Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid
Abstract
1. Introduction
2. Materials and Methods
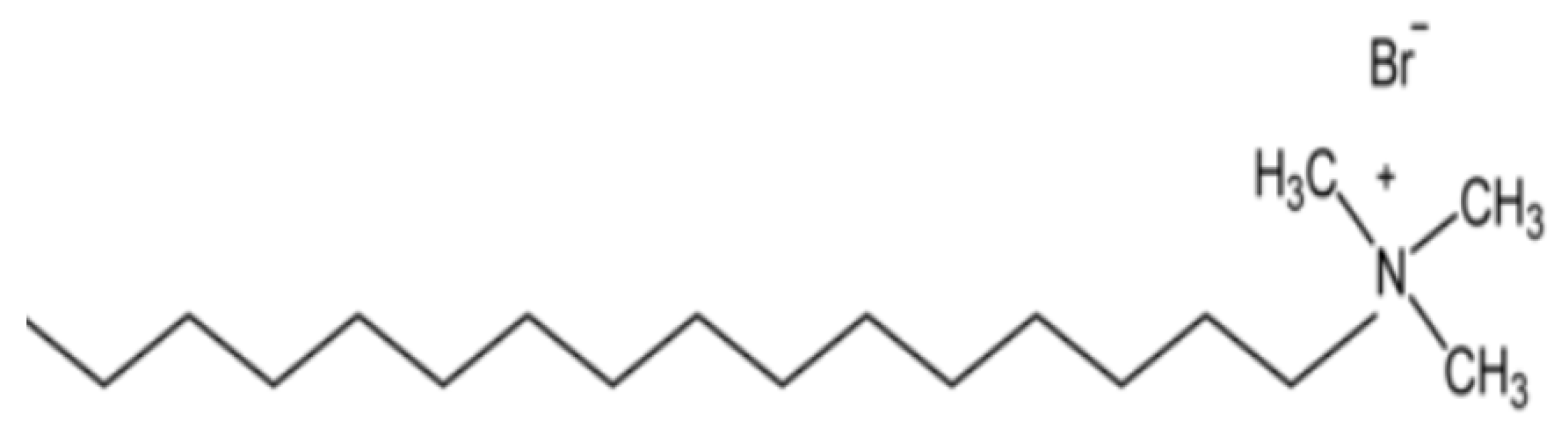
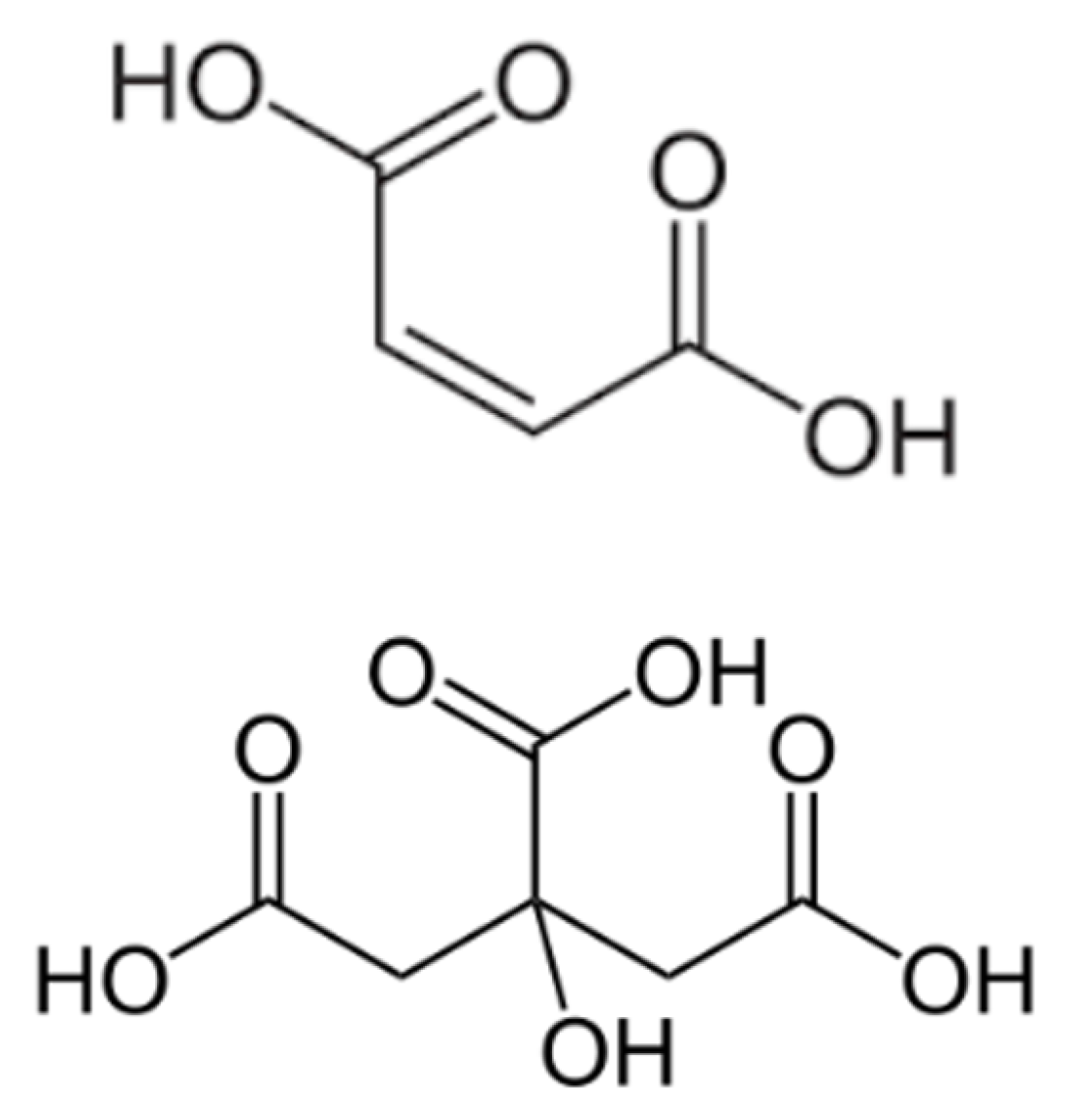
2.1. Materials
2.2. Methods
2.2.1. Samples Preparation
2.2.2. Performance Evaluation Experiments
2.2.3. The Dynamic and Steady Rheological Tests
2.2.4. Temperature and Shear Resistance Test
2.2.5. Sand Suspension Ability Test
2.2.6. Gel Breaking Capability Test
2.2.7. Core damage Evaluation Test
3. Results and Discussion
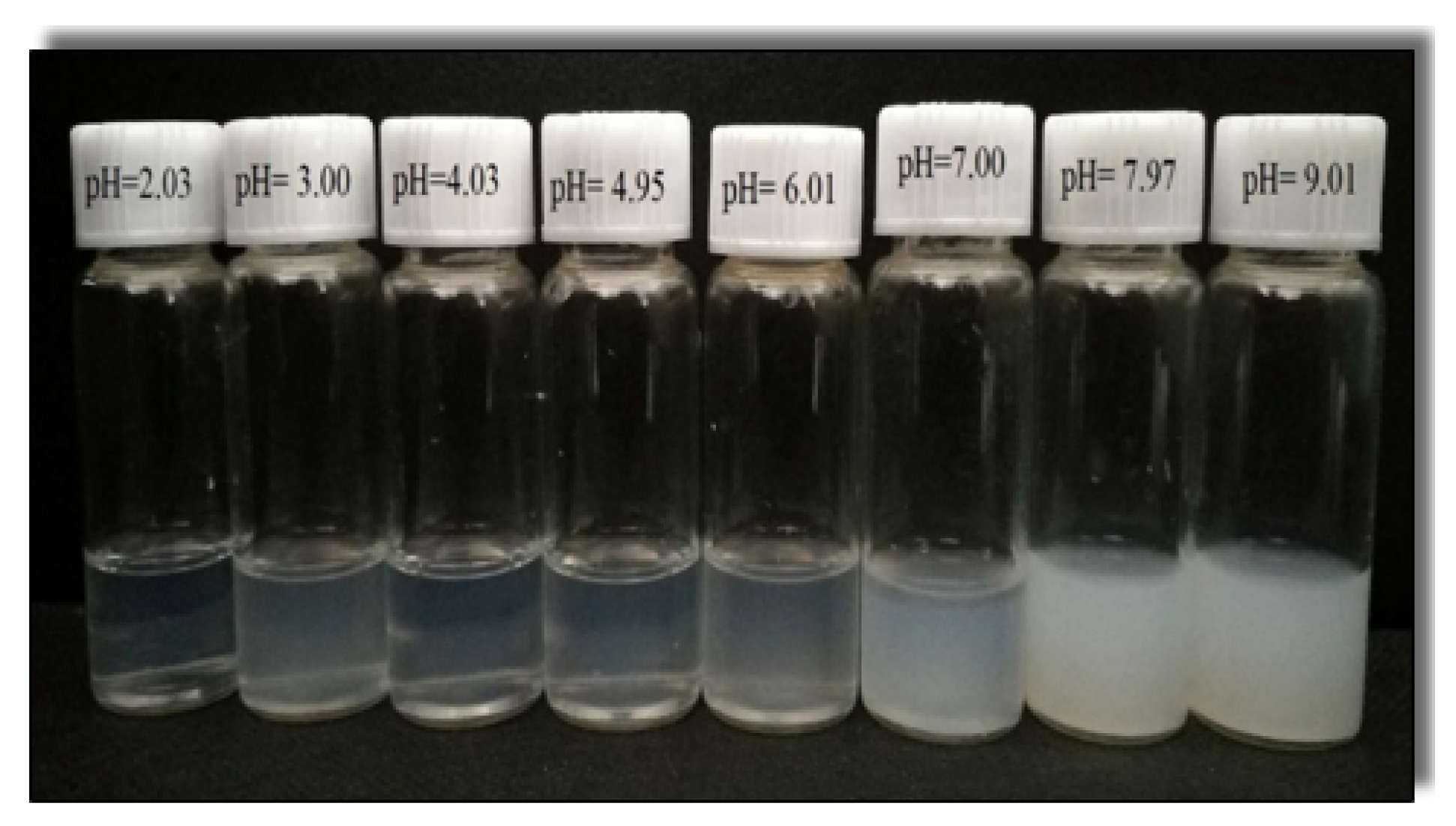
3.1. Phase Behavior
3.2. Rheological Properties
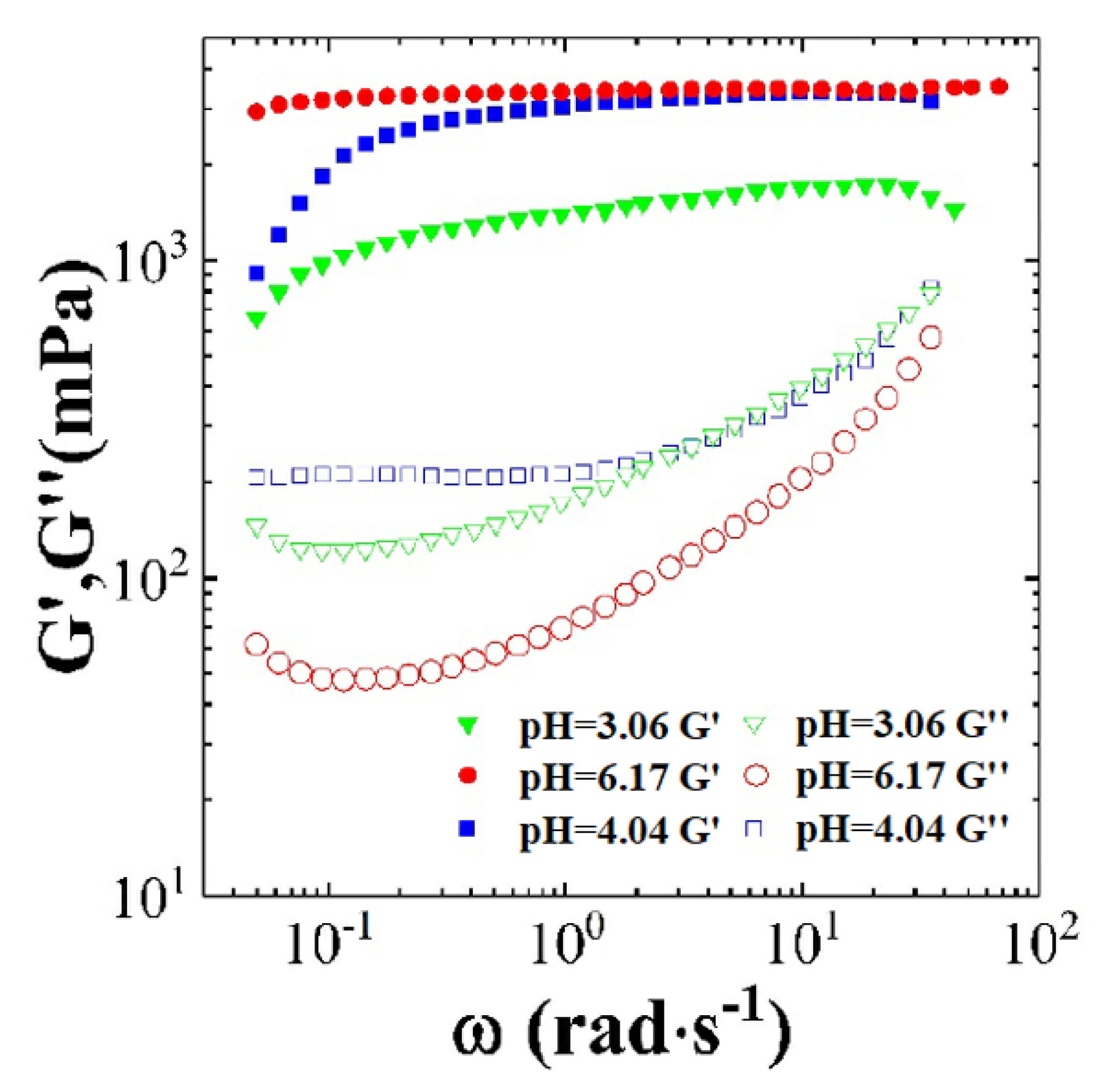
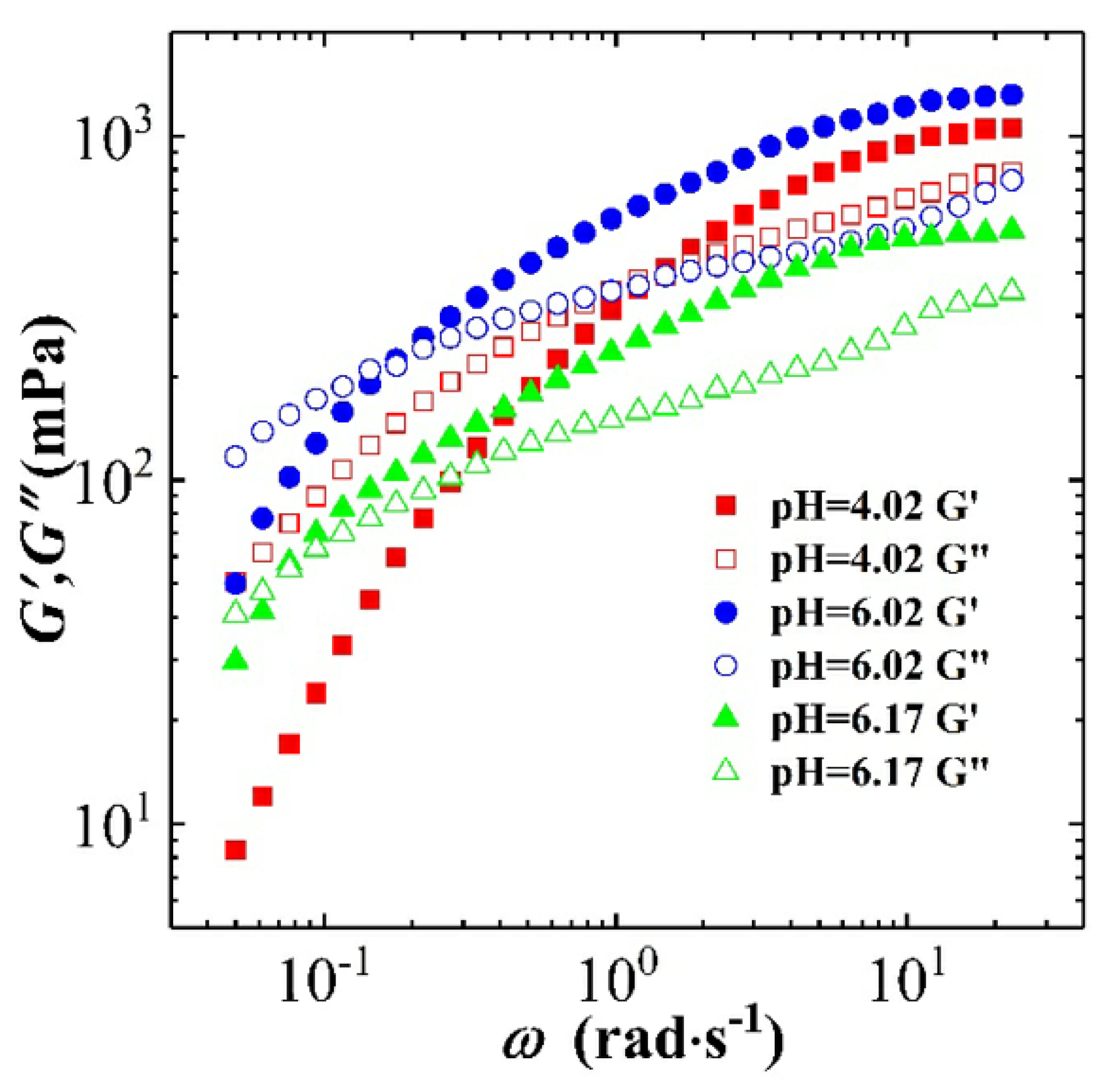
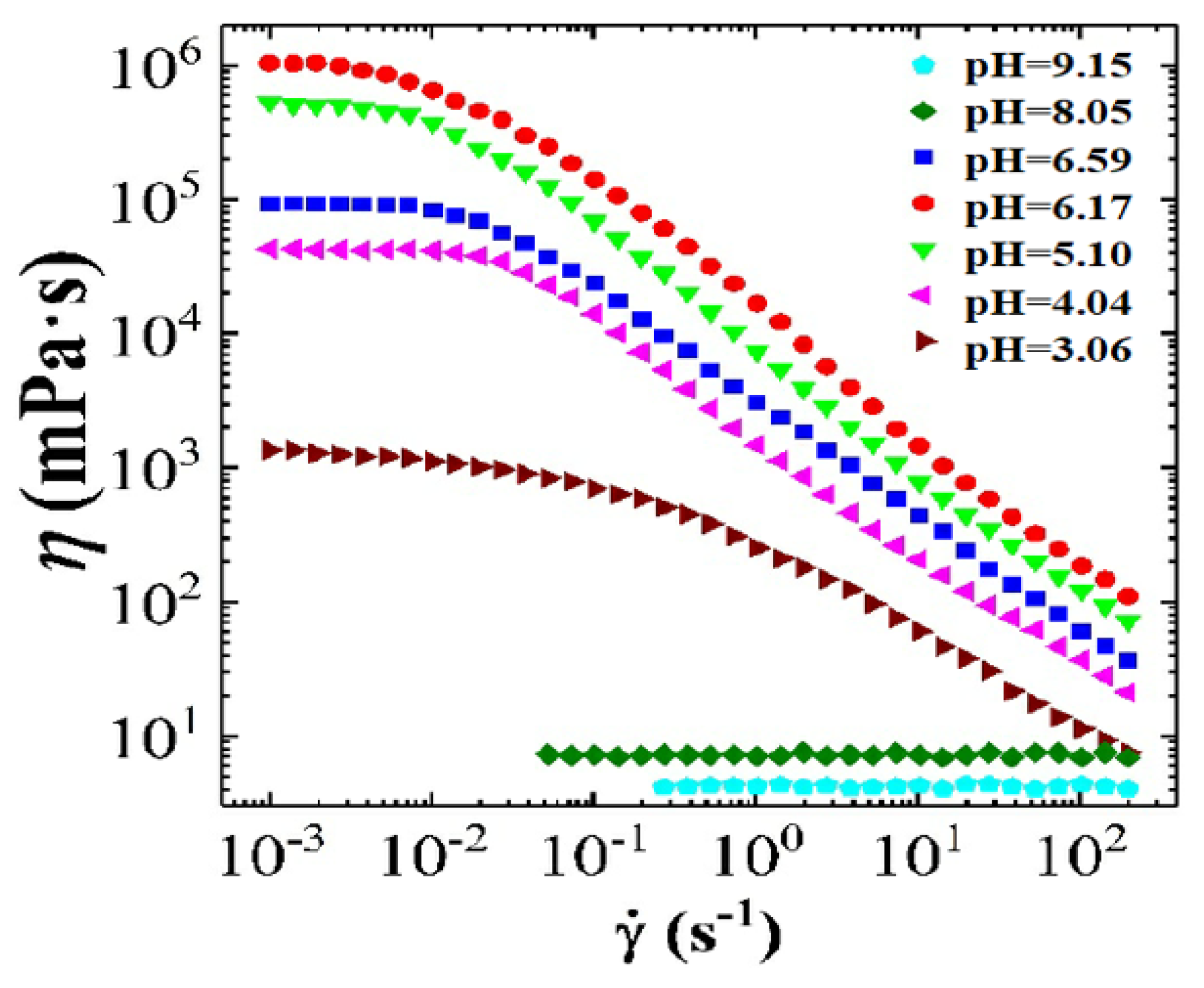
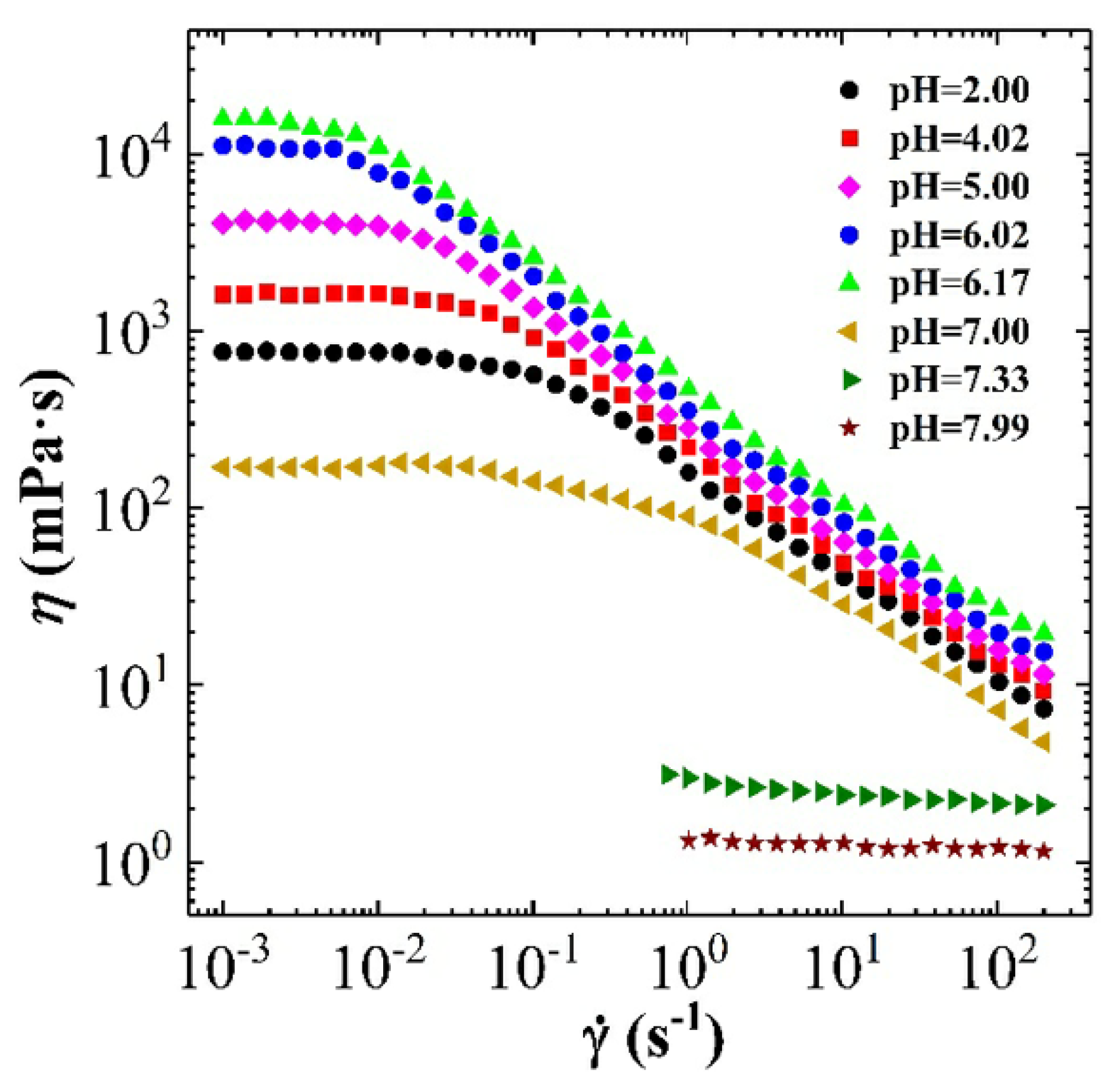
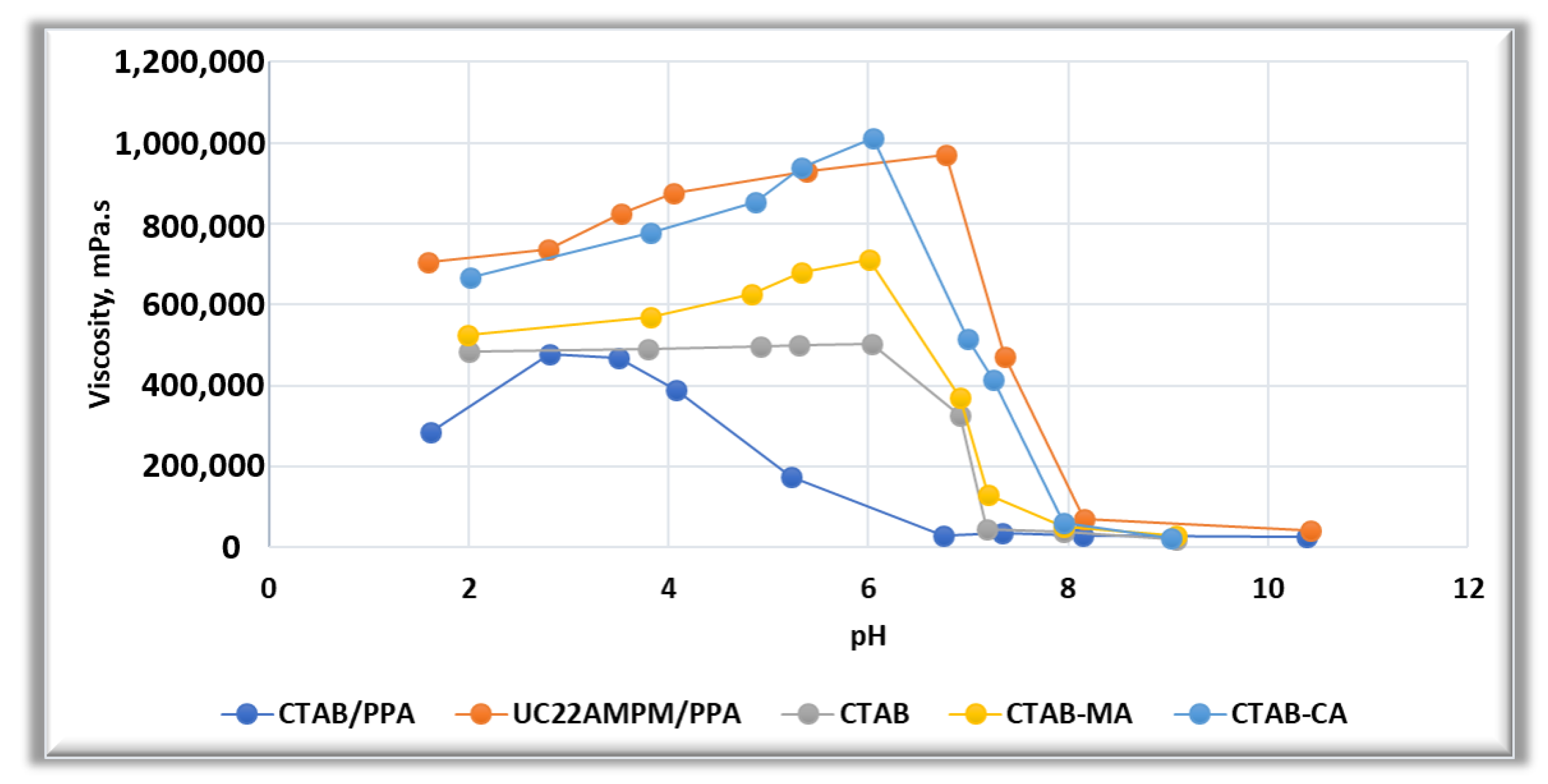
3.2.1. The Effect of pH on Dynamic and Steady Rheological Tests
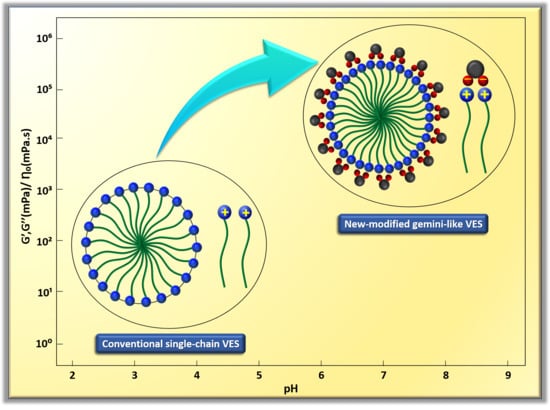
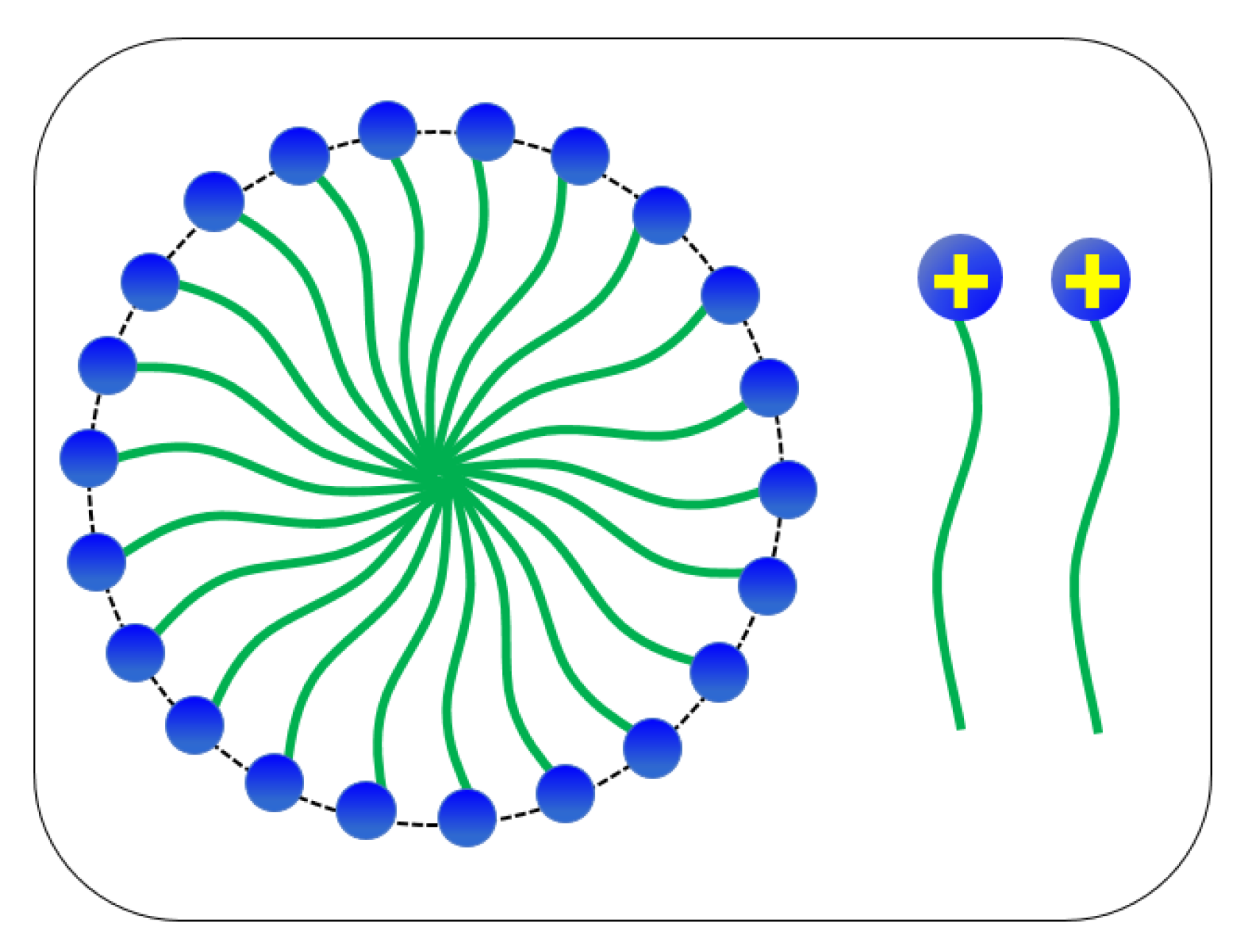
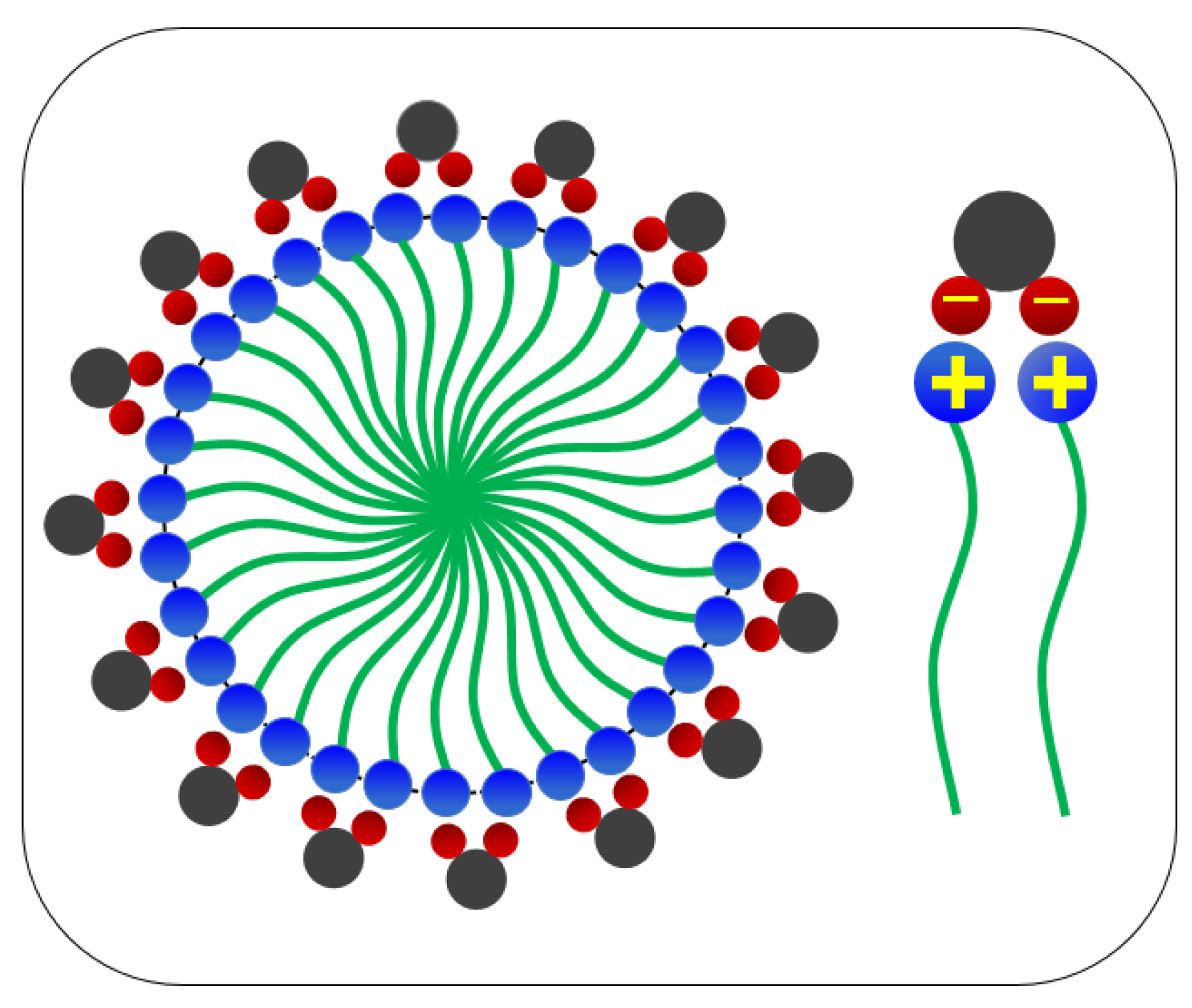
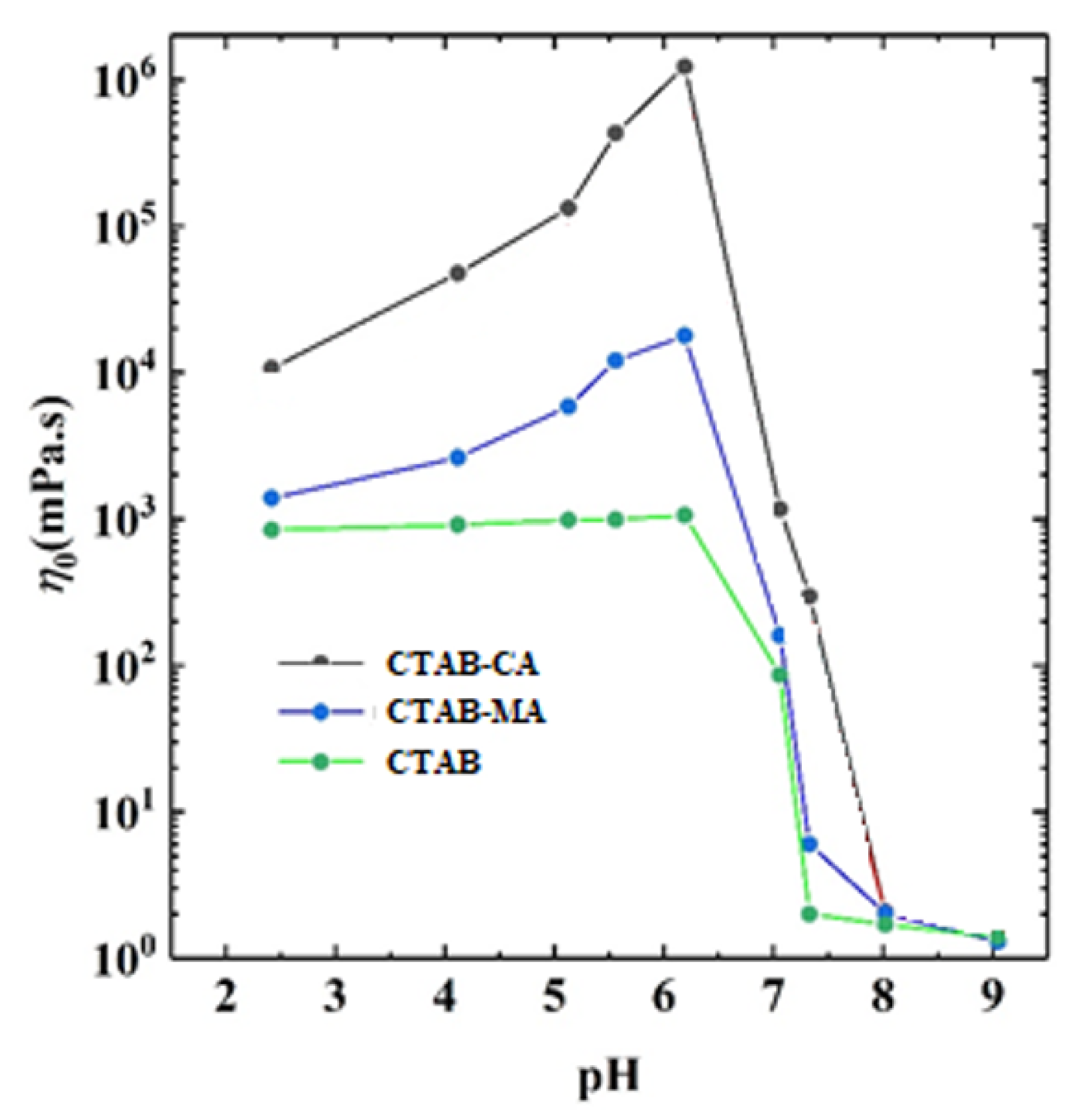
3.2.2. Combining the Effect of pH on Rheological Tests Results with Phase-Behaviour Study
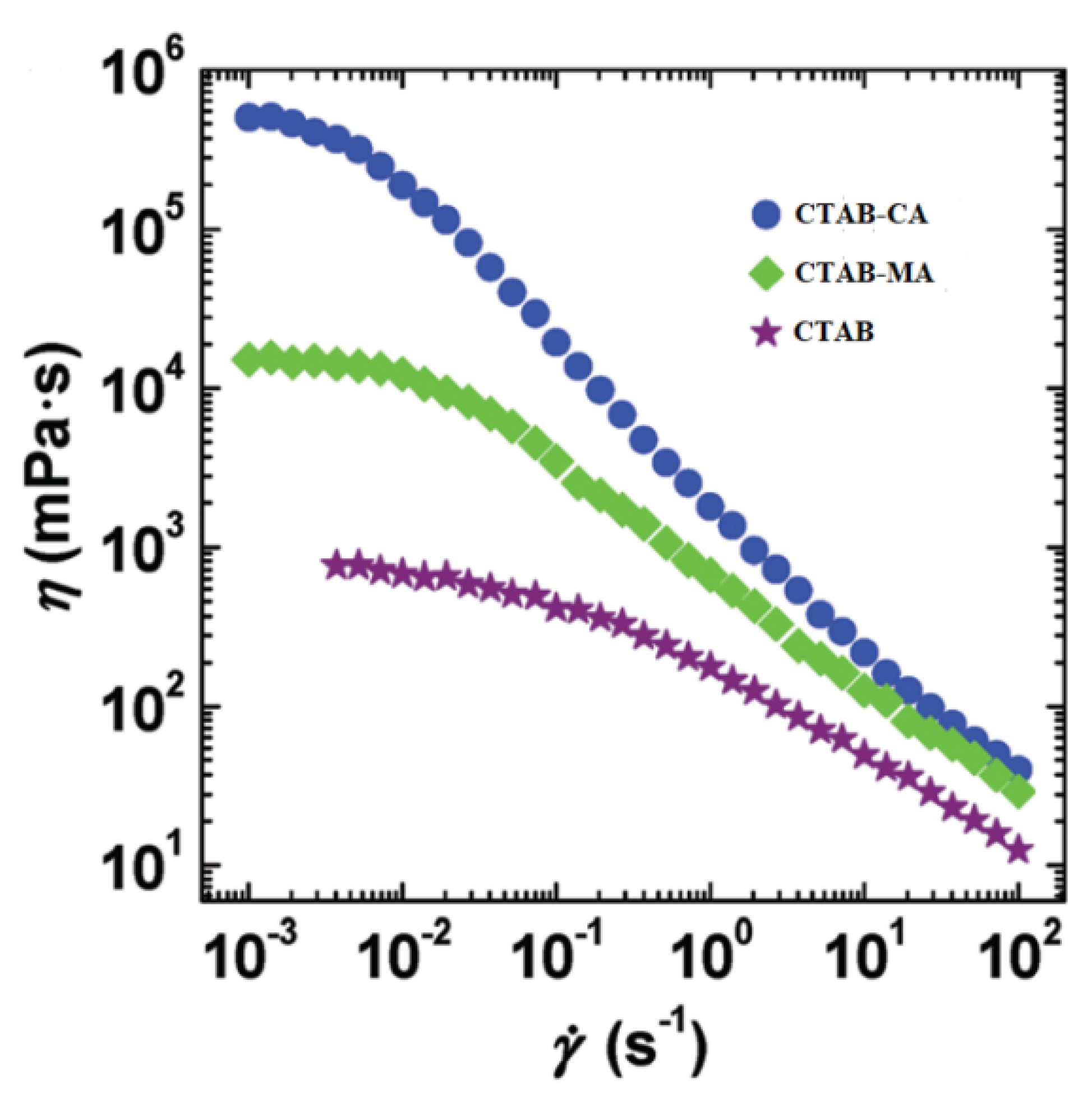
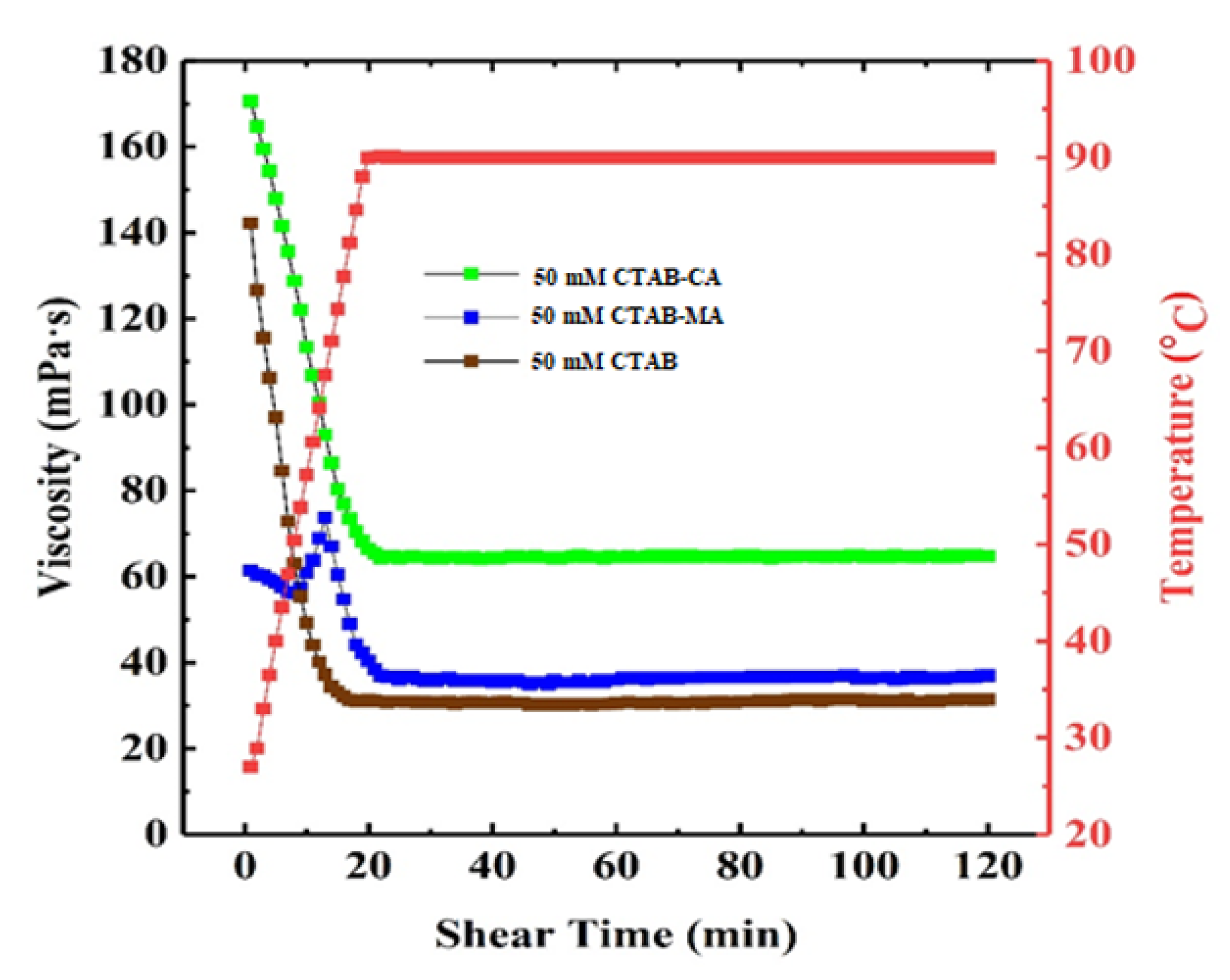
3.3. Temperature and Shear Resistance
3.4. Sand Suspension Ability
3.5. Gel Breaking Capability
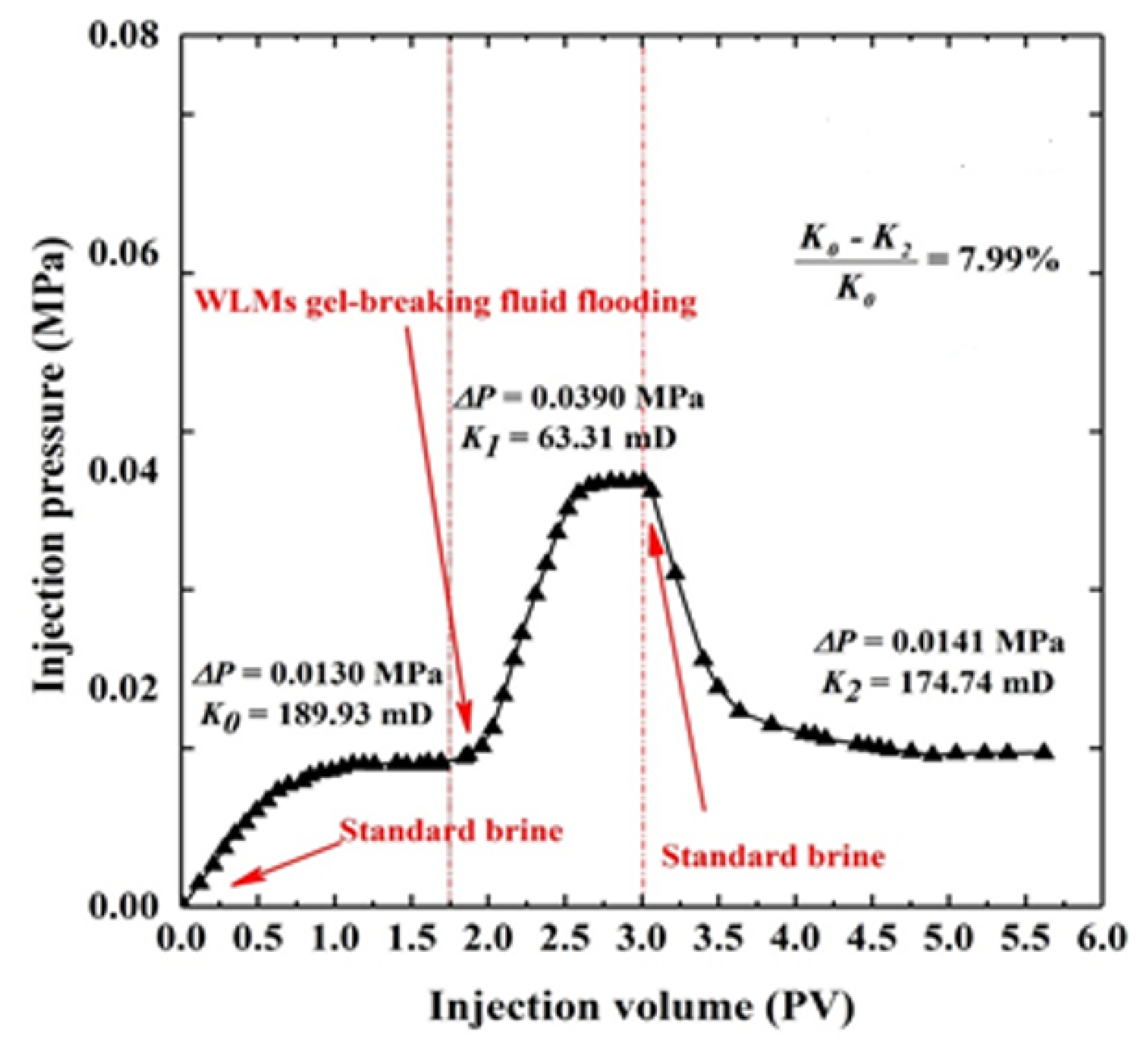
3.6. Core Flooding Evaluation Test
4. Conclusions
- The dynamic and steady state rheological investigation of CTAB-CA and CTAB-MA VES-fluids showed that the synthesized VES fluids mostly exhibit shear-dependant viscoelastic non-Newtonian behaviour.
- In general, both CTAB-CA and CTAB-MA VES-fluids are found to be pH responsive at both acidic and alkaline conditions.
- The effect of the pH shows higher viscosity and more structured solid-like behaviour at acidic range of pH (i.e., pH < 7).
- Whereas, at an alkaline range of pH (i.e., pH > 7), the effect of the pH shows lower viscosity and more liquid-like behaviour is observed.
- CTAB-CA VES-fluid shows more viscoelasticity and solid-like nature compared to CTAB-MA.
- At 25 The viscosity of CTAB-CA can reach up to 106 mPa.s at pH-value of 6.17, whereas the maximum viscosity of CTAB-MA is 10 mPa.s.
- CTAB-CA VES-fluid has the best temperature and shear resistance, its apparent viscosity remains at 65 mPa.s; after continuous shearing for 2 h at 90 C and shear rate of 170 (s).
- CTAB-CA VES-fluid exhibits excellent sand suspension and gel breaking ability; At 90 oc, the sand suspension velocity of CTAB-CA was found to be 1.67 mm/s and complete gel breaking was achieved within 2 h after mixing with the ethanol at the ratio of 10:1.
- The core flooding tests show that, after injecting CTAB-CA fluid into the core sample, the core damage rate is 7.99%, indicating that it doesn’t cause much damage.
- Based on the performance evaluation results, it is expected that CTAB-CA VES-fluid under high-temperature will make the proposed new VES-fluid an attractive thickening fracturing fluid.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallegos, T.J.; Varela, B.A. Trends in Hydraulic Fracturing Distributions and Treatment Fluids, Additives, Proppants, and Water Volumes Applied to Wells Drilled in The United States from 1947 Through 2010: Data Analysis and Comparison to the Literature; USGS: Reston, VA, USA, 2014.
- Bunger, A.P.; McLennan, J.; Jeffrey, R. Effective and Sustainable Hydraulic Fracturing; IntechOpen: London, UK, 2013. [Google Scholar]
- Montgomery, C.T.; Smith, M.B. Hydraulic fracturing: history of an enduring technology. J. Pet. Technol. 2010, 62, 26–40. [Google Scholar] [CrossRef]
- Yew, C.H.; Weng, X. Mechanics of Hydraulic Fracturing; Gulf Professional Publishing: Houston, TX, USA, 2014. [Google Scholar]
- Barbati, A.C.; Desroches, J.; Robisson, A.; McKinley, G.H. Complex fluids and hydraulic fracturing. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 415–453. [Google Scholar] [CrossRef] [PubMed]
- Osiptsov, A.A. Fluid mechanics of hydraulic fracturing: A review. J. Pet. Sci. Eng. 2017, 156, 513–535. [Google Scholar] [CrossRef]
- Howard, G.C.; Fast, C.R. Hydraulic Fracturing; Society of Petroleum Engineers of Aime: New York, NY, USA, 1970; 210p. [Google Scholar]
- Grebe, J.J.; Sanford, R.T. Treatment of Deep Wells. U.S. Patent 1,877,504, 13 September 1932. [Google Scholar]
- Frenier, W.; Ziauddin, M. Chemistry for Enhancing the Production of Oil and Gas Richardson; Society of Petroleum Engineers: Richardson, TX, USA, 2013. [Google Scholar]
- Ingraffea, A.R.; Wells, M.T.; Santoro, R.L.; Shonkoff, S.B. Assessment and risk analysis of casing and cement impairment in oil and gas wells in Pennsylvania, 2000–2012. Proc. Natl. Acad. Sci. USA 2014, 111, 10955–10960. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, H.; Qi, X.; Wang, C.; Liu, H.; Yun, Z. Research progress of cleaning sanitization methods and effect detection for dairy production equipment. China Dairy Ind. 2015, 43, 49–51. [Google Scholar]
- Armstrong, K. Advanced fracturing fluids improve well economics. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1996, 5, 221A. [Google Scholar]
- Armstrong, K.; Card, R.; Navarette, R.; Nelson, E.; Nimerick, K.; Samuelson, M.; Collins, J.; Dumont, G.; Priaro, M.; Wasylycia, N.; et al. Advanced fracturing fluids improve well economics. Oilfield Rev. 1995, 7, 34–51. [Google Scholar]
- Tiab, D.; Donaldson, E.C. Petrophysics: Theory and Practice of Measuring Reservoir Rock and Fluid Transport Properties; Gulf Professional Publishing: Houston, TX, USA, 2015. [Google Scholar]
- Hassan, A.M.; Ayoub, M.; Eissa, M.; Musa, T.; Bruining, H.; Farajzadeh, R. Exergy return on exergy investment analysis of natural-polymer (Guar-Arabic gum) enhanced oil recovery process. Energy 2019, 181, 162–172. [Google Scholar] [CrossRef]
- Hassan, A.M.; Ayoub, M.; Eissa, M.; Musa, T.; Bruining, H.; Zitha, P. Development of an integrated RFID-IC technology for on-line viscosity measurements in enhanced oil recovery processes. J. Pet. Explor. Prod. Technol. 2019, 9, 2605–2612. [Google Scholar] [CrossRef]
- Alohaly, M.; BinGhanim, A.; Rahal, R.; Rahim, S. Seawater Fracturing Fluid Development Challenges: A Comparison between Seawater-Based and Freshwater-Based Fracturing Fluids Using Two Types of Guar Gum Polymers; SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition; Society of Petroleum Engineers: London, UK, 2016. [Google Scholar]
- Marec, A.; Thomas, J.H.; El Guerjouma, R. Damage characterization of polymer-based composite materials: Multivariable analysis and wavelet transform for clustering acoustic emission data. Mech. Syst. Signal Process. 2008, 22, 1441–1464. [Google Scholar] [CrossRef]
- Thomas, R.; Morgenthaler, L. Introduction to matrix treatments. In Reservoir Stimulation, 3rd ed.; Economides, M.J., Nolte, K.G., Eds.; Wiley: New York, NY, USA, 1999; pp. 1–38. [Google Scholar]
- Huang, T.; Crews, J.B. Nanotechnology applications in viscoelastic surfactant stimulation fluids. SPE Prod. Oper. 2008, 23, 512–517. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhang, Z.; Yang, B.; Zhang, Y.; Zhao, J. Study of a novel gemini viscoelastic surfactant with high performance in clean fracturing fluid application. Polymers 2018, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Dai, C.; Zhao, M.; Sun, Y.; Zhao, G. Development, formation mechanism and performance evaluation of a reusable viscoelastic surfactant fracturing fluid. J. Ind. Eng. Chem. 2016, 37, 115–122. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, J.; Mao, J.; Yang, X.; Zhang, H.; Zhang, W. High performance clean fracturing fluid using a new tri-cationic surfactant. Polymers 2018, 10, 535. [Google Scholar] [CrossRef] [PubMed]
- Eoff, L.S. Improvements to Hydrophobically Modified Water-Soluble Polymer Technology to Extend the Range of Oilfield Applications. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 11–13 April 2011. [Google Scholar]
- Mao, J.; Yang, X.; Wang, D.; Li, Y.; Zhao, J. A novel gemini viscoelastic surfactant (VES) for fracturing fluids with good temperature stability. RSC Adv. 2016, 6, 88426–88432. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, W.; Xu, D.; Feng, H.; Zhang, P.; Li, Z.; Lu, Y.; Wu, H. The rheological characteristics for the mixtures of cationic surfactant and anionic–nonionic surfactants: The role of ethylene oxide moieties. Rsc Adv. 2017, 7, 13032–13040. [Google Scholar] [CrossRef]
- Liu, Y.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Lahann, J.; Mitragotri, S.; Tran, T.N.; Kaido, H.; Sundaram, J.; Choi, I.S.; Hoffer, S.; Somorjai, G.A.; Langer, R. A reversibly switching surface. Science 2003, 299, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Hao, J. Reversibly switchable wettability. Chem. Soc. Rev. 2010, 39, 769–782. [Google Scholar] [CrossRef]
- Mohyaldinn, M.E.; Hassan, A.M.; Ayoub, M.A. Application of emulsions and microemulsions in enhanced oil recovery and well stimulation. In Microemulsion—A Chemical Nanoreactor; IntechOpen: London, UK, 2019. [Google Scholar]
- Hassan, A.; Ayoub, M.; Eissa, M.; Bruining, H.; Al-Mansour, A.; Al-Guraishi, A. A Novel Hybrid Enhanced Oil Recovery Method by Smart Water-Injection and Foam-Flooding in Carbonate Reservoirs. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Bali, Indonesia, 29–31 October 2019; Society of Petroleum Engineers: Richardson, TX, USA, 2019. [Google Scholar]
- Alakbari, F.S.; Mohyaldinn, M.E.; Muhsan, A.S.; Hasan, N.; Ganat, T. Chemical Sand Consolidation: From Polymers to Nanoparticles. Polymers 2020, 12, 1069. [Google Scholar] [CrossRef]
- Chauhan, G.; Ojha, K.; Baruah, A. Effects of nanoparticles and surfactant charge groups on the properties of VES gel. Braz. J. Chem. Eng. 2017, 34, 241–251. [Google Scholar] [CrossRef]
- Yang, J.; Guan, B.; Lu, Y.; Cui, W.; Qiu, X.; Yang, Z.; Qin, W. Viscoelastic evaluation of gemini surfactant gel for hydraulic fracturing. In Proceedings of the SPE European Formation Damage Conference & Exhibition, Noordwijk, The Netherlands, 5–7 June 2020; Society of Petroleum Engineers: Richardson, TX, USA, 2013. [Google Scholar]
- Lu, Y.; Yang, Z.; Guan, B.; Qiu, X.; Qin, W.; Yang, J.; Cui, W. Viscoelastic evaluation of gemini surfactant gel for hydraulic fracturing. In Proceedings of the 10th SPE International Conference and Exhibition on European Formation Damage, Noordwijk, The Netherlands, 5–6 June 2013. [Google Scholar]
- Yang, C.; Song, Z.; Zhao, J.; Hu, Z.; Zhang, Y.; Jiang, Q. Self-assembly properties of ultra-long-chain gemini surfactants bearing multiple amide groups with high performance in fracturing fluid application. Colloids Surf. Physicochem. Eng. Asp. 2017, 523, 62–70. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Z.; Xu, F.; Tai, S.; Liu, X.; Mo, S.; Niu, F. Surface tension and aggregation properties of novel cationic gemini surfactants with diethylammonium headgroups and a diamido spacer. Langmuir 2012, 28, 11979–11987. [Google Scholar] [CrossRef] [PubMed]
- Crews, J.B.; Huang, T. Performance enhancements of viscoelastic surfactant stimulation fluids with nanoparticles. In Proceedings of the Europec/EAGE Conference and Exhibition, Rome, Italy, 9–12 June 2008; Society of Petroleum Engineers: London, UK, 2008. [Google Scholar]
- Yang, J. Viscoelastic wormlike micelles and their applications. Curr. Opin. Colloid Interface Sci. 2002, 7, 276–281. [Google Scholar] [CrossRef]
- Chen, F.; Wu, Y.; Wang, M.; Zha, R. Self-assembly networks of wormlike micelles and hydrophobically modified polyacrylamide with high performance in fracturing fluid application. Colloid Polym. Sci. 2015, 293, 687–697. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, J.; Peng, H.; Pan, R.; Aliu, A.O.; Lu, Q.; Yang, J. Synthesis and evaluation of a novel clean hydraulic fracturing fluid based on star-dendritic polymer. J. Nat. Gas Sci. Eng. 2017, 43, 179–189. [Google Scholar] [CrossRef]
- Kang, W.; Wang, P.; Fan, H.; Yang, H.; Dai, C.; Yin, X.; Zhao, Y.; Guo, S. A pH-responsive wormlike micellar system of a noncovalent interaction-based surfactant with a tunable molecular structure. Soft Matter 2017, 13, 1182–1189. [Google Scholar] [CrossRef]
- Wang, P.; Kang, W.; Yang, H.; Yin, X.; Zhao, Y.; Zhu, Z.; Zhang, X. pH-Responsive wormlike micelles based on microstructural transition in a C 22-tailed cationic surfactant–aromatic dibasic acid system. RSC Adv. 2017, 7, 37699–37705. [Google Scholar] [CrossRef]
- Meister, J.J. Hydraulic Fracturing Method Using Viscosified Surfactant Solutions. U.S. Patent 4,007,792, 15 February 1977. [Google Scholar]
- Patel, V.; Dharaiya, N.; Ray, D.; Aswal, V.K.; Bahadur, P. pH controlled size/shape in CTAB micelles with solubilized polar additives: A viscometry, scattering and spectral evaluation. Colloids Surf. Physicochem. Eng. Asp. 2014, 455, 67–75. [Google Scholar] [CrossRef]
- Kjøniksen, A.L.; Nyström, B.; Nakken, T.; Palmgren, O.; Tande, T. Effect of surfactant concentration, pH, and shear rate on the rheological properties of aqueous systems of a hydrophobically modifed chitosan and its unmodified analogue. Polym. Bull. 1997, 38, 71–79. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Pham, T.D.; Pham, T.T.; Phan, M.N.; Ngo, T.M.V.; Vu, C.M. Adsorption characteristics of anionic surfactant onto laterite soil with differently charged surfaces and application for cationic dye removal. J. Mol. Liq. 2020, 301, 112456. [Google Scholar] [CrossRef]
- Dorshow, R.; Briggs, J.; Bunton, C.; Nicoli, D. Dynamic light scattering from cetyltrimethylammonium bromide micelles. Intermicellar interactions at low ionic strengths. J. Phys. Chem. 1982, 86, 2388–2395. [Google Scholar] [CrossRef]
- Kuperkar, K.C.; Mata, J.P.; Bahadur, P. Effect of 1-alkanols/salt on the cationic surfactant micellar aqueous solutions—A dynamic light scattering study. Colloids Surf. Physicochem. Eng. Asp. 2011, 380, 60–65. [Google Scholar] [CrossRef]
- Vautier-Giongo, C.; Bales, B.L. Estimate of the ionization degree of ionic micelles based on Krafft temperature measurements. J. Phys. Chem. 2003, 107, 5398–5403. [Google Scholar] [CrossRef]
- Goyal, P.; Dasannacharya, B.; Kelkar, V.; Manohar, C.; Rao, K.S.; Valaulikar, B. Shapes and sizes of micelles in CTAB solutions. Phys. Condens. Matter 1991, 174, 196–199. [Google Scholar] [CrossRef]
- Kuperkar, K.; Abezgauz, L.; Danino, D.; Verma, G.; Hassan, P.; Aswal, V.; Varade, D.; Bahadur, P. Viscoelastic micellar water/CTAB/NaNO3 solutions: Rheology, SANS and cryo-TEM analysis. J. Colloid Interface Sci. 2008, 323, 403–409. [Google Scholar] [CrossRef]
- Kim, W.J.; Yang, S.M. Microstructures and rheological responses of aqueous CTAB solutions in the presence of benzyl additives. Langmuir 2000, 16, 6084–6093. [Google Scholar] [CrossRef]
- Inoue, T.; Inoue, Y.; Watanabe, H. Nonlinear rheology of CTAB/NaSal aqueous solutions: finite extensibility of a network of wormlike micelles. Langmuir 2005, 21, 1201–1208. [Google Scholar] [CrossRef]
- Chu, Z.; Dreiss, C.A.; Feng, Y. Smart wormlike micelles. Chem. Soc. Rev. 2013, 42, 7174–7203. [Google Scholar] [CrossRef]
- Hassan, A.; Bruining, H.; Musa, T.; Chahardowli, M. The use of RFID technology to measure the compositions of diethyl ether-oil-brine mixtures in enhanced imbibition experiments. J. Pet. Sci. Eng. 2017, 156, 769–779. [Google Scholar] [CrossRef][Green Version]
- Ghosh, S.; Khatua, D.; Dey, J. Interaction between zwitterionic and anionic surfactants: spontaneous formation of zwitanionic vesicles. Langmuir 2011, 27, 5184–5192. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Pathak, A.K.; Ojha, K. Phase behaviour and thermodynamic properties of lamellar liquid crystal developed for viscoelastic surfactant based fracturing fluid. Chem. Eng. Sci. 2015, 131, 146–154. [Google Scholar] [CrossRef]
- Acharya, D.P.; Hossain, M.K.; Sakai, T.; Kunieda, H. Phase and rheological behaviour of viscoelastic wormlike micellar solutions formed in mixed nonionic surfactant systems. Phys. Chem. Chem. Phys. 2004, 6, 1627–1631. [Google Scholar] [CrossRef]
- Haas, S.; Hoffmann, H.; Thunig, C.; Hoinkis, E. Phase and aggregation behaviour of double-chain cationic surfactants from the class of N-alkyl-N-alkyl′-N,N-dimethylammonium bromide surfactants. Colloid Polym. Sci. 1999, 277, 856–867. [Google Scholar] [CrossRef]
- Martin, F.; Oxley, J. Effect of various alkaline chemicals on phase behavior of surfactant/brine/oil mixtures. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Phoenix, AZ, USA, 9–11 March 1985; Society of Petroleum Engineers: London, UK, 1985. [Google Scholar]
- Kang, W.; Mushi, S.J.; Yang, H.; Wang, P.; Hou, X. Development of smart viscoelastic surfactants and its applications in fracturing fluid: A review. J. Pet. Sci. Eng. 2020, 190, 107107. [Google Scholar] [CrossRef]
- Del Giudice, F.; Haward, S.J.; Shen, A.Q. Relaxation time of dilute polymer solutions: A microfluidic approach. J. Rheol. 2017, 61, 327–337. [Google Scholar] [CrossRef]
- Yousufi, M.M.; Elhaj, M.E.M.; Moniruzzaman, M.; Ayoub, M.A.; Nazri, A.B.M.; binti Husin, H.; bin Mohd Saaid, I. Synthesis and evaluation of Jatropha oil-based emulsified acids for matrix acidizing of carbonate rocks. J. Pet. Explor. Prod. Technol. 2019, 9, 1119–1133. [Google Scholar] [CrossRef]
- Khair, E.M.M.; Shicheng, Z.; Shanbo, M.; Mei, Z. Performance and application of new anionic D3F-AS05 viscoelastic fracturing fluid. J. Pet. Sci. Eng. 2011, 78, 131–138. [Google Scholar] [CrossRef]
- Fang, B.; Cao, D.h.; Jiang, T.q. Rheological properties of novel viscoelastic micelle systems containing anionic-nonionic dimeric surfactant. J. Cent. South Univ. Technol. 2008, 15, 176–180. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Lin, W.; Kang, Z.; You, Q. Formula optimization and rheology study of clean fracturing fluid. J. Mol. Liq. 2017, 241, 563–569. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, Z.; Dai, L.; Liu, L.; Shi, W. Introducing hydroxyl into cationic surfactants as viscoelastic surfactant fracturing fluid with high temperature resistance. Russ. J. Appl. Chem. 2016, 89, 2016–2026. [Google Scholar] [CrossRef]



| VES-Fluid | Temperature [C] | Height [mm] | Average Suspension Time [s] | Average Suspension Time [mm/s] |
|---|---|---|---|---|
| 30 mM CTAB | 30 | 240 | - | - |
| 30 mM CTAB | 90 | 240 | 54.5 | 4.37 |
| 30 mM CTAB-MA | 30 | 240 | - | - |
| 30 mM CTAB-MA | 70 | 240 | 103.4 | 2.32 |
| 30 mM CTAB-CA | 30 | 240 | - | - |
| 30 mM CTAB-CA | 70 | 240 | 143.6 | 1.67 |
| VES-Fluid | Ethanol to Fluid Ratio | Breaking Temperature [C] | Breaking Time [min] | Viscosity after Breaking [mPa.s] |
|---|---|---|---|---|
| 30 mM CTAB | 15:100 | 30 | 60 | 3.4 |
| 30 mM CTAB | 15:100 | 80 | 20 | - |
| 30 mM CTAB-MA | 15:100 | 30 | 152 | 2.5 |
| 30 mM CTAB-MA | 15:100 | 80 | 60 | 4.4 |
| 30 mM CTAB-CA | 15:100 | 30 | 120 | 3.9 |
| 30 mM CTAB-CA | 15:100 | 80 | - | 3.2 |
| VES-Fluid | Breaking Temperature [C] | Surface Tension [mN/m] | Viscosity after Breaking [mPa.s] |
|---|---|---|---|
| 30 mM CTAB | 30 | 27.4 | 0.4373 |
| 30 mM CTAB | 80 | 27.4 | 0.4373 |
| 30 mM CTAB-MA | 30 | 29.1 | 0.518 |
| 30 mM CTAB-MA | 80 | 29.1 | 0.518 |
| 30 mM CTAB-CA | 30 | 23.6 | 0.414 |
| 30 mM CTAB-CA | 80 | 23.6 | 0.414 |
| Condition | Length [mm] | Diameter [mm] | Dry Weight [g] | Porosity [%] | Perm [mD] | Pore Volume [cc] | Bulk Volume |
|---|---|---|---|---|---|---|---|
| Before VES Fluid Flooding | 75.8 | 38.1 | 187.4 | 17.4 | 189.9 | 14.8 | 85.1 |
| After VES fluid flooding | 75.8 | 38.1 | 182.9 | 19.9 | 174.7 | 16.6 | 87.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chieng, Z.H.; Mohyaldinn, M.E.; Hassan, A.M.; Bruining, H. Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid. Polymers 2020, 12, 1470. https://doi.org/10.3390/polym12071470
Chieng ZH, Mohyaldinn ME, Hassan AM, Bruining H. Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid. Polymers. 2020; 12(7):1470. https://doi.org/10.3390/polym12071470
Chicago/Turabian StyleChieng, Z. H., Mysara Eissa Mohyaldinn, Anas. M. Hassan, and Hans Bruining. 2020. "Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid" Polymers 12, no. 7: 1470. https://doi.org/10.3390/polym12071470
APA StyleChieng, Z. H., Mohyaldinn, M. E., Hassan, A. M., & Bruining, H. (2020). Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid. Polymers, 12(7), 1470. https://doi.org/10.3390/polym12071470