Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing
Abstract
1. Introduction
2. Materials and Methods
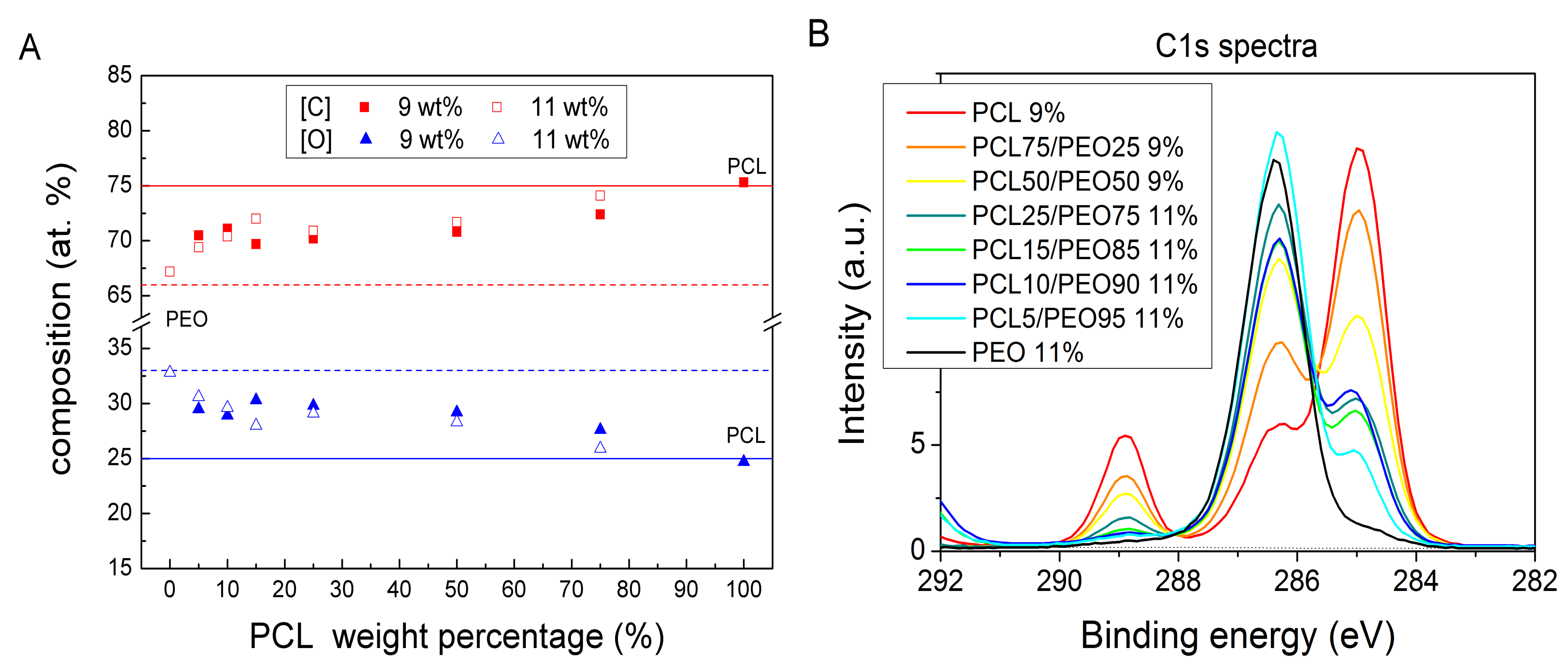
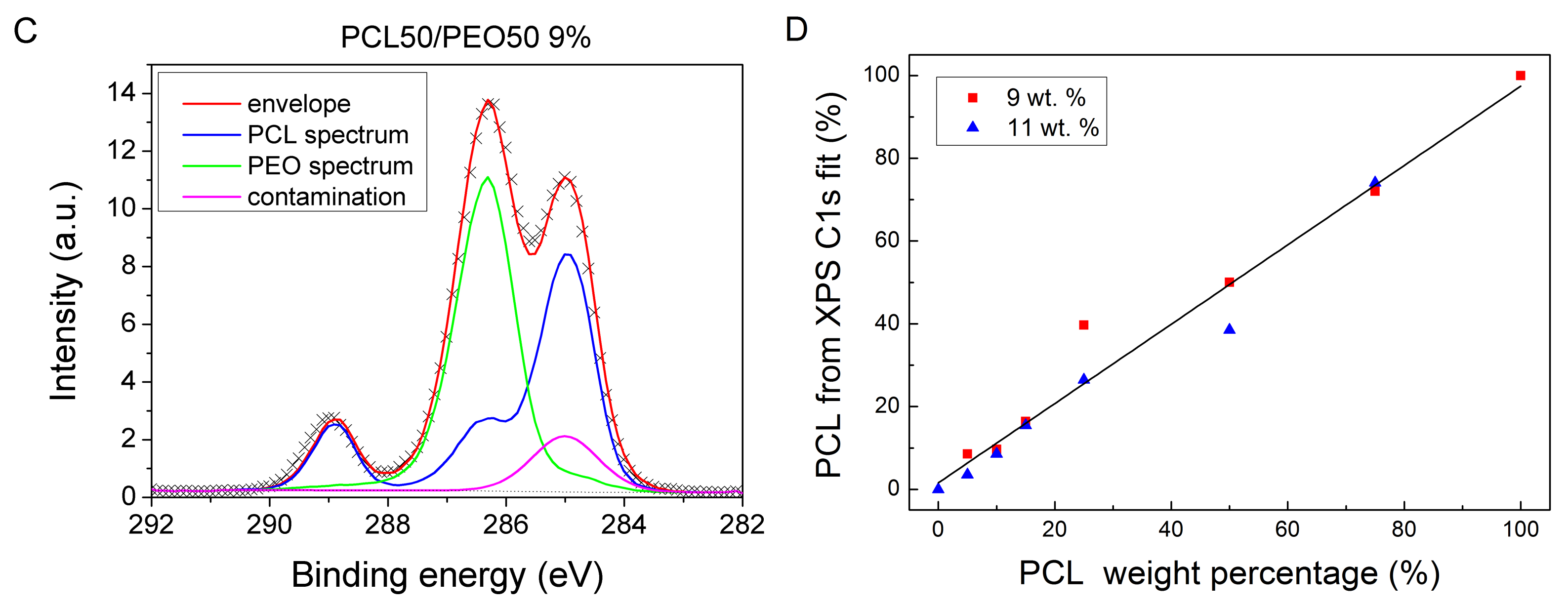
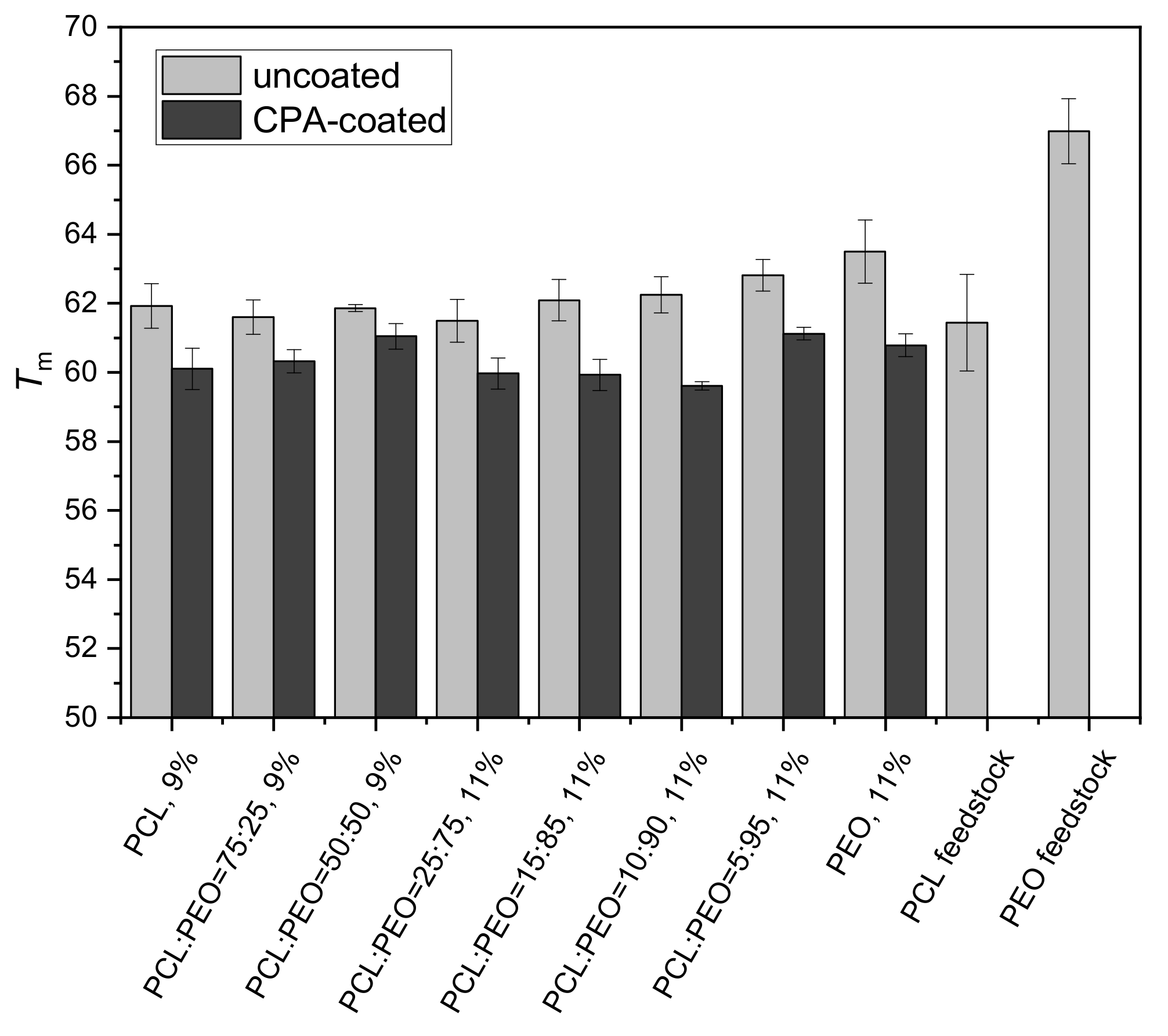
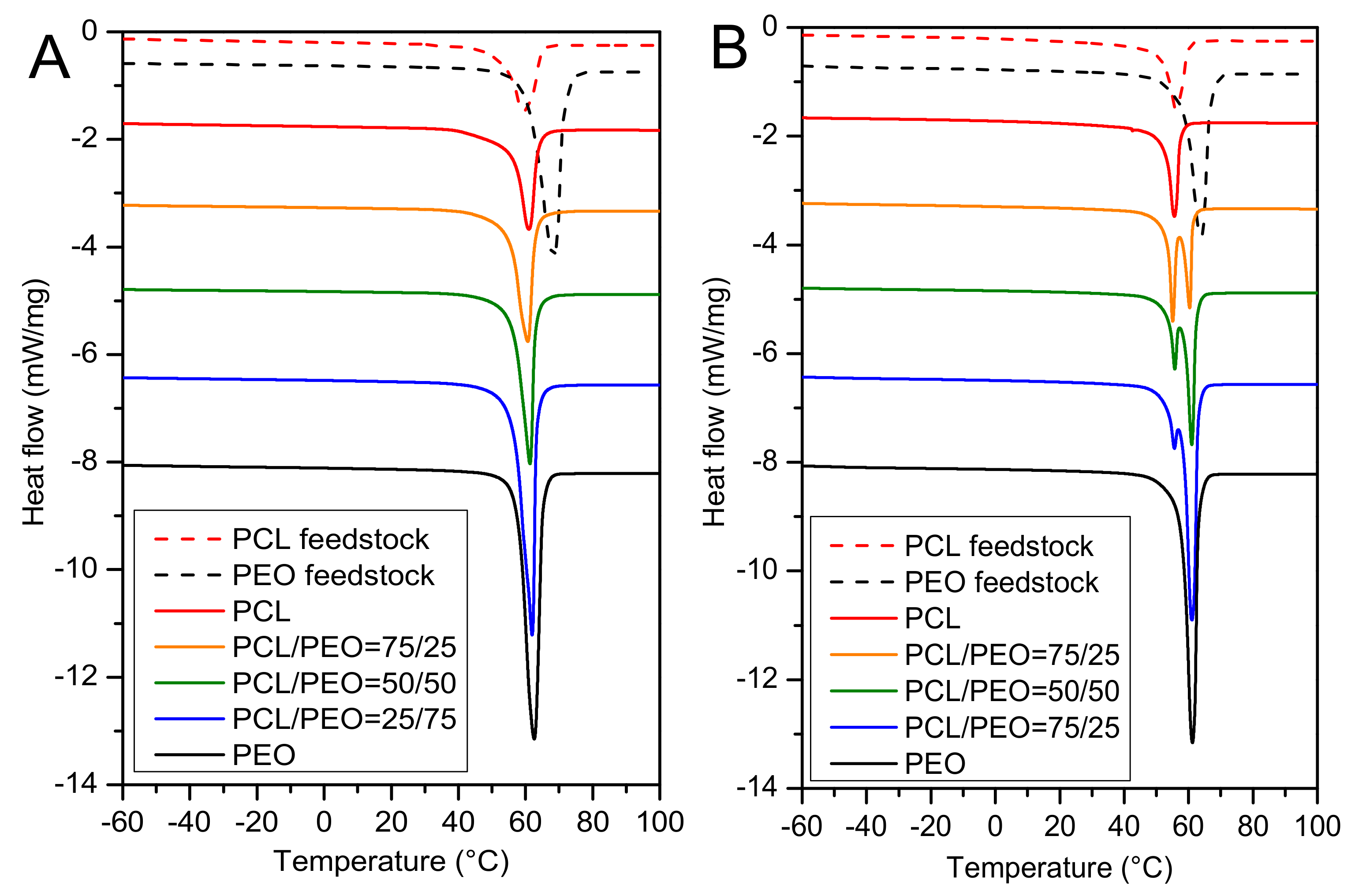
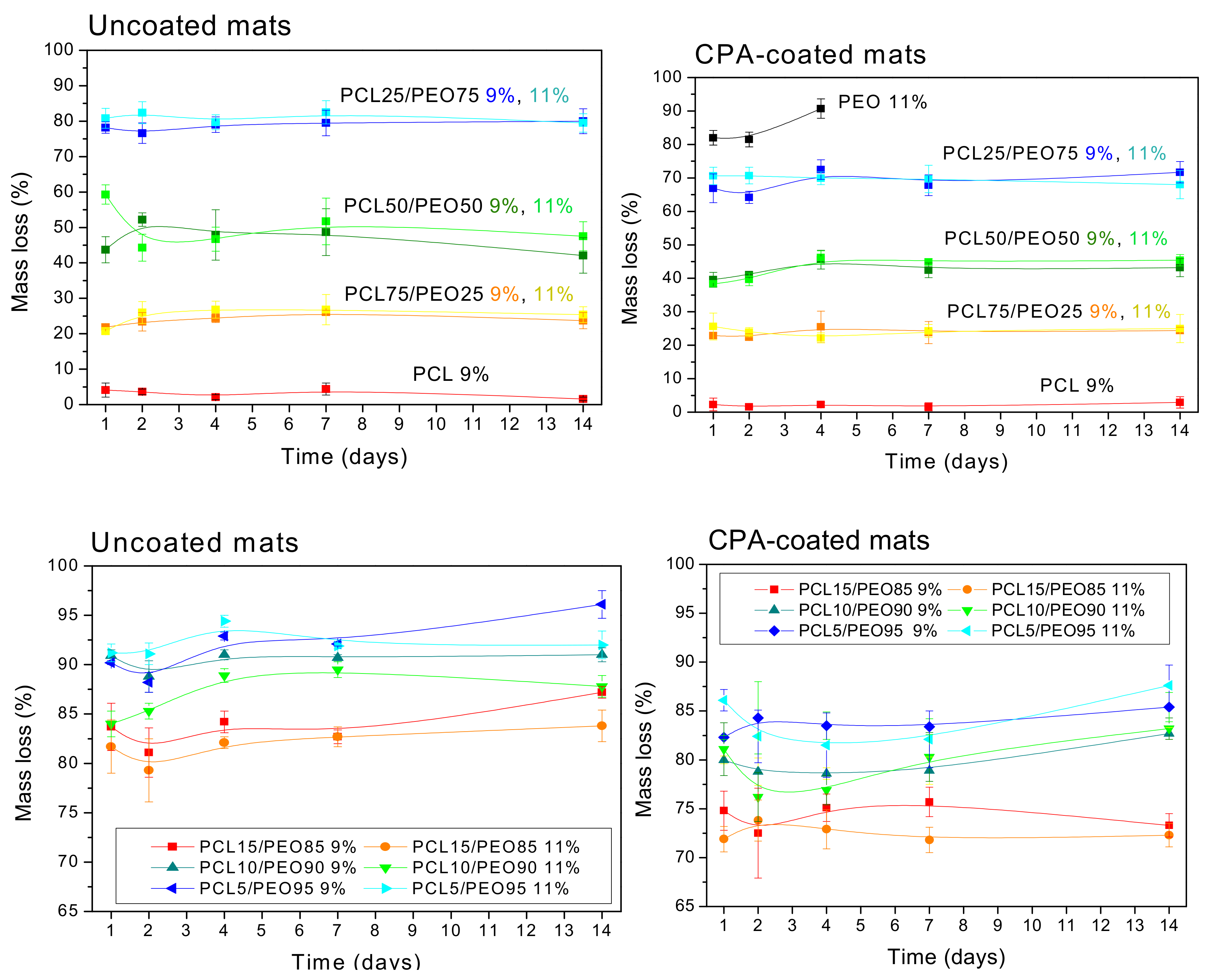
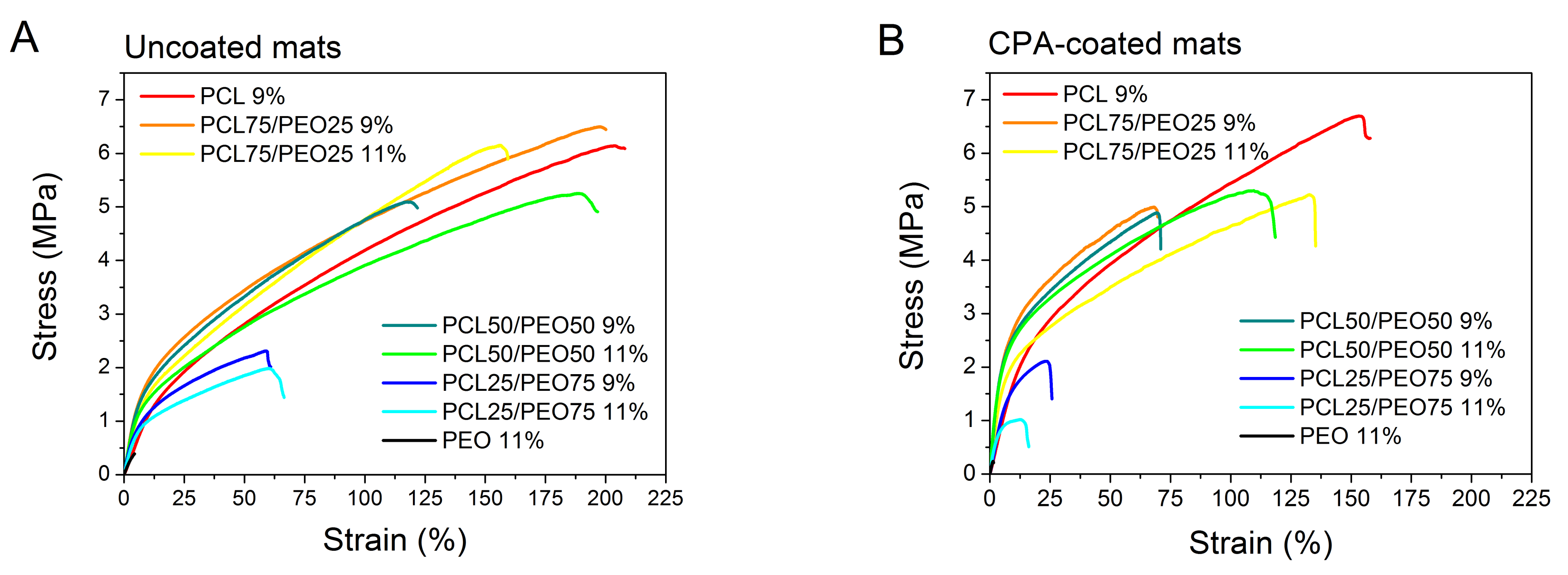
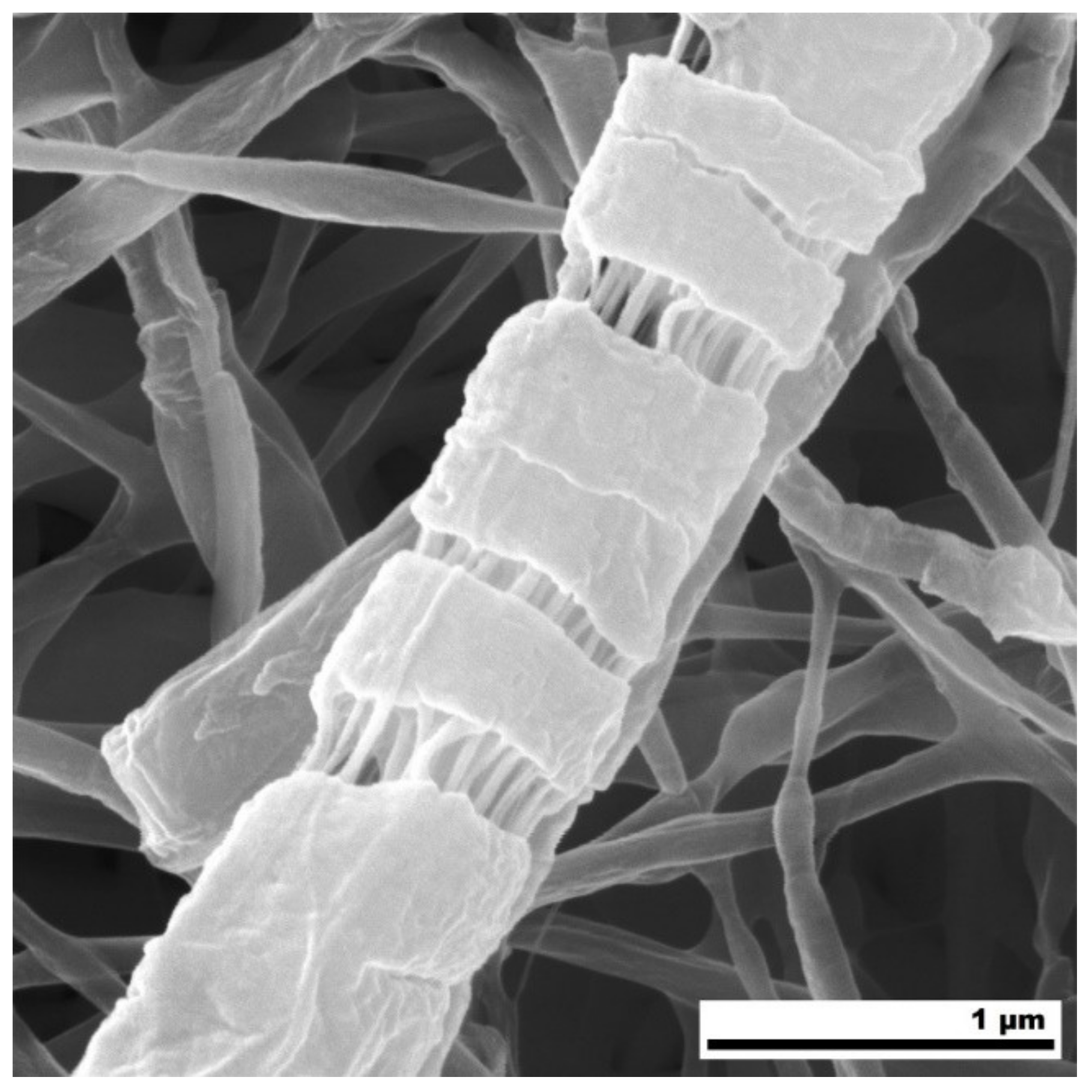
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
References
- Shang, L.; Yu, Y.; Liu, Y.; Chen, Z.; Kong, T.; Zhao, Y. Spinning and Applications of Bioinspired Fiber Systems. ACS Nano 2019, 13, 2749–2772. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Yin, C.; Tu, H.; Jiang, S.; Wang, Q.; Zhou, X.; Xing, X.; Xie, C.; Shi, X.; Du, Y.; et al. Controlled Co-delivery of Growth Factors through Layer-by-Layer Assembly of Core–Shell Nanofibers for Improving Bone Regeneration. ACS Nano 2019, 13, 6372–6382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. 2004, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luo, J.; Huang, X.; Lin, L.; Wang, L.; Hu, M.; Tang, L.; Xue, H.; Gao, J.-F.; Mai, Y.-W. A highly stretchable, super-hydrophobic strain sensor based on polydopamine and graphene reinforced nanofiber composite for human motion monitoring. Compos. Part B Eng. 2020, 181, 107580. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Bechelany, M.; Pal, K.; Rahier, H.; Uludag, H.; Kim, I.S.; Bechelany, M. Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Appl. Mater. Today 2019, 17, 1–35. [Google Scholar] [CrossRef]
- Sell, S.A.; Barnes, C.; Smith, M.; McClure, M.; Madurantakam, P.; Grant, J.; McManus, M.; Bowlin, G.L. Extracellular matrix regenerated: Tissue engineering via electrospun biomimetic nanofibers. Polym. Int. 2007, 56, 1349–1360. [Google Scholar] [CrossRef]
- Repanas, A.; Andriopoulou, S.; Glasmacher, B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J. Drug Deliv. Sci. Technol. 2016, 31, 137–146. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Xun, X.; Zhang, W.; Xu, Y.; Gu, D. Three-Dimensional Porous Scaffolds with Biomimetic Microarchitecture and Bioactivity for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 36359–36370. [Google Scholar] [CrossRef]
- Miszuk, J.M.; Xu, T.; Yao, Q.; Fang, F.; Childs, J.D.; Hong, Z.; Tao, J.; Fong, H.; Sun, H. Functionalization of PCL-3D electrospun nanofibrous scaffolds for improved BMP2-induced bone formation. Appl. Mater. Today 2018, 10, 194–202. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Kumar, K.S.; Rajini, N.; Siengchin, S.; Ayrilmis, N.; Rajulu, A.V. A comprehensive review of electrospun nanofibers: Food and packaging perspective. Compos. Part B Eng. 2019, 175, 107074. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core-Shell Fibers: Design, Roles, and Controllable Release Strategies in Tissue Engineering and Drug Delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef]
- Wang, M.; Hai, T.; Feng, Z.; Yu, D.-G.; Yang, Y.; Bligh, S.A. The Relationships between the Working Fluids, Process Characteristics and Products from the Modified Coaxial Electrospinning of Zein. Polymers 2019, 11, 1287. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, W.; Yang, Y.; Wang, K.; Yu, D.-G. From Taylor cone to solid nanofiber in tri-axial electrospinning: Size relationships. Results Phys. 2019, 15, 102770. [Google Scholar] [CrossRef]
- Wang, K.; Wang, P.; Wang, M.; Yu, D.-G.; Wan, F.; Bligh, S.W.A. Comparative study of electrospun crystal-based and composite-based drug nano depots. Mater. Sci. Eng. C 2020, 113, 110988. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Yu, D.-G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef]
- Yu, D.-G.; Wang, M.; Li, X.; Liu, X.; Zhu, L.-M.; Bligh, S.W.A. Multifluid electrospinning for the generation of complex nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1601. [Google Scholar] [CrossRef]
- Wang, K.; Wen, H.-F.; Yu, D.-G.; Yang, Y.; Zhang, D. Electrosprayed hydrophilic nanocomposites coated with shellac for colon-specific delayed drug delivery. Mater. Des. 2018, 143, 248–255. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H. Conductive Bicomponent Fibers Containing Polyaniline Produced via Side-by-Side Electrospinning. Polymers 2019, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, J.; Zheng, X.; Wang, M.; Liu, Y.; Yu, D.-G. A nanofiber-based drug depot with high drug loading for sustained release. Int. J. Pharm. 2020, 583, 119397. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Wang, M.; Zhang, F.; Liu, Y.; Liu, X.; Yu, D.-G.; Shen, H. Sheath-separate-core nanocomposites fabricated using a trifluid electrospinning. Mater. Des. 2020, 192, 108782. [Google Scholar] [CrossRef]
- Wang, M.; Wang, K.; Yang, Y.; Liu, Y.; Yu, D.-G. Electrospun Environment Remediation Nanofibers Using Unspinnable Liquids as the Sheath Fluids: A Review. Polymers 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Stevenson, A.T.; Jankus, D.J.; Tarshis, M.A.; Whittington, A.; Stevenson, J.A.T. The correlation between gelatin macroscale differences and nanoparticle properties: Providing insight into biopolymer variability. Nanoscale 2018, 10, 10094–10108. [Google Scholar] [CrossRef]
- Miroshnichenko, S.; Timofeeva, V.; Permyakova, E.; Ershov, S.; Kiryukhantsev-Korneev, F.V.; Dvořaková, E.; Shtansky, D.V.; Zajíčková, L.; Solovieva, A.; Manakhov, A.; et al. Plasma-Coated Polycaprolactone Nanofibers with Covalently Bonded Platelet-Rich Plasma Enhance Adhesion and Growth of Human Fibroblasts. Nanomaterials 2019, 9, 637. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun Scaffolds—A review. J. Mater. Chem. 2011, 21, 9419. [Google Scholar] [CrossRef]
- Metwally, S.; Karbowniczek, J.; Szewczyk, P.; Marzec, M.M.; Gruszczyński, A.; Bernasik, A.; Stachewicz, U. Single-Step Approach to Tailor Surface Chemistry and Potential on Electrospun PCL Fibers for Tissue Engineering Application. Adv. Mater. Interfaces 2018, 6, 1801211. [Google Scholar] [CrossRef]
- Llorens, E.; del Valle, L.J.; Ferrán, R.; Rodríguez-Galán, A.; Puiggali, J. Scaffolds with tuneable hydrophilicity from electrospun microfibers of polylactide and poly(ethylene glycol) mixtures: Morphology, drug release behavior, and biocompatibility. J. Polym. Res. 2014, 21, 360. [Google Scholar] [CrossRef]
- Pavliňáková, V.; Vojtova, L.; Pavlinak, D.; Vojtek, L.; Sedlakova, V.; Hyršl, P.; Alberti, M.; Jaros, J.; Hampl, A.; Jančař, J.; et al. Novel electrospun gelatin/oxycellulose nanofibers as a suitable platform for lung disease modeling. Mater. Sci. Eng. C 2016, 67, 493–501. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.; Morshed, M.; Nasr-Esfahani, M.; Ramakrishna, S. Electrospun poly (ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; He, W.; Yong, T.; Ramakrishna, S. Grafting of Gelatin on Electrospun Poly(caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Eng. 2005, 11, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.R.; Ferreira, P.; Vaz, R.; Alves, P.; Figueiredo, M.; Correia, I.J.; Coimbra, P. Development of UV cross-linked gelatin coated electrospun poly(caprolactone) fibrous scaffolds for tissue engineering. Int. J. Boil. Macromol. 2016, 93, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Zhang, C.; Advincula, A.A.; Baer, E.; Pokorski, J.K. Protein and Bacterial Antifouling Behavior of Melt-Coextruded Nanofiber Mats. ACS Appl. Mater. Interfaces 2016, 8, 8928–8938. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. Compos. Part A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Li, Y.-F.; Rubert, M.; Aslan, H.; Yu, Y.; Howard, K.A.; Dong, M.; Besenbacher, F.; Chen, M. Ultraporous interweaving electrospun microfibers from PCL–PEO binary blends and their inflammatory responses. Nanoscale 2014, 6, 3392. [Google Scholar] [CrossRef]
- Asadian, M.; Dhaenens, M.; Onyshchenko, Y.; de Waele, S.; Declercq, H.; Cools, P.; Devreese, B.; Deforce, D.; Morent, R.; de Geyter, N. Plasma Functionalization of Polycaprolactone Nanofibers Changes Protein Interactions with Cells, Resulting in Increased Cell Viability. ACS Appl. Mater. Interfaces 2018, 10, 41962–41977. [Google Scholar] [CrossRef]
- Santos, F.G.; Bonkovoski, L.C.; Garcia, F.P.; Cellet, T.S.P.; Witt, M.A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Antibacterial Performance of a PCL–PDMAEMA Blend Nanofiber-Based Scaffold Enhanced with Immobilized Silver Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 9304–9314. [Google Scholar] [CrossRef]
- Patelli, A.; Mussano, F.; Brun, P.; Genova, T.; Ambrosi, E.; Michieli, N.T.; Mattei, G.; Scopece, P.; Moroni, L. Nanoroughness, Surface Chemistry, and Drug Delivery Control by Atmospheric Plasma Jet on Implantable Devices. ACS Appl. Mater. Interfaces 2018, 10, 39512–39523. [Google Scholar] [CrossRef]
- Sardella, E.; Salama, R.; Waly, G.H.; Habib, A.N.; Favia, P.; Gristina, R. Improving Internal Cell Colonization of Porous Scaffolds with Chemical Gradients Produced by Plasma Assisted Approaches. ACS Appl. Mater. Interfaces 2017, 9, 4966–4975. [Google Scholar] [CrossRef]
- Wörz, A.; Berchtold, B.; Moosmann, K.; Prucker, O.; Rühe, J. Protein-resistant polymer surfaces. J. Mater. Chem. 2012, 22, 19547. [Google Scholar] [CrossRef]
- Bridges, A.W.; García, A.J. Anti-Inflammatory Polymeric Coatings for Implantable Biomaterials and Devices. J. Diabetes Sci. Technol. 2008, 2, 984–994. [Google Scholar] [CrossRef]
- Tan, S.; Huang, X.; Wu, B. Some fascinating phenomena in electrospinning processes and applications of electrospun nanofibers. Polym. Int. 2007, 56, 1330–1339. [Google Scholar] [CrossRef]
- Reneker, D.; Kataphinan, W.; Théron, A.; Zussman, E.; Yarin, A. Nanofiber garlands of polycaprolactone by electrospinning. Polymers 2002, 43, 6785–6794. [Google Scholar] [CrossRef]
- Mo, X.; Xu, C.Y.; Kotaki, M.; Ramakrishna, S. Electrospun P(LLA-CL) nanofiber: A biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials 2004, 25, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.R.; Bhattarai, N.; Viswanathamurthi, P.; Yi, H.K.; Hwang, P.H.; Kim, H.Y. Hydrophilic nanofibrous structure of polylactide; fabrication and cell affinity. J. Biomed. Mater. Res. Part A 2006, 78, 247–257. [Google Scholar] [CrossRef]
- Bui, H.T.; Chung, O.H.; Cruz, J.D.; Park, J.S. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol. Res. 2014, 22, 1288–1296. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Harrison, B.S. Morphology impact on oxygen sensing ability of Ru(dpp)3Cl2 containing biocompatible polymers. Mater. Sci. Eng. C 2015, 53, 280–285. [Google Scholar] [CrossRef]
- Hrib, J.; Širc, J.; Hobzova, R.; Hampejsova, Z.; Bosakova, Z.; Munzarova, M.; Michálek, J. Nanofibers for drug Delivery—Incorporation and release of model molecules, influence of molecular weight and polymer structure. Beilstein J. Nanotechnol. 2015, 6, 1939–1945. [Google Scholar] [CrossRef]
- Nadri, S.; Nasehi, F.; Barati, G. Effect of parameters on the quality of core-shell fibrous scaffold for retinal differentiation of conjunctiva mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2016, 105, 189–197. [Google Scholar] [CrossRef]
- Manakhov, A.; Nečas, D.; Čechal, J.; Pavlinak, D.; Eliáš, M.; Zajíčková, L. Deposition of stable amine coating onto polycaprolactone nanofibers by low pressure cyclopropylamine plasma polymerization. Thin Solid Films 2015, 581, 7–13. [Google Scholar] [CrossRef]
- Manakhov, A.; Kedroňová, E.; Medalová, J.; Černochová, P.; Obrusník, A.; Michlicek, M.; Shtansky, D.V.; Zajíčková, L. Carboxyl-anhydride and amine plasma coating of PCL nanofibers to improve their bioactivity. Mater. Des. 2017, 132, 257–265. [Google Scholar] [CrossRef]
- Permyakova, E.; Polčak, J.; Slukin, P.V.; Ignatov, S.; Gloushankova, N.A.; Zajíčková, L.; Shtansky, D.V.; Manakhov, A. Antibacterial biocompatible PCL nanofibers modified by COOH-anhydride plasma polymers and gentamicin immobilization. Mater. Des. 2018, 153, 60–70. [Google Scholar] [CrossRef]
- Martins, A.; Pinho, E.D.; Faria, S.; Pashkuleva, I.; Marques, A.P.; Reis, R.L.; Neves, N.M. Surface Modification of Electrospun Polycaprolactone Nanofiber Meshes by Plasma Treatment to Enhance Biological Performance. Small 2009, 5, 1195–1206. [Google Scholar] [CrossRef]
- Makhneva, E.; Farka, Z.; Skládal, P.; Zajíčková, L. Cyclopropylamine plasma polymer surfaces for label-free SPR and QCM immunosensing of Salmonella. Sens. Actuators B Chem. 2018, 276, 447–455. [Google Scholar] [CrossRef]
- Andrady, A.L. Science and Technology of Polymer Nanofibers; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Khajavi, R.; Abbasipour, M. Electrospinning as a versatile method for fabricating coreshell, hollow and porous nanofibers. Sci. Iran. 2012, 19, 2029–2034. [Google Scholar] [CrossRef]
- Bide, M.; Phaneuf, M.D.; Phaneuf, T.; Brown, P. Controlled Drug Release from Nanofibrous Polyester Materials. In Medical and Healthcare Textiles; Elsevier BV: Amsterdam, The Netherlands, 2010; pp. 198–205. [Google Scholar]
- Lavielle, N.; Popa, A.-M.; de Geus, M.; Hébraud, A.; Schlatter, G.; Thöny-Meyer, L.; Rossi, R.M. Controlled formation of poly(ε-caprolactone) ultrathin electrospun nanofibers in a hydrolytic degradation-assisted process. Eur. Polym. J. 2013, 49, 1331–1336. [Google Scholar] [CrossRef]
- Manakhov, A.; Landová, M.; Medalová, J.; Michlicek, M.; Polčak, J.; Nečas, D.; Zajíčková, L. Cyclopropylamine plasma polymers for increased cell adhesion and growth. Plasma Process. Polym. 2016, 14, 1600123. [Google Scholar] [CrossRef]
- Favia, P.; Stendardo, M.V.; D’Agostino, R. Selective grafting of amine groups on polyethylene by means of NH3−H2 RF glow discharges. Plasmas Polym. 1996, 1, 91–112. [Google Scholar] [CrossRef]
- Michlíček, M.; Manakhov, A.; Dvořáková, E.; Zajíčková, L. Homogeneity and penetration depth of atmospheric pressure plasma polymerization onto electrospun nanofibrous mats. Appl. Surf. Sci. 2019, 471, 835–841. [Google Scholar] [CrossRef]
- Manakhov, A.; Michlicek, M.; Felten, A.; Pireaux, J.-J.; Nečas, D.; Zajíčková, L. XPS depth profiling of derivatized amine and anhydride plasma polymers: Evidence of limitations of the derivatization approach. Appl. Surf. Sci. 2017, 394, 578–585. [Google Scholar] [CrossRef]
- Vandenabeele, C.R.; Buddhadasa, M.; Girard-Lauriault, P.-L.; Snyders, R. Comparison between single monomer versus gas mixture for the deposition of primary amine-rich plasma polymers. Thin Solid Films 2017, 630, 100–107. [Google Scholar] [CrossRef]
- Kweon, H. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K.; Pielichowska, K. Differential scanning calorimetry studies on poly(ethylene glycol) with different molecular weights for thermal energy storage materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D. Beaded nanofibers formed during electrospinning. Polymers 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymers 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; Du Toit, L.C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Miscibility and crystallization of poly(ethylene oxide) and poly(ε-caprolactone) blends. Polymers 2003, 44, 3101–3106. [Google Scholar] [CrossRef]
- Samanta, P.; Srivastava, R.; Nandan, B.; Chen, H.-L. Crystallization behavior of crystalline/crystalline polymer blends under confinement in electrospun nanofibers of polystyrene/poly(ethylene oxide)/poly(?-caprolactone) ternary mixtures. Soft Matter 2017, 13, 1569–1582. [Google Scholar] [CrossRef]
- Samanta, P.; Singh, S.; Srivastava, R.; Nandan, B.; Liu, C.-L.; Chen, H.-L.; Velmayil, T. Crystallization behaviour of poly(ethylene oxide) under confinement in the electrospun nanofibers of polystyrene/poly(ethylene oxide) blends. Soft Matter 2016, 12, 5110–5120. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F. Polymer Physics: Applications to Molecular Association and Thermoreversible Gelation; Cambridge University Press: Cambridge, UK, 2011; ISBN 978-0-521-86429-9. [Google Scholar]
- Hegemann, D. Plasma Polymer Deposition and Coatings on Polymers. In Comprehensive Materials Processing; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 2014, pp. 201–228. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupka, V.; Dvořáková, E.; Manakhov, A.; Michlíček, M.; Petruš, J.; Vojtová, L.; Zajíčková, L. Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing. Polymers 2020, 12, 1403. https://doi.org/10.3390/polym12061403
Kupka V, Dvořáková E, Manakhov A, Michlíček M, Petruš J, Vojtová L, Zajíčková L. Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing. Polymers. 2020; 12(6):1403. https://doi.org/10.3390/polym12061403
Chicago/Turabian StyleKupka, Vojtěch, Eva Dvořáková, Anton Manakhov, Miroslav Michlíček, Josef Petruš, Lucy Vojtová, and Lenka Zajíčková. 2020. "Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing" Polymers 12, no. 6: 1403. https://doi.org/10.3390/polym12061403
APA StyleKupka, V., Dvořáková, E., Manakhov, A., Michlíček, M., Petruš, J., Vojtová, L., & Zajíčková, L. (2020). Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing. Polymers, 12(6), 1403. https://doi.org/10.3390/polym12061403








