 | 0.3198 | 6.39 | 2.45 | |
| 0.3 | −0.1673 | 167.3 | 0.3535 | 6.70 | 5.01 | |
| 0.45 | −0.1514 | 151.4 | 0.3906 | 7.03 | 10.72 | |
| CoZIF | 0.033 | −0.3721 | 372.1 | 0.1589 | 5.95 | 0.89 |
| 0.075 | −0.1913 | 191.3 | 0.3091 | 6.20 | 1.58 | |
| 0.15 | −0.1675 | 167.5 | 0.3531 | 6.54 | 3.47 | |
| 0.3 | −0.1638 | 163.8 | 0.3610 | 6.73 | 5.37 | |
| 0.45 | −0.1672 | 167.2 | 0.3537 | 6.95 | 8.91 | |
| PABA/CoZIF | 0.033 | −0.3721 | 372.1 | 0.1589 | 6.07 | 1.17 |
| 0.075 | −0.1837 | 183.7 | 0.3219 | 6.24 | 1.74 | |
| 0.15 | −0.1577 | 157.7 | 0.3750 | 6.52 | 3.31 | |
| 0.3 | −0.165 | 165.0 | 0.3584 | 6.81 | 6.46 | |
| 0.45 | −0.1559 | 155.9 | 0.3793 | 7.00 | 10.0 | |
| Pd@CuPc/MOF on Pt electrode [25] | 0.300 | −0.177 | 177.0 | 0.3341 | 6.95 | 8.91 |
| 20% Pt/C on GCE electrode [2] | 0.500 | −0.0306 | 30.6 | 1.9326 | - | - |
| Ru/VC on GCE electrode [2] | 0.500 | −0.0867 | 86.7 | 0.6821 | - | - |
| Pt/VC on GCE electrode [2] | 0.500 | −0.1328 | 132.8 | 0.4453 | - | - |
| Pt/Ru/VC on GCE electrode [2] | 0.500 | −0.0306 | 30.6 | 1.9326 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modibane, K.D.; Waleng, N.J.; Ramohlola, K.E.; Maponya, T.C.; Monama, G.R.; Makgopa, K.; Hato, M.J. Poly(3-aminobenzoic acid) Decorated with Cobalt Zeolitic Benzimidazolate Framework for Electrochemical Production of Clean Hydrogen. Polymers 2020, 12, 1581. https://doi.org/10.3390/polym12071581
Modibane KD, Waleng NJ, Ramohlola KE, Maponya TC, Monama GR, Makgopa K, Hato MJ. Poly(3-aminobenzoic acid) Decorated with Cobalt Zeolitic Benzimidazolate Framework for Electrochemical Production of Clean Hydrogen. Polymers. 2020; 12(7):1581. https://doi.org/10.3390/polym12071581
Chicago/Turabian StyleModibane, Kwena Desmond, Ngwako Joseas Waleng, Kabelo Edmond Ramohlola, Thabiso Carol Maponya, Gobeng Release Monama, Katlego Makgopa, and Mpitloane Joseph Hato. 2020. "Poly(3-aminobenzoic acid) Decorated with Cobalt Zeolitic Benzimidazolate Framework for Electrochemical Production of Clean Hydrogen" Polymers 12, no. 7: 1581. https://doi.org/10.3390/polym12071581
APA StyleModibane, K. D., Waleng, N. J., Ramohlola, K. E., Maponya, T. C., Monama, G. R., Makgopa, K., & Hato, M. J. (2020). Poly(3-aminobenzoic acid) Decorated with Cobalt Zeolitic Benzimidazolate Framework for Electrochemical Production of Clean Hydrogen. Polymers, 12(7), 1581. https://doi.org/10.3390/polym12071581





