Towards a Deeper Understanding of Creep and Physical Aging Behavior of the Emulsion Polymer Isocyanate
Abstract
1. Introduction
2. Experimental
2.1. Materials and Samples
- (1)
- Aqueous polymer emulsion (EPI main component): a vinyl acetate based tri-copolymer latex (xPVAc, EP203), containing approximately 30% (wt.) of CaCO3, as well as 10% (wt.) of PVOH as a protective colloid and the main provider of hydroxyl groups. The aqueous vinyl latex was prepared by Shanghai Junyan Chemical Material Co. Ltd. (Shanghai, China) and was used as received. It had a solid content of 51.0%, viscosity of 7100 mPa·s (at 25 °C), and pH of 6.5.
- (2)
- Polymer isocyanate (EPI crosslinkers): EMDI (Rubinate 9259) and p-MDI (Rubinate 5005), of which characteristics as listed in Table 1, were synthesized by Huntsman Polyurethanes (China) Ltd. and also were employed as received.
2.2. Test Methods
2.2.1. Uniaxial Tensile Creep
2.2.2. PALS Measurements
3. Results and Discussion
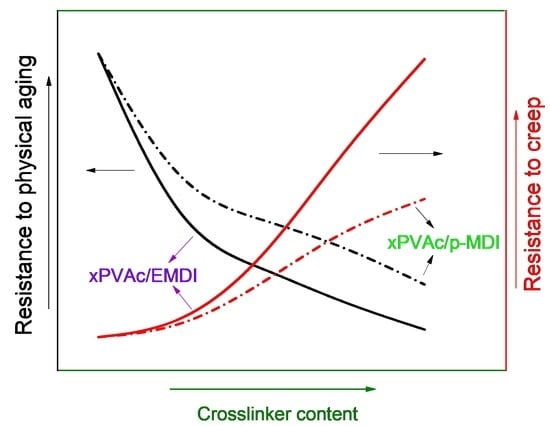
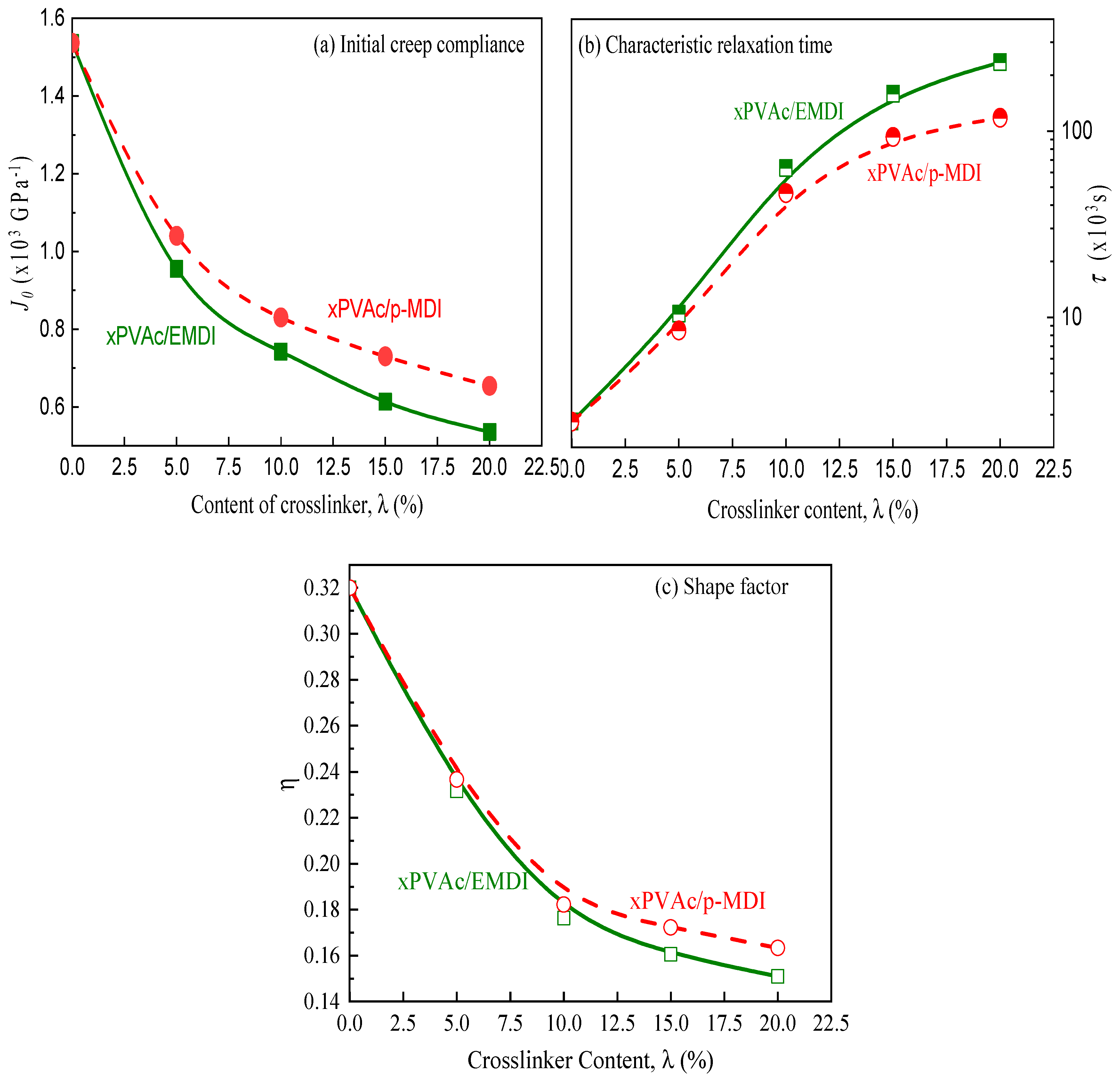
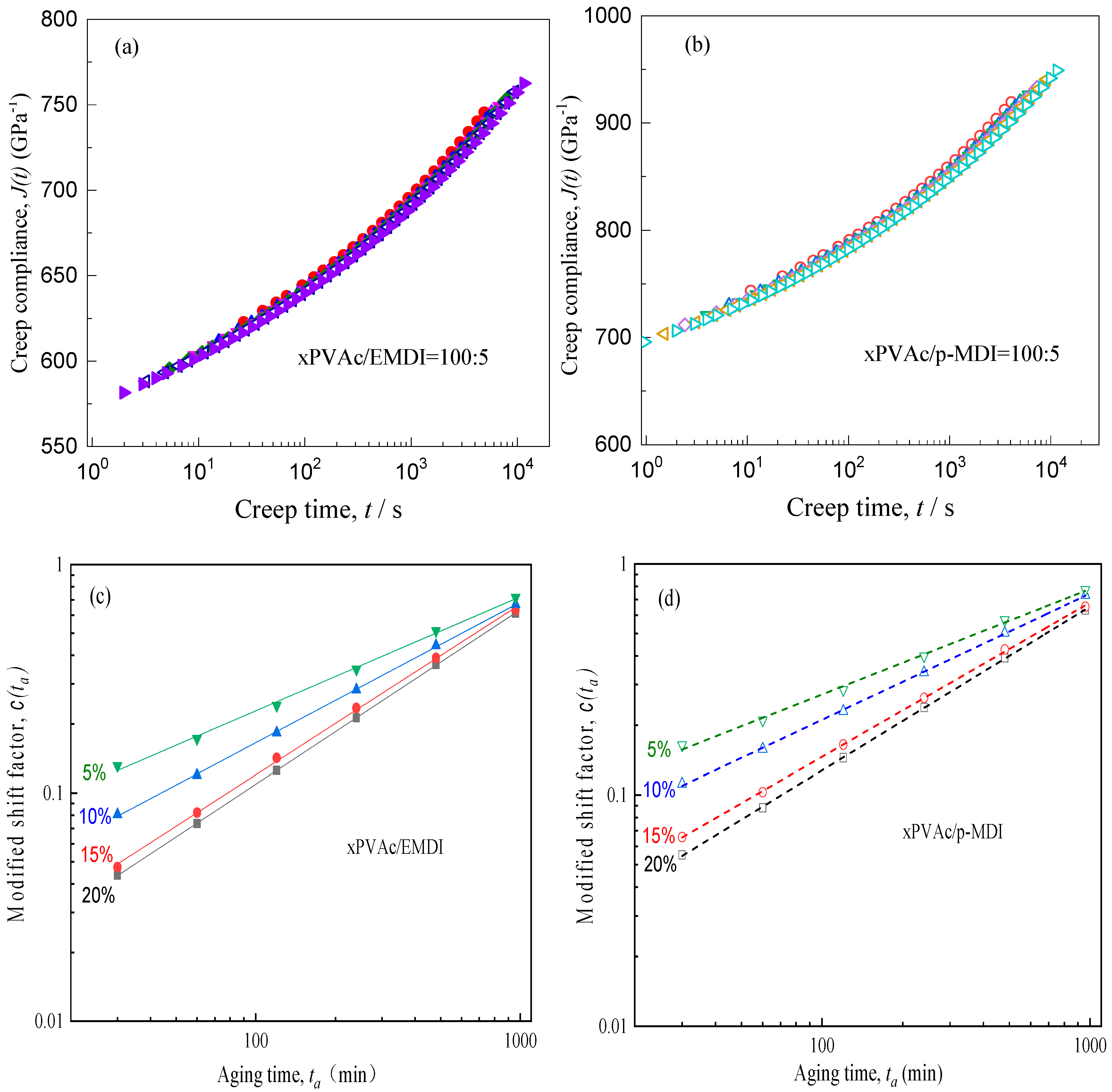
3.1. Crosslinker Dependence of Creep Characteristics
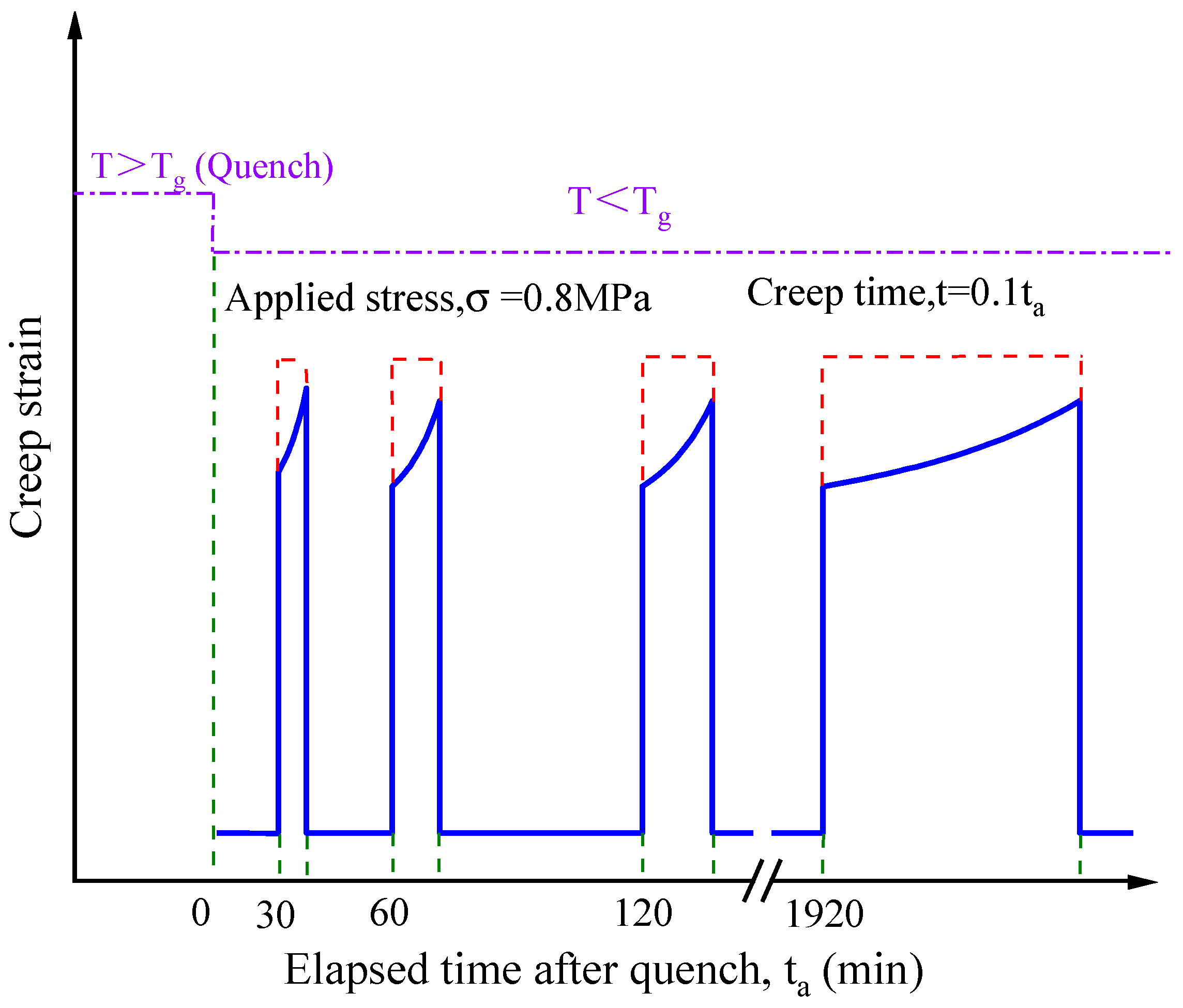
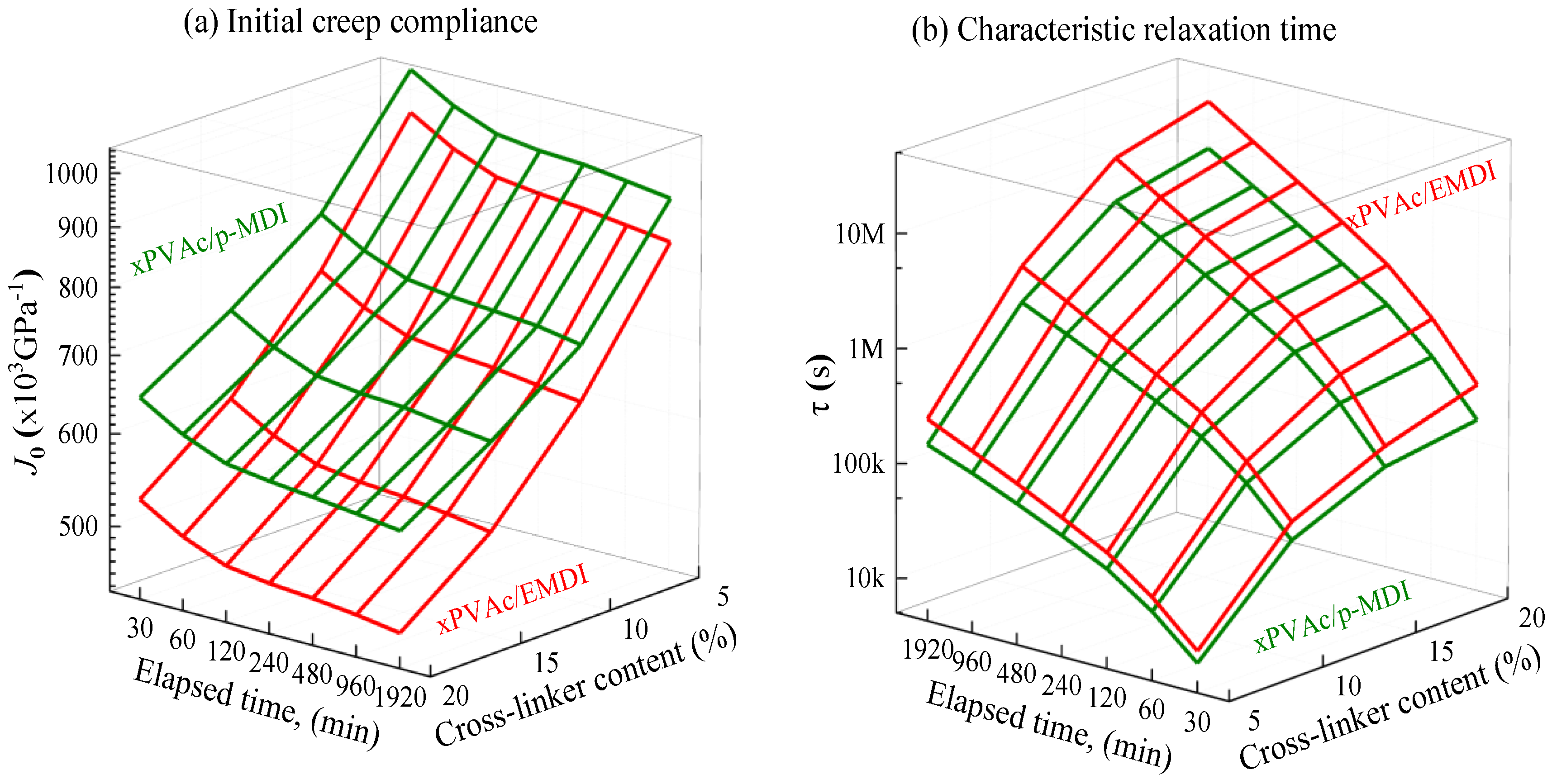
3.2. Effect of Physical Aging on Creep Behaviour

3.3. Relaxation Mechanism during Physical Aging
4. Conclusions
- (1)
- The addition of isocyanate crosslinker can significantly enhance the creep resistance of the waterborne polymer xPVAc, and the positive effect can boost with increasing isocyanate content.
- (2)
- All of the cured EPI film with different crosslinkers exhibit the pronounced physical aging phenomena. However, the relaxation rate would be greatly accelerated by the introduction of the higher amount of EMD or p-MDI.
- (3)
- Physical aging contributed to the decrease in elastic creep compliance and shape factor, while an increase in retardation time. Accordingly, the ideal momentary creep master curve can be obtained using modified horizontal shift for aging EPI film.
- (4)
- The size and number of free volume cavities in cured EPI adhesive layers are highly dependent upon the crosslinker concentration and elapsed time, and the mechanism of vacancy diffusion controls the relaxation behavior of glassy EPI films.
- (5)
- As aqueous emulsified isocyanate has the stronger crosslinking capacity with the functional groups available in/on the main component than the unmodified polymeric isocyanate, xPVAc/EMDI films are much better resistant to creep performance compared to xPVAc/p-MDI at the same crosslinker loading. However, it exhibits the worse resistance of physical aging, which may be credited to the higher fractional free volume in the EPI film.
Author Contributions
Funding
Conflicts of Interest
References
- Grøstad, K.; Pedersen, A. Emulsion Polymer Isocyanates as Wood Adhesive: A Review. J. Adhes. Sci. Technol. 2010, 24, 1357–1381. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Zhao, J.; Li, J. Investigation of Adhesion Performance of Aqueous Polymer Latex Modified by Polymeric Methylene Diisocyanate. J. Adhes. 2006, 82, 93–114. [Google Scholar] [CrossRef]
- Sakurada, S.; Miyazaki, H.; Hattori, T.; Shiraishi, M.; Inoue, T. Adhesive Composition Consisting of Polyvinylalcohol Solution or Polyvinylacetate Latex Modified with Hydrophobic Solution of Isocyanate Compound. U.S. Patent 3,931,088, 6 January 1976. [Google Scholar]
- Qiao, L.; Easteal, A.J.; Bolt, C.J.; Coveny, P.K.; Franich, R.A. Thermomechanical analysis and performance tests of some EPI wood adhesives. Pigment Resin Technol. 2000, 29, 229–237. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, W.; Liu, H.; Zhao, J. Investigation on fracture performance of aqueous polymer isocyanates in terms of energy release rate. J. Adhes. Sci. Technol. 2006, 20, 161–174. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Zhao, Z.; Guo, M. Novel whey protein-based aqueous polymer-isocyanate adhesive for glulam. J. Appl. Polym. Sci. 2010, 120, 220–225. [Google Scholar] [CrossRef]
- Krystofiak, T.; Proszyk, S.; Jozwiak, M. Studies of some properties of EPI adhesives. For. Wood Technol. 2003, 53, 214–217. [Google Scholar]
- Bastani, A.; Adamopoulos, S.; Koddenberg, T.; Militz, H. Study of adhesive bondlines in modified wood with fluorescence microscopy and X-ray micro-computed tomography. Int. J. Adhes. Adhes. 2016, 68, 351–358. [Google Scholar] [CrossRef]
- Taki, K.; Tomita, B.; Mizumachi, H. Studies on aqueous vinyl polymer solution-isocyanate adhesives. Part I. Mechanical properties of base polymers and bond strength over a wide temperature range. Mokuzai Gakkaishi 1982, 28, 143–149. [Google Scholar]
- Taki, K.; Tomita, B.; Mizumachi, H. Studies on aqueous vinyl polymer solution-isocyanate adhesives. Part II. Dependence of mechanical properties and bond strength on the concentration of crosslinks in cured adhesives over a wide temperature range. Mokuzai Gakkaishi 1982, 28, 150–155. [Google Scholar]
- Umemura, K.; Takahashi, A.; Kawai, S. Durability of isocyanate resin adhesives for wood I: Thermal properties of isocyanate resin cured with water. J. Wood Sci. 1998, 44, 204–210. [Google Scholar] [CrossRef]
- Guo, J.; Hu, H.; Zhang, K.; He, Y.; Guo, X. Revealing the Mechanical Properties of Emulsion Polymer Isocyanate Film in Humid Environments. Polymers 2018, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Hori, N.; Takemura, A. Effect of postcure conditions on the dynamic mechanical behavior of water-based polymer-isocyanate adhesive for wood. J. Wood Sci. 2008, 54, 377–382. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, H.; Li, S.; He, Y.; Guo, J. Effect of sodium dodecyl sulfate (SDS) on mechanical performance of polyvinyl-acetate-based emulsion polymer isocyanate. Int. J. Adhes. Adhes. 2020, 98, 102539. [Google Scholar] [CrossRef]
- Rindler, A.; Pöll, C.; Hansmann, C.; Müller, U.; Konnerth, J. Moisture related elastic and viscoelastic behaviour of wood adhesives by means of in-situ nanoindentation. Int. J. Adhes. Adhes. 2018, 85, 123–129. [Google Scholar] [CrossRef]
- Aicher, S.; Christian, Z.; Stapf, G. Creep Testing of One-Component Polyurethane and Emulsion Polymer Isocyanate Adhesives for Structural Timber Bonding. For. Prod. J. 2015, 65, 60–71. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Elsevier Scientific Publishing Company: New York, NY, USA, 1978. [Google Scholar]
- Zhang, X.; Hu, H.; Guo, M. Relaxation of a hydrophilic polymer induced by moisture desorption through the glass transition. Phys. Chem. Chem. Phys. 2015, 17, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Dorkenoo, K.D.; Pfromm, P.H. Experimental evidence and theoretical analysis of physical aging in thin and thick amorphous glassy polymer films. J. Polym. Sci. Pol. Phys. 1999, 37, 2239–2251. [Google Scholar] [CrossRef]
- Rittigstein, P.; Torkelson, J.M. Polymer–nanoparticle interfacial interactions in polymer nanocomposites: Confinement effects on glass transition temperature and suppression of physical aging. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2935–2943. [Google Scholar] [CrossRef]
- Cangialosi, D.; Boucher, V.; Alegria, A.; Colmenero, J. Enhanced physical aging of polymer nanocomposites: The key role of the area to volume ratio. Polymer 2012, 53, 1362–1372. [Google Scholar] [CrossRef]
- Soma, H.; Nishitsuji, S.; Inoue, T. Molecular weight dependence in a relaxation phenomenon at glassy state: Physical aging of polycarbonate. Polymer 2012, 53, 895–896. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; He, Y. A Coupled Thermodynamic Model for Transport Properties of Thin Films during Physical Aging. Polymers 2019, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koros, W.; Paul, D.R. Effects of CO2 exposure and physical aging on the gas permeability of thin 6FDA-based polyimide membranes—Part 2. with crosslinking. J. Membr. Sci. 2006, 282, 32–43. [Google Scholar] [CrossRef]
- Flory, A.; McKenna, G.B. Physical aging behavior of the normal force and torque in polymer glasses. Mech. Time Depend. Mater. 2010, 14, 347–357. [Google Scholar] [CrossRef]
- Hutchinson, J.M. Physical aging of polymers. Prog. Polym. Sci. 1995, 20, 703–760. [Google Scholar] [CrossRef]
- Cangialosi, D.; Boucher, V.; Alegria, A.; Colmenero, J. Physical aging in polymers and polymer nanocomposites: Recent results and open questions. Soft Matter 2013, 9, 8619–8630. [Google Scholar] [CrossRef]
- Ling, Z.; Omura, Y.; Hori, N.; Iwata, T.; Takemura, A. In-situ chemical structure analysis of aqueous vinyl polymer solution-isocyanate adhesive in post-cure process by using Fourier transform near infrared spectroscopy. Int. J. Adhes. Adhes. 2018, 81, 56–64. [Google Scholar] [CrossRef]
- Kirkegaard, P.; Pederson, N.J.; Eldrup, M. PATFIT-88: A Date-Processing System for Positron Annihilation Spectra on Mainframe and Personal Computers; Ris ϕ National Laboratory: Roskilde, Denmark, 1989. [Google Scholar]
- Li, X.S.; Boyce, M.C. On the measurement of structural relaxation in polymers using positron annihilation lifetime spectroscopy. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 869–873. [Google Scholar] [CrossRef]
- Cangialosi, D.; Schut, H.; Wübbenhorst, M.; Van Turnhout, J.; Van Veen, A. Accumulation of charges in polycarbonate due to positron irradiation. Radiat. Phys. Chem. 2003, 68, 507–510. [Google Scholar] [CrossRef]
- Curro, J.G.; Lagasse, R.R.; Simha, R. Diffusion model for volume recovery in glasses. Macromolecules 1982, 15, 1621–1626. [Google Scholar] [CrossRef]
- Thornton, A.W.; Hill, A.J.; Nairn, K.M.; Hill, J.M. Predicting particle transport through an aging polymer using vacancy diffusion. Curr. Appl. Phys. 2008, 8, 501–503. [Google Scholar] [CrossRef]
- McCaig, M.; Paul, D.; Barlow, J. Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical agingPart II. Mathematical model. Polymer 2000, 41, 639–648. [Google Scholar] [CrossRef]
- Cangialosi, D.; Boucher, V.M.; Alegria, A.; Colmenero, J. Free volume holes diffusion to describe physical aging in poly (mehtyl methacrylate)/silica nanocomposites. J. Chem. Phys. 2011, 135, 014901. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, A.K. Studies in Newtonian Flow. II. The Dependence of the viscosity of liquids on free-Space. J. Appl. Phys. 1951, 22, 1471. [Google Scholar] [CrossRef]





| Crosslinker | Solid Content (%) | NCO (%) | Functionality | Modification Method |
|---|---|---|---|---|
| Rubinate 9259 | 100 | 30.6 | 2.7 | Aqueous emulsifiable |
| Rubinate 5005 | 100 | 30.5–32.5 | 2.6–2.7 | Standard industrial |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Fang, X.; He, Y.; Hu, H. Towards a Deeper Understanding of Creep and Physical Aging Behavior of the Emulsion Polymer Isocyanate. Polymers 2020, 12, 1425. https://doi.org/10.3390/polym12061425
Zhou S, Fang X, He Y, Hu H. Towards a Deeper Understanding of Creep and Physical Aging Behavior of the Emulsion Polymer Isocyanate. Polymers. 2020; 12(6):1425. https://doi.org/10.3390/polym12061425
Chicago/Turabian StyleZhou, Shihao, Xuansheng Fang, Yaolong He, and Hongjiu Hu. 2020. "Towards a Deeper Understanding of Creep and Physical Aging Behavior of the Emulsion Polymer Isocyanate" Polymers 12, no. 6: 1425. https://doi.org/10.3390/polym12061425
APA StyleZhou, S., Fang, X., He, Y., & Hu, H. (2020). Towards a Deeper Understanding of Creep and Physical Aging Behavior of the Emulsion Polymer Isocyanate. Polymers, 12(6), 1425. https://doi.org/10.3390/polym12061425