Preparation of RGO and Anionic Polyacrylamide Composites for Removal of Pb(II) in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO, GO/APAM, E-RGO, E-RGO/APAM
2.3. Characterization
2.4. Methylene Blue Adsorption Experiments to Determine the Specific Surface Area
2.5. Pb(II) Adsorption Experiments
3. Results and Discussion
3.1. Characterization of E-RGO/APAM Composites
3.1.1. Particle Size Analysis
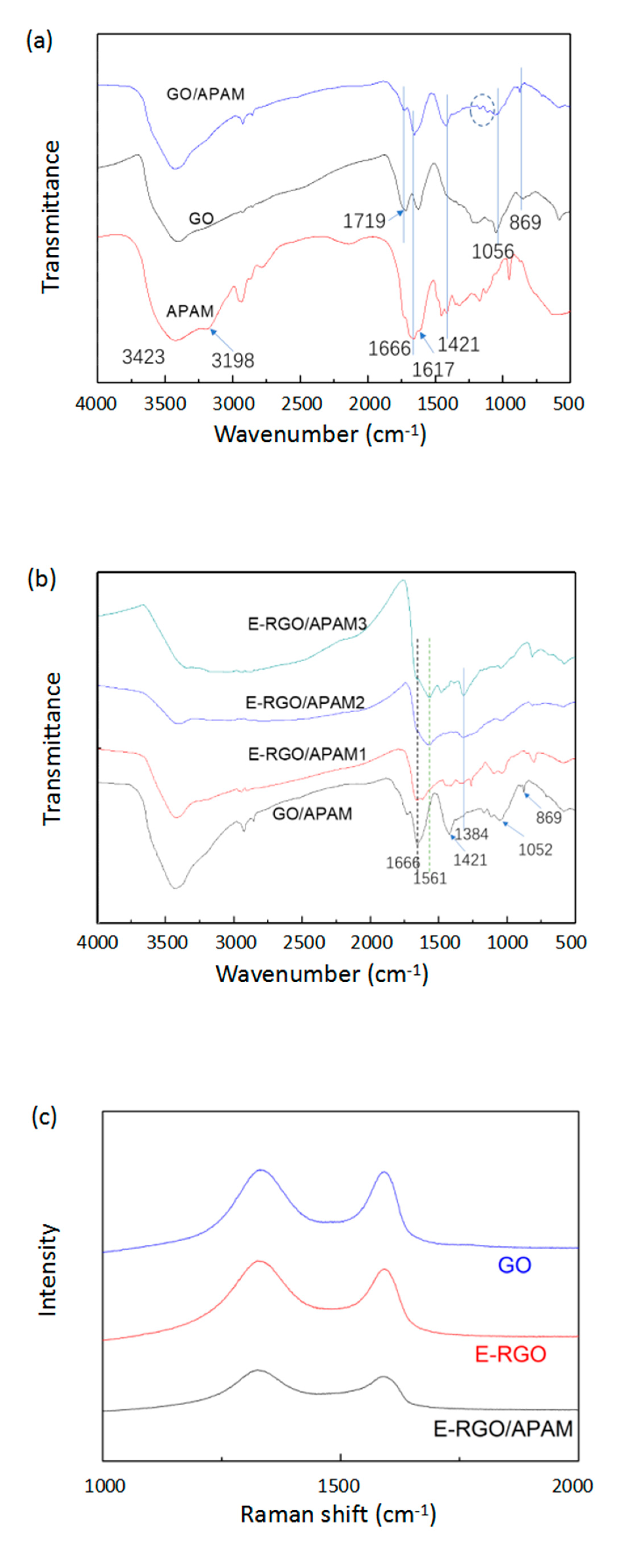
3.1.2. Analysis of Infrared Spectrum and Raman Spectrum
3.1.3. Morphology Analysis
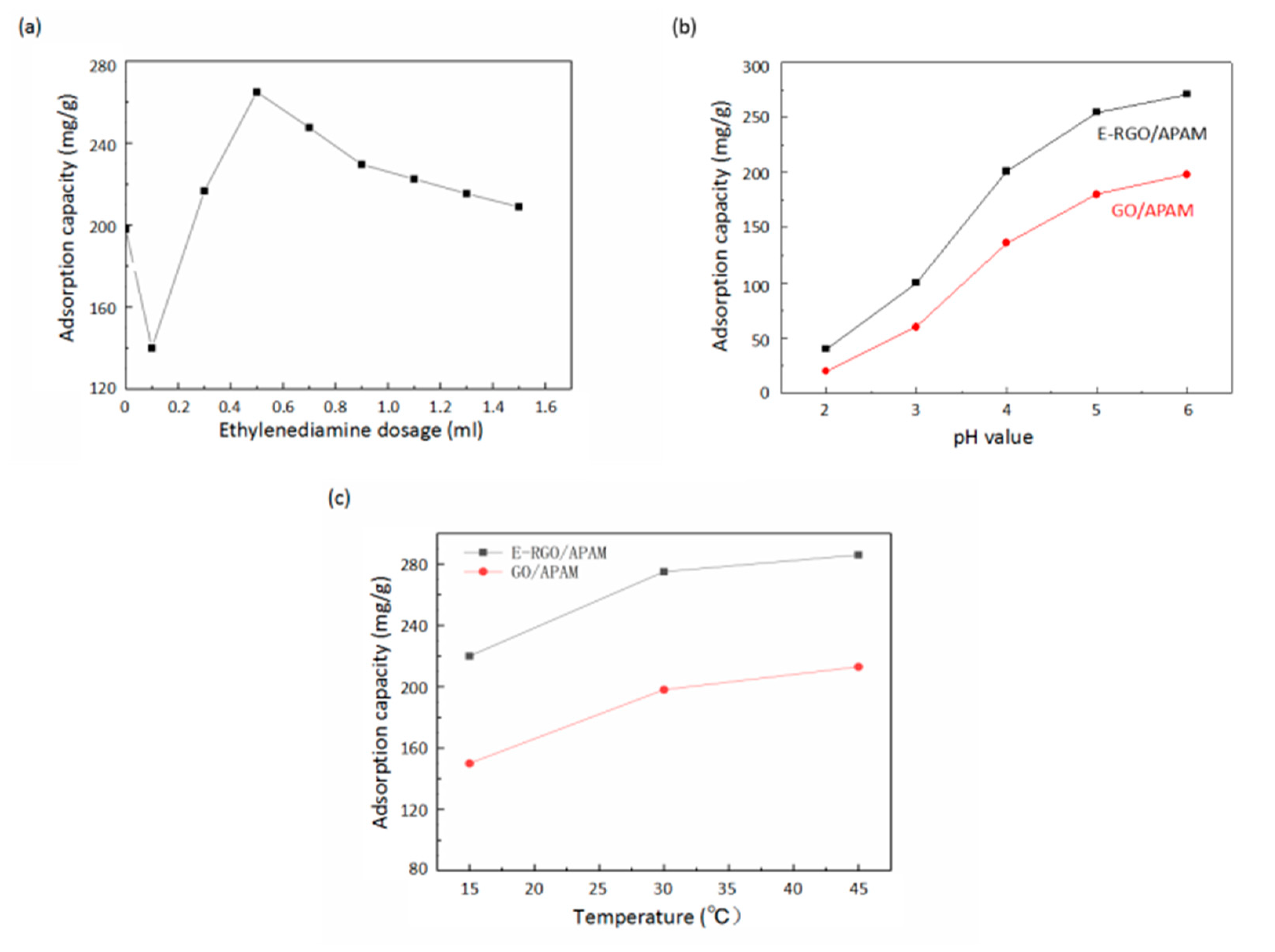
3.2. Characterization of Pb(II) Adsorption Properties of E-RGO/APAM
3.3. Adsorption Kinetics and Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dzhardimalieva, G.I.; Baimuratova, R.K.; Knerelman, E.I.; Davydova, G.I.; Kudaibergenov, S.E.; Kharissova, O.V.; Zhinzhilo, V.A.; Uflyand, I.E. Synthesis of Copper(II) Trimesinate Coordination Polymer and Its Use as a Sorbent for Organic Dyes and a Precursor for Nanostructured Material. Polymers 2020, 12, 1024. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Popescu, M.-C.; Bulgariu, L. Adsorption of Cu(II) Ions on Adsorbent Materials Obtained from Marine Red Algae Callithamnion corymbosum sp. Water 2020, 12, 372. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Bogatyrov, V.M.; Galaburda, M.; Sokolowska, Z. Study on Adsorption and Aggregation in the Mixed System of Polyacrylamide, Cu(II) Ions and Innovative Carbon-Silica Composite. Polymers 2020, 12, 961. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Zheng, X.; Lv, W.; Wang, M.; Yang, Q.H.; Kang, F. Adsorption of lead(II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 2011, 27, 7558–7562. [Google Scholar] [CrossRef] [PubMed]
- Bhatluri, K.K.; Manna, M.S.; Ghoshal, A.K.; Saha, P. Supported liquid membrane based removal of lead(II) and cadmium(II) from mixed feed: Conversion to solid waste by precipitation. J. Hazard. Mater. 2015, 299, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J. The application of graphene-based materials for the removal of heavy metals and radionuclides from water and wastewater. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1042–1105. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Erratum: “Low-Level Environmental Lead Exposure and Children’s Intellectual Function: An International Pooled Analysis”. Environ. Health Perspect. 2019, 127, 99001. [Google Scholar] [CrossRef]
- Bulgariu, L.; Bulgariu, D. Functionalized soy waste biomass—A novel environmental-friendly biosorbent for the removal of heavy metals from aqueous solution. J. Clean. Prod. 2018, 197, 875–885. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I.; Martinez, M.; Miralles, N.; Poch, J.; Serarols, J. Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from aqueous solution by olive stone waste. Sep. Purif. Technol. 2006, 50, 132–140. [Google Scholar] [CrossRef]
- Park, H.G.; Kim, T.W.; Chae, M.Y.; Yoo, I.K. Activated carbon-containing alginate adsorbent for the simultaneous removal of heavy metals and toxic organics. Process Biochem. 2007, 42, 1371–1377. [Google Scholar] [CrossRef]
- Ge, H.; Fan, X. Adsorption of Pb2+ and Cd2+ onto a Novel Activated Carbon-Chitosan Complex. Chem. Eng. Technol. 2011, 34, 1745–1752. [Google Scholar] [CrossRef]
- Yang, S.T.; Chang, Y.; Wang, H.; Liu, G.; Chen, S.; Wang, Y.; Liu, Y.; Cao, A. Folding/aggregation of graphene oxide and its application in Cu2+ removal. J Colloid Interface Sci 2010, 351, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Meng, Q.; Zhang, X.; Ma, R.; Cao, B. Green synthesis of carbon nanotube–graphene hybrid aerogels and their use as versatile agents for water purification. J. Mater. Chem. 2012, 22, 8767–8771. [Google Scholar] [CrossRef]
- Chang, H.; Wu, H. Graphene-based nanocomposites: Preparation, functionalization, and energy and environmental applications. Energy Environ. Sci. 2013, 6, 3483–3507. [Google Scholar] [CrossRef]
- Su, H.; Zhang, C.; Li, X.; Wu, L.; Chen, Y. Aggregation prevention: Reduction of graphene oxide in mixed medium of alkylphenol polyoxyethylene (7) ether and 2-methoxyethanol. RSC Adv. 2018, 8, 39140–39148. [Google Scholar] [CrossRef]
- Li, S.; Yang, P.; Liu, X.; Zhang, J.; Xie, W.; Wang, C.; Liu, C.; Guo, Z. Graphene oxide based dopamine mussel-like cross-linked polyethylene imine nanocomposite coating with enhanced hexavalent uranium adsorption. J. Mater. Chem. A 2019, 7, 16902–16911. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Tawfik, A.M.; Ezzat, A.O. Application of Super-Amphiphilic Silica-Nanogel Composites for Fast Removal of Water Pollutants. Molecules 2016, 21, 1392. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Boggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption behavior of EDTA-graphene oxide for Pb (II) removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Huang, Z. Magnetic dithiocarbamate functionalized reduced graphene oxide for the removal of Cu(II), Cd(II), Pb(II), and Hg(II) ions from aqueous solution: Synthesis, adsorption, and regeneration. Chemosphere 2018, 209, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Abu Haija, M. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H.; Wang, C.; Zhang, J.; Zhang, Z. Adsorption of hexavalent chromium from aqueous solutions by graphene modified with cetyltrimethylammonium bromide. J. Colloid Interface Sci. 2013, 394, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, A.; Yang, J.; Ma, F.; Wu, D.; Xing, J.; Zhou, X.; Zhao, D. Synergetic effects and flocculation behavior of anionic polyacrylamide and extracellular polymeric substrates extracted from Klebsiella sp. J1 on improving soluble cadmium removal. Bioresour. Technol. 2015, 175, 34–41. [Google Scholar] [CrossRef]
- Wu, L.-L.; Deng, F.-R.; Zhang, C.-C. Preparation via flocculation of reduced graphene oxide/cationic polyacrylamide composites and their electrochemical properties. RSC Adv. 2016, 6, 103944–103947. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, H.; Gao, B.; Zhang, S.; Zhao, C.; Zhou, Y.; Xu, B. Fabricating an anionic polyacrylamide (APAM) with an anionic block structure for high turbidity water separation and purification. RSC Adv. 2017, 7, 28918–28930. [Google Scholar] [CrossRef]
- Gu, S.; Wang, L.; Mao, X.; Yang, L.; Wang, C. Selective Adsorption of Pb(II) from Aqueous Solution by Triethylenetetramine-Grafted Polyacrylamide/Vermiculite. Materials 2018, 11, 514. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Shen, L.; Xiao, Y. A new approach to fabricate graphene nanosheets in organic medium: Combination of reduction and dispersion. J. Mater. Chem. 2010, 20, 1722–1727. [Google Scholar] [CrossRef]
- Feng, L.; Zheng, H.; Wang, Y.; Zhang, S.; Xu, B. Ultrasonic-template technology inducing and regulating cationic microblocks in CPAM: Characterization, mechanism and sludge flocculation performance. RSC Adv. 2017, 7, 23444–23456. [Google Scholar] [CrossRef]
- Guo, L.; Ye, P.; Wang, J.; Fu, F.; Wu, Z. Three-dimensional Fe3O4-graphene macroscopic composites for arsenic and arsenate removal. J. Hazard. Mater. 2015, 298, 28–35. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Li, J.; Zhao, G.; Ren, X.; Wang, X. Graphene oxide-iron oxide and reduced graphene oxide-iron oxide hybrid materials for the removal of organic and inorganic pollutants. RSC Adv. 2012, 2, 8821–8826. [Google Scholar] [CrossRef]



| Pseudo Second Order Kinetic Equation | |||
|---|---|---|---|
| Qe | k2 | R2 | |
| E-RGO/APAM | 290.6 mg/g | 0.0017 | 0.9996 |
| GO/APAM | 168.3 mg/g | 0.0024 | 0.9982 |
| Langmuir Adsorption Model | Freundlich Adsorption Model | ||||
|---|---|---|---|---|---|
| 1/n | |||||
| 400.8 mg/g | 0.030 | 0.984 | 0.3187 | 69.55 | 0.948 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Li, M.; Li, M.; Sun, Q.; Zhang, C. Preparation of RGO and Anionic Polyacrylamide Composites for Removal of Pb(II) in Aqueous Solution. Polymers 2020, 12, 1426. https://doi.org/10.3390/polym12061426
Wu L, Li M, Li M, Sun Q, Zhang C. Preparation of RGO and Anionic Polyacrylamide Composites for Removal of Pb(II) in Aqueous Solution. Polymers. 2020; 12(6):1426. https://doi.org/10.3390/polym12061426
Chicago/Turabian StyleWu, Lili, Mengke Li, Mu Li, Qiyue Sun, and Chaocan Zhang. 2020. "Preparation of RGO and Anionic Polyacrylamide Composites for Removal of Pb(II) in Aqueous Solution" Polymers 12, no. 6: 1426. https://doi.org/10.3390/polym12061426
APA StyleWu, L., Li, M., Li, M., Sun, Q., & Zhang, C. (2020). Preparation of RGO and Anionic Polyacrylamide Composites for Removal of Pb(II) in Aqueous Solution. Polymers, 12(6), 1426. https://doi.org/10.3390/polym12061426






