Effects of Chemical Modifications on the Thermoresponsive Behavior of a PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
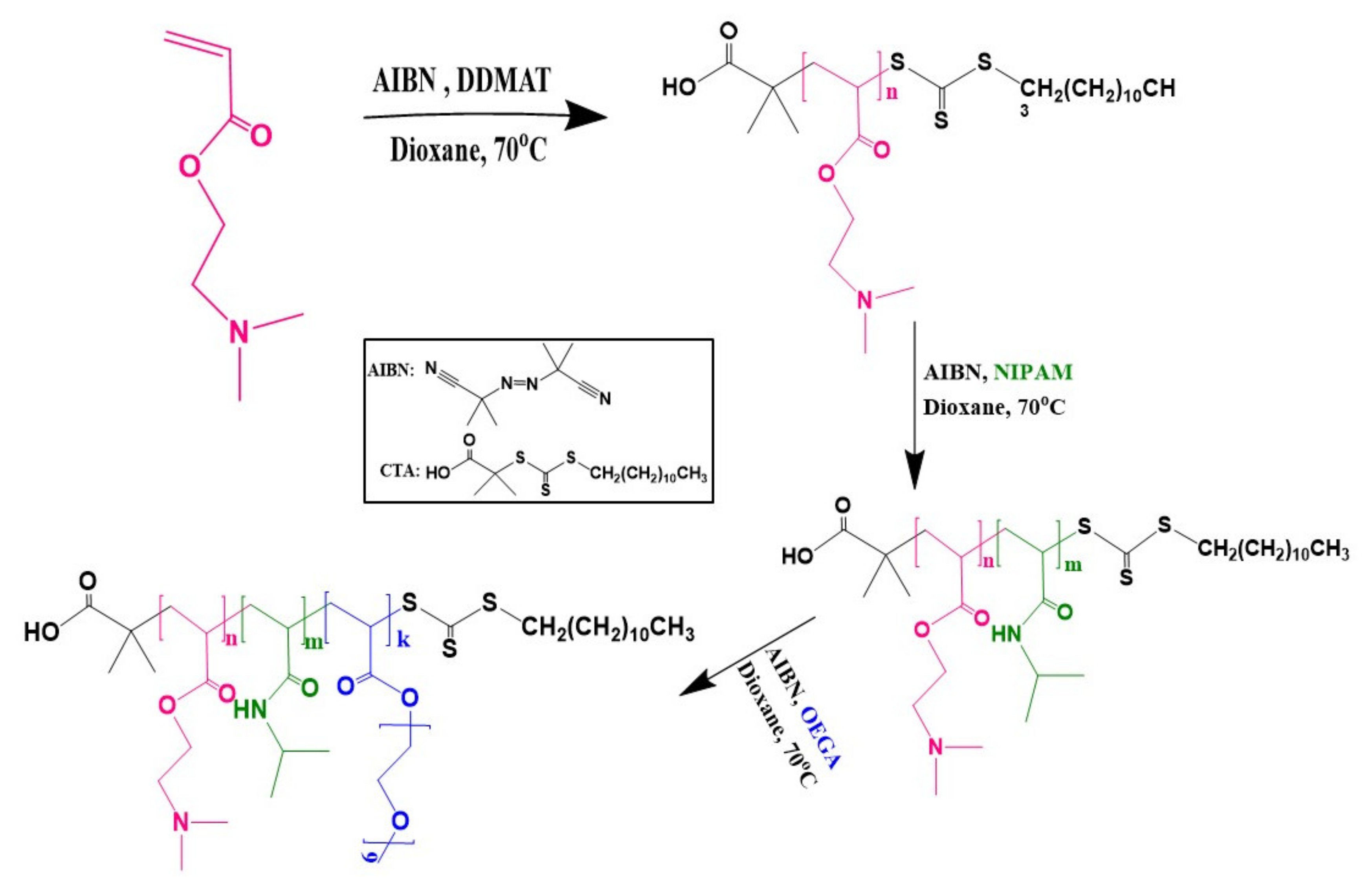
2.2. Synthesis of PDMAEA Homopolymer
2.3. Synthesis of PDMAEA-b-PNIPAM Block Copolymer
2.4. Synthesis of PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer
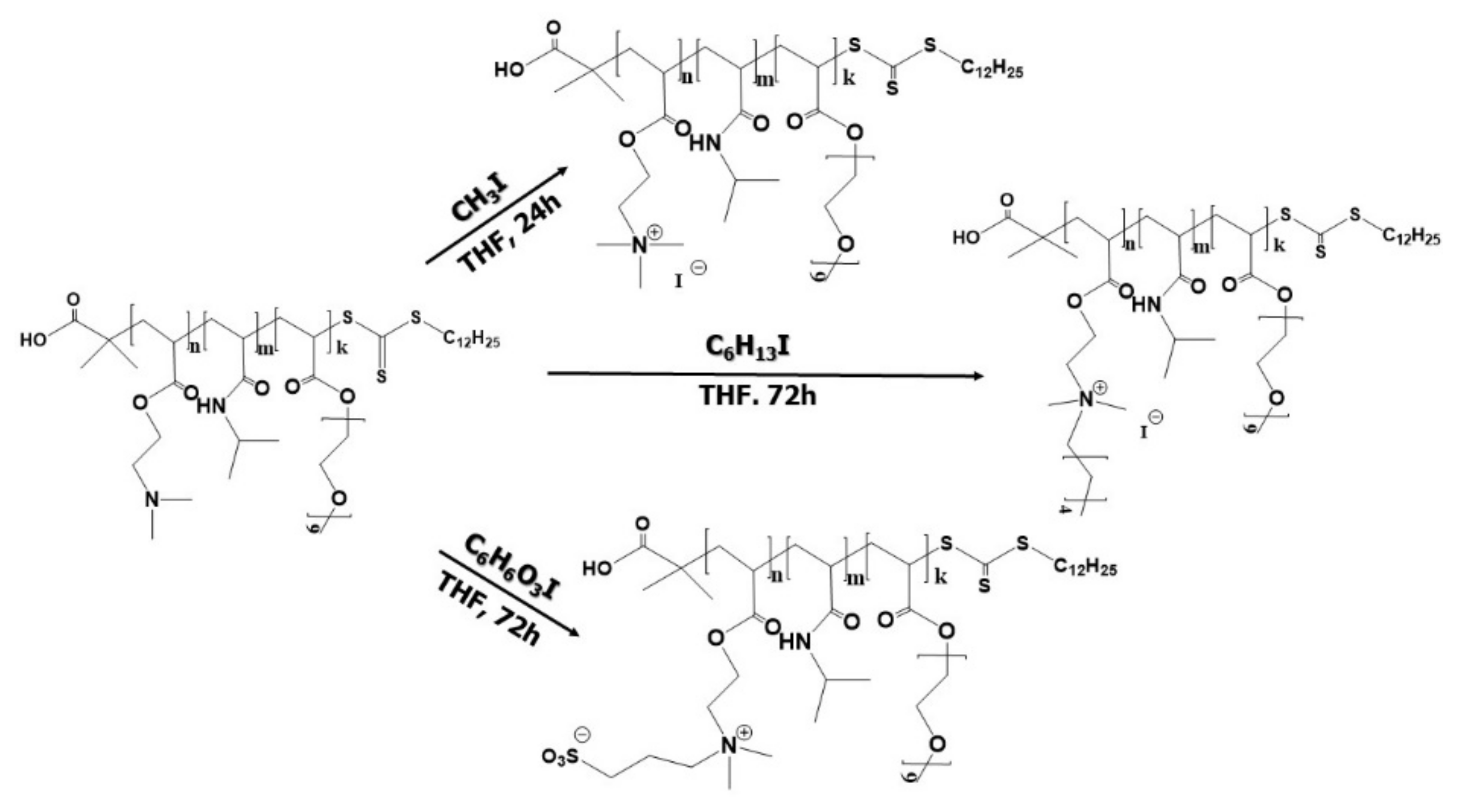
2.5. Chemical Modifications of Triblock Terpolymer
2.5.1. Synthesis of Q1PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer
2.5.2. Synthesis of Q6PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer
2.5.3. Synthesis of SPDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer
2.6. Self-Assembly of Triblock Terpolymer and Its Derivatives in Aqueous Solutions
2.7. Characterization Methods
3. Results and Discussion
3.1. PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer Synthesis, Chemical Modifications and Molecular Characterization
3.2. Aqueous Solution Properties of Triblock Terpolymers
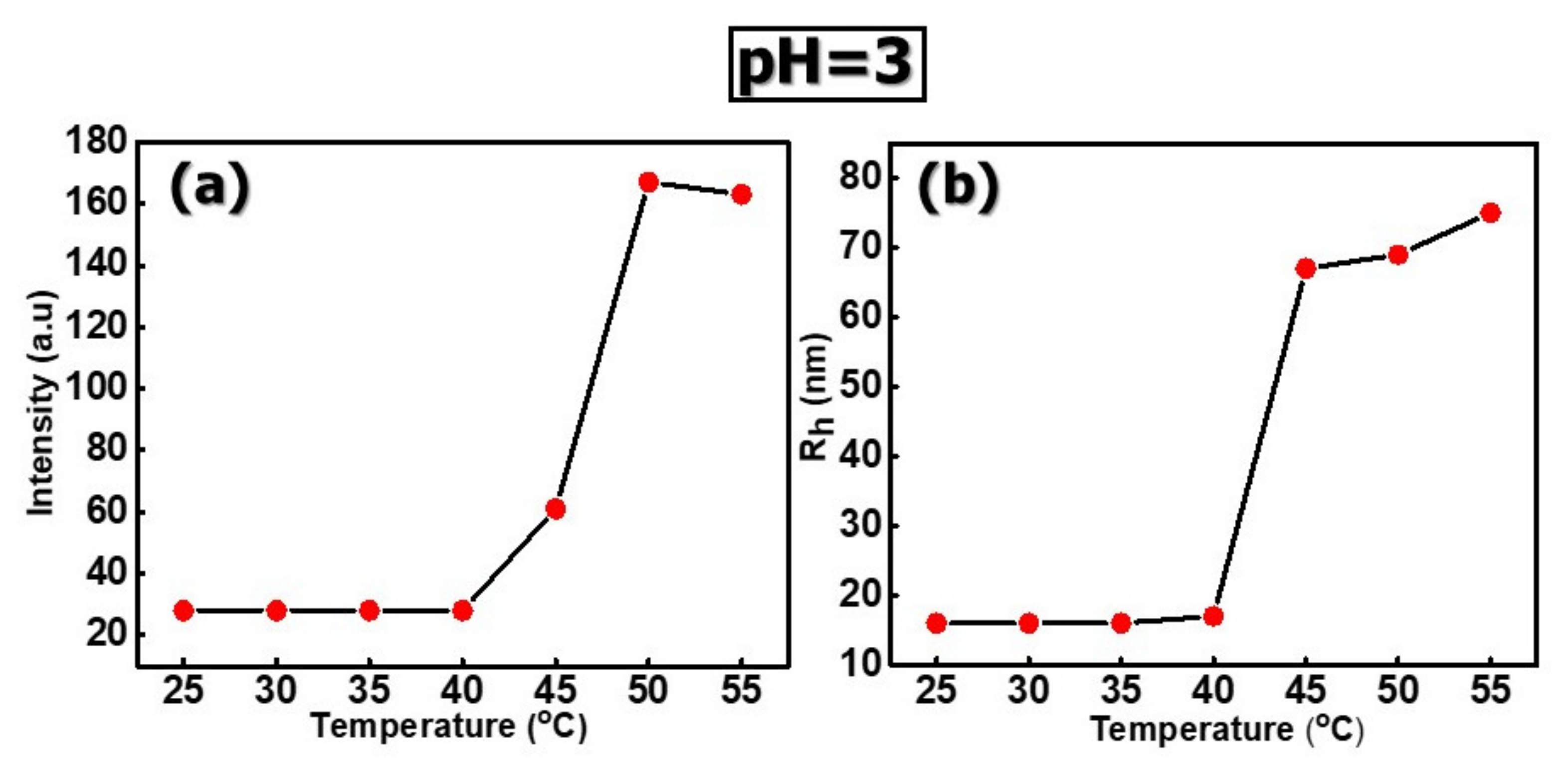
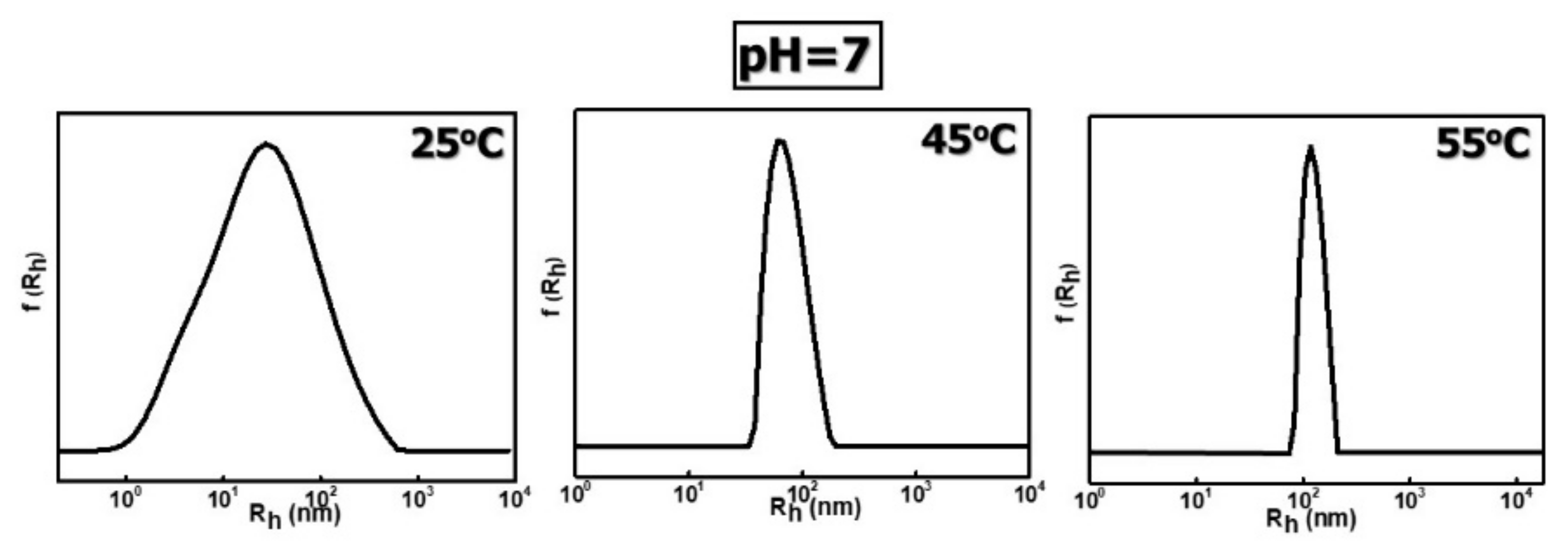
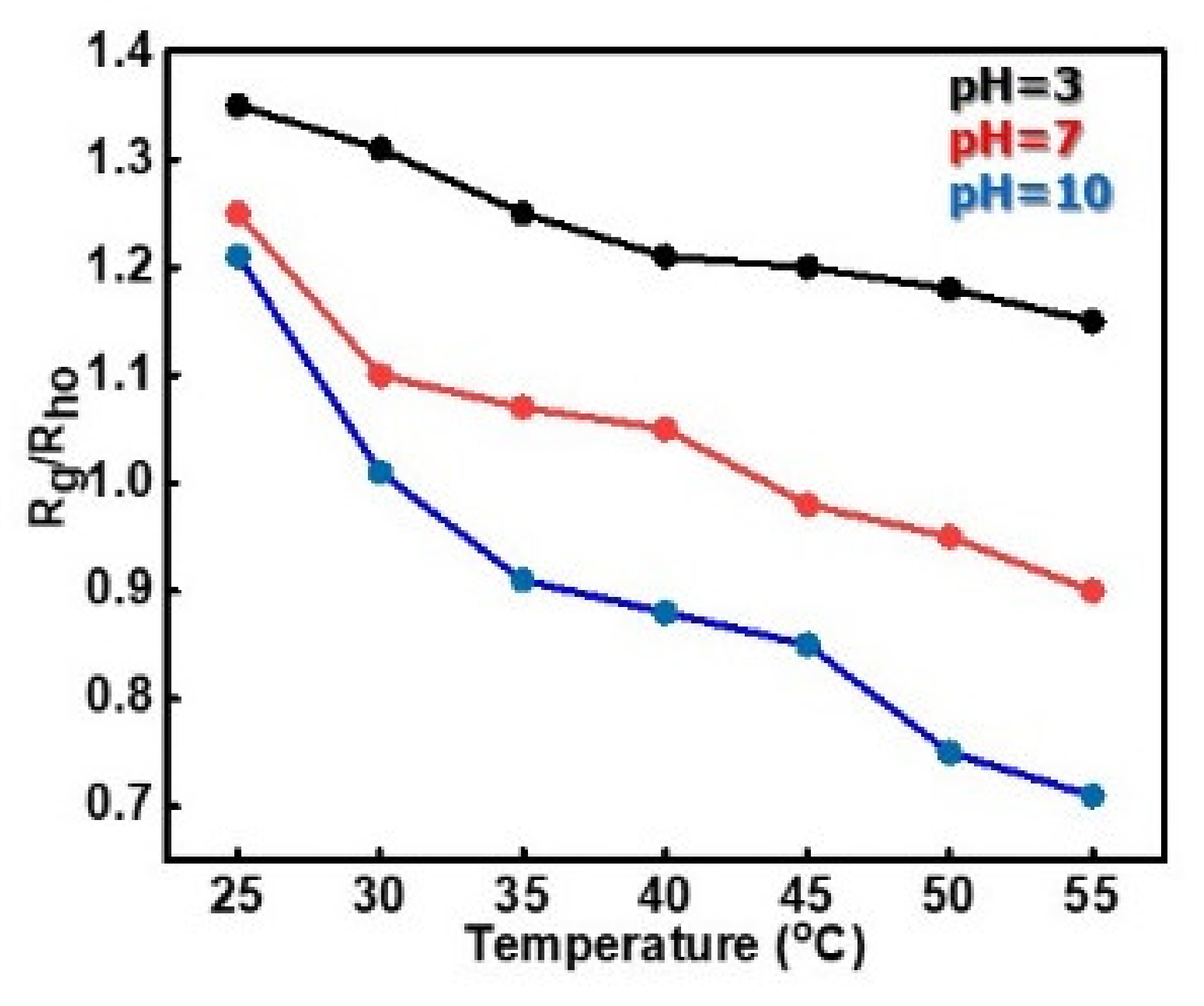
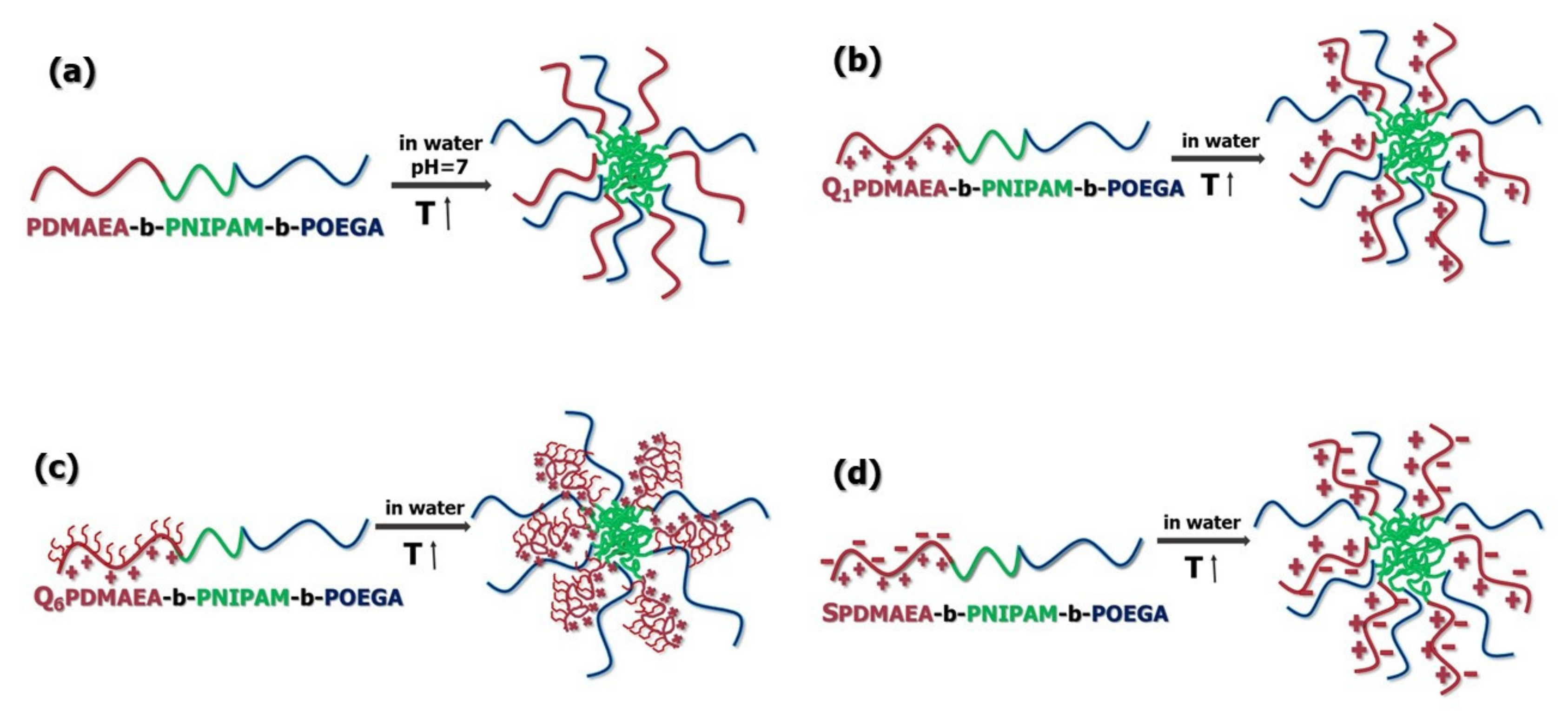
3.2.1. Effects of Temperature and pH on the Self-Assembly Behavior of PDMAEA-b-PNIPAM-b-POEGA Terpolymers
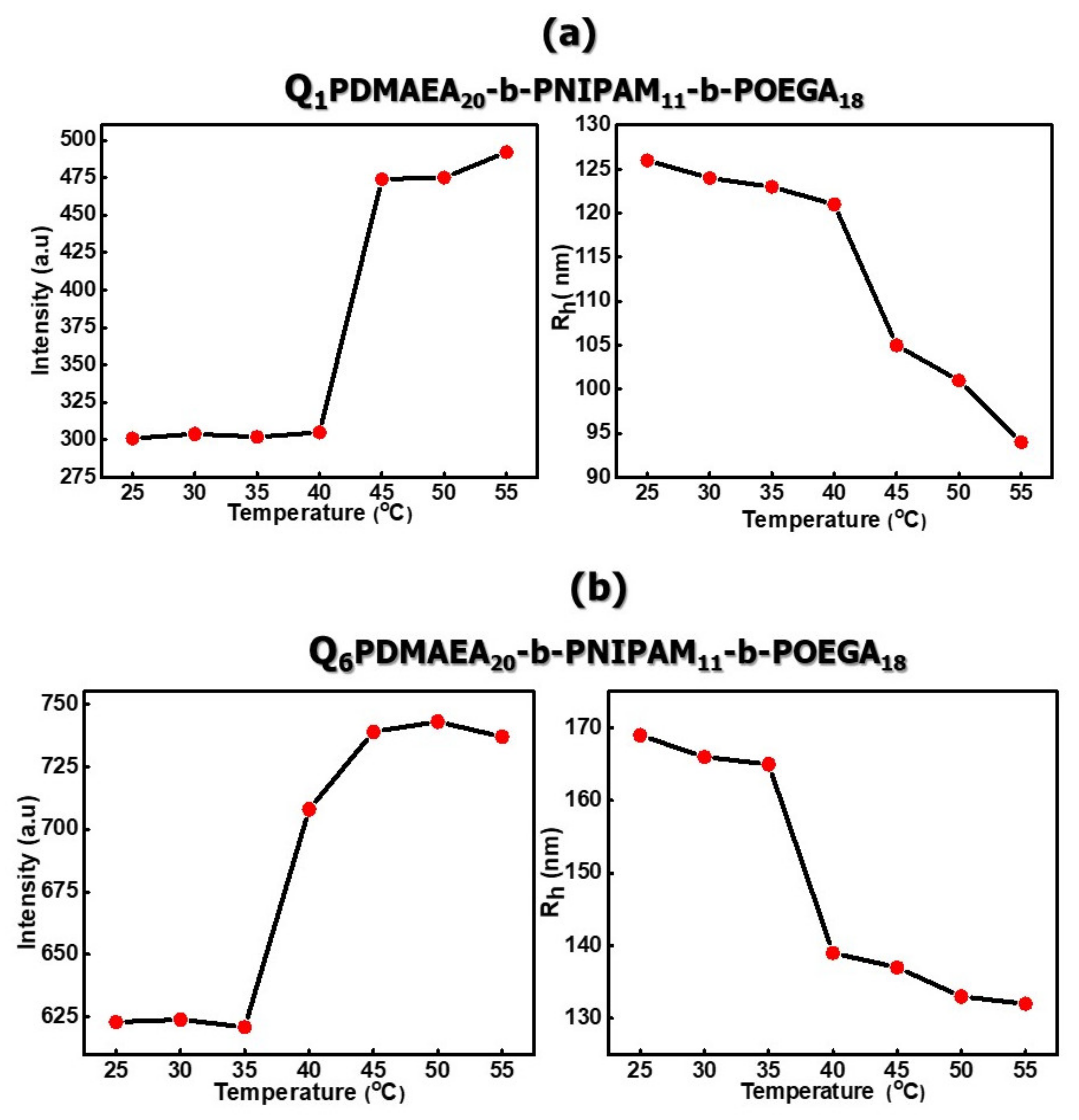
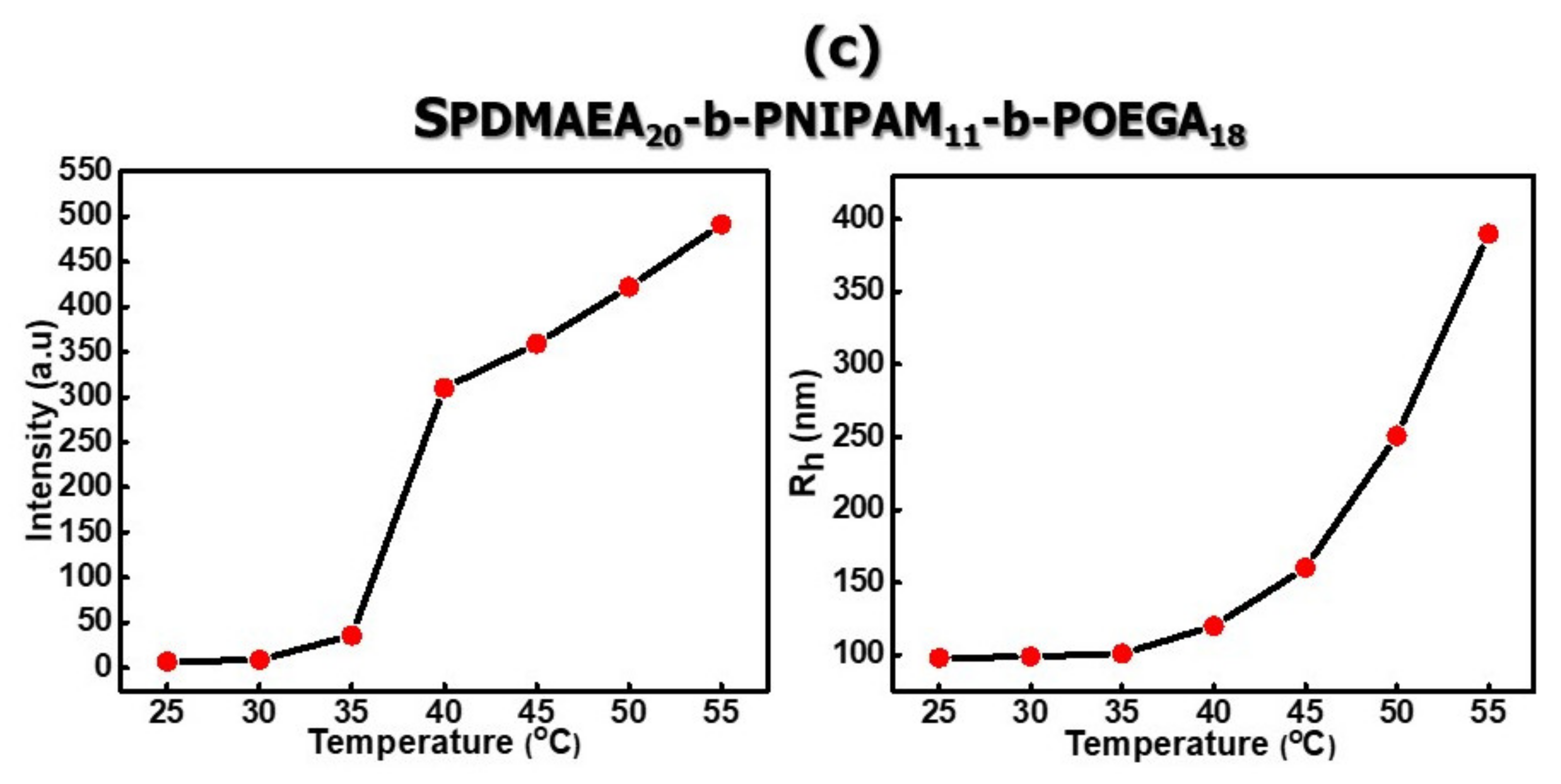
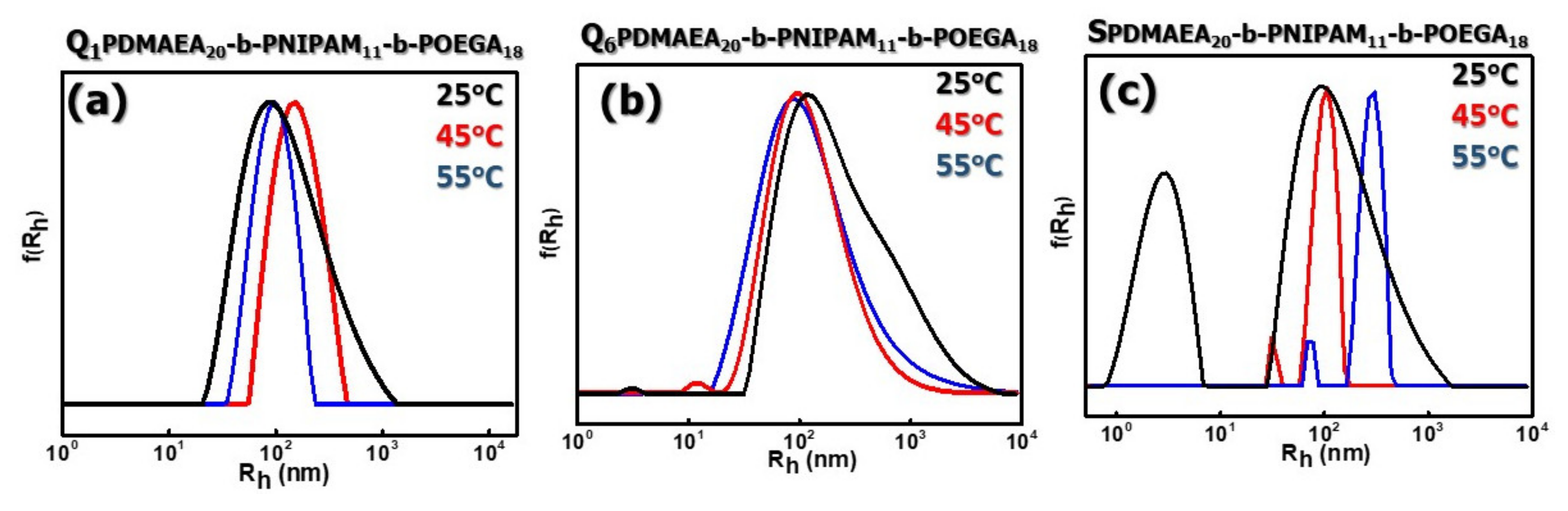
3.2.2. Self-Assembly Properties of Chemically Modified PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymers in Aqueous Media as a Function of Temperature
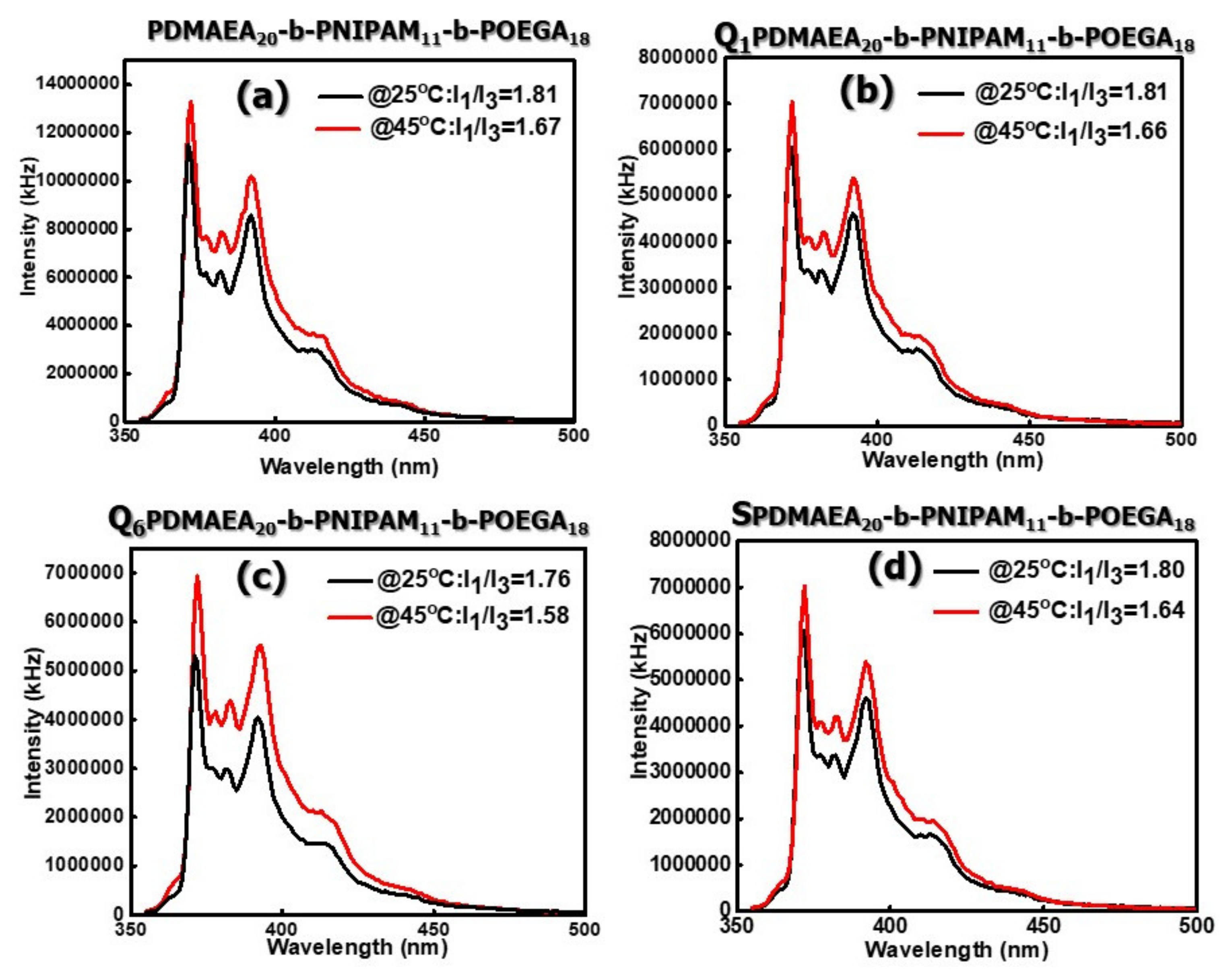
3.2.3. Surface Charge and Micropolarity of the Formed Aggregates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cölfen, H. Double-hydrophilic block copolymers: Synthesis and application as novel surfactants and crystal growth modifiers. Macromol. Rapid Commun. 2001, 22, 219–252. [Google Scholar] [CrossRef]
- Mahajan, S.; Renker, S.; Simon, P.F.; Gutmann, J.S.; Jain, A.; Gruner, S.M.; Fetters, L.J.; Coates, G.W.; Wiesner, U. Synthesis and Characterization of Amphiphilic Poly(ethylene oxide)-block-poly(hexyl methacrylate) Copolymers. Macromol. Chem. Phys. 2003, 204, 1047–1055. [Google Scholar] [CrossRef]
- Schmidt, B.V. Double Hydrophilic Block Copolymer Self-Assembly in Aqueous Solution. Macromol. Chem. Phys. 2018, 219, 1700494. [Google Scholar] [CrossRef]
- Ge, Z.; Liu, S. Supramolecular self-assembly of nonlinear amphiphilic and double hydrophilic block copolymers in aqueous solutions. Macromol. Rapid Commun. 2009, 30, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.M.; Bates, F.S. 50th Anniversary Perspective: Block Polymers Pure Potential. Macromolecules 2017, 50, 3–22. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Hadjichristidis, N.; Pispas, S.; Floudas, G. Block Copolymers: Synthetic Strategies, Physical Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003; Chapter 1,3. [Google Scholar]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block copolymer micelles in nanomedicine applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H. Synthesis of block copolymers. In Block Copolymers I; Springer: New York, NY, USA, 2005; pp. 1–124. [Google Scholar]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Pispas, S.; Avgeropoulos, A. Linear and non-linear triblock terpolymers. Synthesis, self-assembly in selective solvents and in bulk. Prog. Polym. Sci. 2005, 30, 725–782. [Google Scholar] [CrossRef]
- Grubbs, R.B. Nitroxide-mediated radical polymerization: Limitations and versatility. Polym. Rev. 2011, 51, 104–137. [Google Scholar] [CrossRef]
- Shunmugam, R.; Smith, C.E.; Tew, G.N. Atrp synthesis of abc lipophilic hydrophilic–fluorophilic triblock copolymers. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2601–2608. [Google Scholar] [CrossRef]
- Davis, K.A.; Matyjaszewski, K. ABC triblock copolymers prepared using atom transfer radical polymerization techniques. Macromolecules 2001, 34, 2101–2107. [Google Scholar] [CrossRef]
- Huang, Y.; Yong, P.; Chen, Y.; Gao, Y.; Xu, W.; Lv, Y.; Yang, L.; Reis, R.L.; Pirraco, R.P.; Chen, J. Micellization and gelatinization in aqueous media of pH-and thermo-responsive amphiphilic ABC (PMMA 82-b-PDMAEMA 150-b-PNIPAM 65) triblock copolymer synthesized by consecutive RAFT polymerization. RSC Adv. 2017, 7, 28711–28722. [Google Scholar] [CrossRef]
- Laaser, J.E.; Lohmann, E.; Jiang, Y.; Reineke, T.M.; Lodge, T.P. Architecture-Dependent Stabilization of Polyelectrolyte Complexes between Polyanions and Cationic Triblock Terpolymer Micelles. Macromolecules 2016, 49, 6644–6654. [Google Scholar] [CrossRef]
- Perrier, S.B. 50th Anniversary Perspective: RAFT Polymerization: A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process. Aust. J. Chem. 2005, 58, 379–410. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process—A first update. Aust. J. Chem. 2006, 59, 669–692. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Toward living radical polymerization. Acc. Chem. Res. 2008, 41, 1133–1142. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process—A second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Moad, G. RAFT polymerization to form stimuli-responsive polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; McCormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- Mertoglu, M.; Garnier, S.; Laschewsky, A.; Skrabania, K.; Storsberg, J. Stimuli responsive amphiphilic block copolymers for aqueous media synthesised via reversible addition fragmentation chain transfer polymerisation (RAFT). Polymer 2005, 46, 7726–7740. [Google Scholar] [CrossRef]
- McCormick, C.L.; Sumerlin, B.S.; Lokitz, B.S.; Stempka, J.E. RAFT-synthesized diblock and triblock copolymers: Thermally-induced supramolecular assembly in aqueous media. Soft Matter 2008, 4, 1760–1773. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S. Bioapplications of RAFT polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, B.D.; Gunatillake, P.A.; Meagher, L. Biomedical applications of polymers derived by reversible addition–fragmentation chain-transfer (RAFT). Adv. Drug Deliv. Rev. 2015, 91, 141–152. [Google Scholar] [CrossRef]
- Tian, Q.; Fei, C.; Yin, H.; Feng, Y. Stimuli-responsive polymer wormlike micelles. Prog. Polym. Sci. 2019, 89, 108–132. [Google Scholar] [CrossRef]
- Li, M.-H.; Keller, P. Stimuli-responsive polymer vesicles. Soft Matter 2009, 5, 927–937. [Google Scholar] [CrossRef]
- Wyman, I.W.; Liu, G. Micellar structures of linear triblock terpolymers: Three blocks but many possibilities. Polymer 2013, 54, 1950–1978. [Google Scholar] [CrossRef]
- Ye, Z.; Li, Y.; An, Z.; Wu, P. Exploration of doubly thermal phase transition process of PDEGA-b-PDMA-b-PVCL in water. Langmuir 2016, 32, 6691–6700. [Google Scholar] [CrossRef]
- Mäkinen, L.; Varadharajan, D.; Tenhu, H.; Hietala, S. Triple hydrophilic UCST–LCST block copolymers. Macromolecules 2016, 49, 986–993. [Google Scholar]
- Guan, C.M.; Luo, Z.H.; Qiu, J.J.; Tang, P.P. Novel fluorosilicone triblock copolymers prepared by two-step RAFT polymerization: Synthesis, characterization, and surface properties. Eur. Polym. J. 2010, 46, 1582–1593. [Google Scholar] [CrossRef]
- Huang, J.; Xu, J.; Chen, K.; Wang, T.; Cui, C.; Wei, X.; Zhang, R.; Li, L.; Guo, X. Synthesis of triblock copolymers via RAFT polymerization and their application as surfactants for crude oil-in-water emulsion. Ind. Eng. Chem. Res. 2015, 54, 1564–1575. [Google Scholar] [CrossRef]
- Vasquez, D.; Einfalt, T.; Meier, W.; Palivan, C.G. Asymmetric Triblock Copolymer Nanocarriers for Controlled Localization and pH-Sensitive Release of Proteins. Langmuir 2016, 32, 10235–10243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, G.; Pispas, S. Morphological transitions in aggregates of thermosensitive poly (ethylene oxide)-b-poly(N-isopropylacrylamide) block copolymers prepared via RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4099–4110. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhu, H.; Yin, Q.; Liu, H.; Hu, Y. Effect of composition of PDMAEMA-b-PAA block copolymers on their pH-and temperature-responsive behaviors. Langmuir 2013, 29, 1024–1034. [Google Scholar] [CrossRef]
- Atanase, L.I.; Reiss, G. Micellization of pH-stimulable poly(2-vinylpyridine)-b-poly(ethylene oxide) copolymers and their complexation with anionic surfactants. J. Colloid Interface Sci. 2013, 395, 190–197. [Google Scholar] [CrossRef]
- Atanase, L.I.; Lerch, J.P.; Caprarescu, S.; Iurciuc, C.E.; Riess, G. Micellization of pH-sensitive poly(butadiene)-block-poly(2-vinylpyridine)-block-poly(ethylene oxide) triblock copolymers: Complex formation with anionic surfactants. J. Appl. Polym. Sci. 2017, 134, 45313. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mat. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 1. [Google Scholar] [CrossRef]
- York, A.W.; Kirkland, S.E.; McCormick, C.L. Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: Stimuli-responsive drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1018–1036. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Brooks, W.L.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Pelton, R. Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic. J. Colloid Interface Sci. 2010, 348, 673–674. [Google Scholar] [CrossRef]
- Virtanen, J.; Holappa, S.; Lemmetyinen, H.; Tenhu, H. Aggregation in aqueous poly(N-isopropylacrylamide)-block-poly (ethylene oxide) solutions studied by fluorescence spectroscopy and light scattering. Macromolecules 2002, 35, 4763–4769. [Google Scholar] [CrossRef]
- Umapathi, R.; Reddy, P.M.; Kumar, A.; Venkatesu, P.; Chang, C.J. The biological stimuli for governing the phase transition temperature of the “smart” polymer PNIPAM in water. Colloids Surf. B 2015, 135, 588–595. [Google Scholar] [CrossRef]
- Liu, S.Q.; Tong, Y.W.; Yang, Y.Y. Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly (D, L-lactide-co-glycolide) with varying compositions. Biomaterials 2005, 26, 5064–5074. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, X.Z.; Zhu, J.L.; Cheng, H.; Cheng, S.X.; Zhuo, R.X. Self-assembled, thermoresponsive micelles based on triblock PMMA-b-PNIPAAm-b-PMMA copolymer for drug delivery. Nanotechnology 2007, 18, 215605. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Yamato, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly (butylmethacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef]
- Liu, R.; Fraylich, M.; Saunders, B.R. Thermoresponsive copolymers: From fundamental studies to applications. Colloid Polym. Sci. 2009, 287, 627–643. [Google Scholar] [CrossRef]
- Donini, C.; Robinson, D.N.; Colombo, P.; Giordano, F.; Peppas, N.A. Preparation of poly (methacrylic acid-g-poly (ethylene glycol)) nanospheres from methacrylic monomers for pharmaceutical applications. Int. J. Pharm. 2002, 24, 83–91. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Cheung, C.Y.; Murthy, N.; Bornstein, P.; Stayton, P.S.; Hoffman, A.S. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J. Control. Release 2002, 78, 295–303. [Google Scholar] [CrossRef]
- De las Heras Alarcón, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Kamada, H.; Tsutsumi, Y.; Yoshioka, Y.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.I.; Okamoto, T.; Mukai, Y.; Shibata, H.; Nakagawa, S.; et al. Design of a pH-sensitive polymeric carrier for drug release and its application in cancer therapy. Clin. Cancer Res. 2004, 10, 2545–2550. [Google Scholar] [CrossRef]
- Cho, S.K.; Dang, C.; Wang, X.; Ragan, R.; Kwon, Y.J. Mixing-sequence-dependent nucleic acid complexation and gene transfer efficiency by polyethylenimine. Biomater. Sci. 2015, 3, 1124–1133. [Google Scholar] [CrossRef]
- Liao, X.; Walden, G.; Falcon, N.D.; Donell, S.; Raxworthy, M.J.; Wormstone, M.; Riley, G.P.; Saeed, A. A direct comparison of linear and star-shaped poly (dimethylaminoethyl acrylate) polymers for polyplexation with DNA and cytotoxicity in cultured cell lines. Eur. Polym. J. 2017, 87, 458–467. [Google Scholar] [CrossRef]
- Truong, N.P.; Jia, Z.; Burges, M.; McMillan, N.A.; Monteiro, M.J. Self-catalyzed degradation of linear cationic poly (2-dimethylaminoethyl acrylate) in water. Biomacromolecules 2011, 12, 1876–1882. [Google Scholar] [CrossRef]
- Sun, F.; Feng, C.; Liu, H.; Huang, X. PHEA-g-PDMAEA well-defined graft copolymers: SET-LRP synthesis, self-catalyzed hydrolysis, and quaternization. Polym. Chem. 2016, 7, 6973–6979. [Google Scholar] [CrossRef]
- Chroni, A.; Pispas, S. Hydrophilic/hydrophobic modifications of a PnBA-b-PDMAEA copolymer and complexation behaviour with short DNA. Eur. Polym. J. 2020, 2020, 109636. [Google Scholar] [CrossRef]
- Giaouzi, D.; Pispas, S. Synthesis and self-assembly of thermoresponsive poly(N-isopropylacrylamide)-b-poly (oligo ethylene glycol methyl ether acrylate) double hydrophilic block copolymers. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1467–1477. [Google Scholar] [CrossRef]
- Chroni, A.; Pispas, S.; Forys, A.; Trzebicka, B. pH-Driven Morphological Diversity in Poly [n-Butyl Acrylate-block-(2-(Dimethylamino) Ethyl Acrylate)] Amphiphilic Copolymer Solutions. Macromol. Rapid Commun. 2019, 40, 1900477. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, V.; Pispas, S. Stimuli-responsive amphiphilic PDMAEMA-b-PLMA copolymers and their cationic and zwitterionic analogs. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 598–610. [Google Scholar] [CrossRef]
- Škvarla, J.; Zedník, J.; Šlouf, M.; Pispas, S.; Štěpánek, M. Poly(N-isopropyl acrylamide)-block-poly(N-butyl acrylate) thermoresponsive amphiphilic copolymers: Synthesis, characterization and self-assembly behavior in aqueous solutions. Eur. Polym. J. 2014, 61, 124–132. [Google Scholar] [CrossRef]
- Zhao, W.; Fonsny, P.; FitzGerald, P.; Warr, G.G.; Perrier, S. Unexpected behavior of polydimethylsiloxane/poly (2-(dimethylamino) ethyl acrylate) (charged) amphiphilic block copolymers in aqueous solution. Polym. Chem. 2013, 4, 2140–2150. [Google Scholar] [CrossRef]
- Doncom, K.E.; Willcock, H.; O’Reilly, R.K. The direct synthesis of sulfobetaine-containing amphiphilic block copolymers and their self-assembly behavior. Eur. Polym. J. 2017, 87, 497–507. [Google Scholar] [CrossRef]
- Qin, S.; Geng, Y.; Discher, D.E.; Yang, S. Temperature-Controlled Assembly and Release from Polymer Vesicles of Poly (ethylene oxide)-block-poly(N-isopropylacrylamide). Adv. Mater. 2006, 18, 2905–2909. [Google Scholar] [CrossRef]
- Liu, G.; Hu, X.; Chen, C.; Jin, Q.; Ji, J. Self-assembly and degradation of poly [(2-methacryloyloxyethyl phosphorylcholine)-block-(D, L-lactide)] diblock copolymers: Large compound micelles to vesicles. Polym. Int. 2011, 60, 578–583. [Google Scholar] [CrossRef]
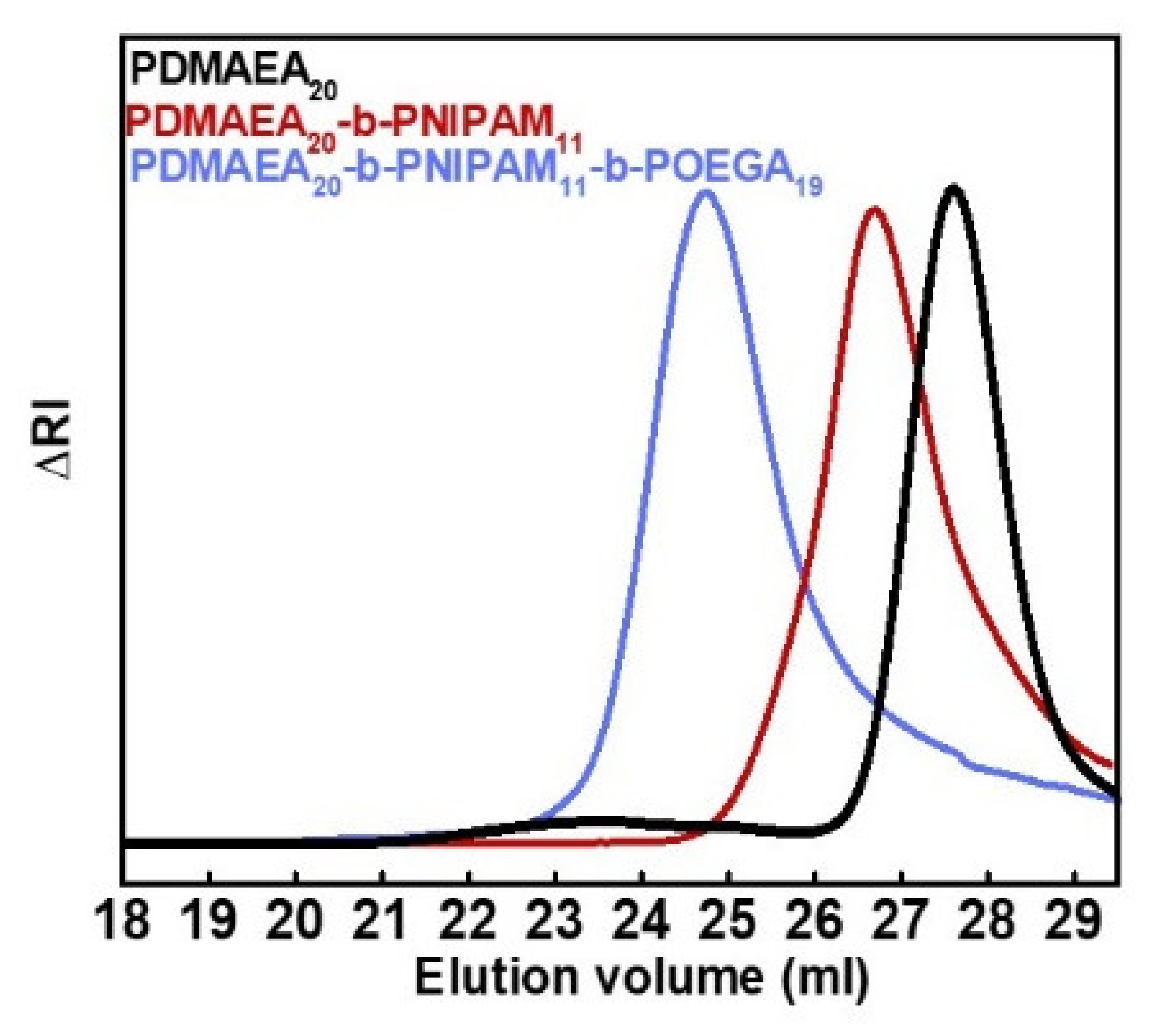


| SAMPLE | Mw a (×104) (g/mol) | Mw/Mna | %wt PDMAEA | %wt PNIPAM | %wt POEGA |
|---|---|---|---|---|---|
| PDMAEA | 0.28 | 1.17 | - | ||
| PDMAEA-b-PNIPAM | 0.41 | 1.28 | 69 b | 31 b | |
| PDMAEA20-b-PNIPAM11-b-POEGA18 | 1.28 | 1.57 | 22 b | 10 b | 68 b |
| QPDMAEA20-b-PNIPAM11-b-POEGA18 | 1.56 c | - | 36 c | 8 c | 55 c |
| Q6PDMAEA20-b-PNIPAM11-b-POEGA18 | 1.70 c | - | 41 c | 8 c | 51 c |
| SPDMAEA20-b-PNIPAM11-b-POEGA18 | 1.52 c | - | 34 c | 8 c | 58 c |
| SAMPLE | T (°C) | Mw, app (×106) (g/mol) a | Nagg a | Rh (nm) b | ζ-Potential (mV) c |
|---|---|---|---|---|---|
| PDMAEA20-b-PNIPAM11-b-POEGA18 | 25 | 0.3 | 23 | 26 | +2.27 |
| 45 | 4.2 | 328 | 73 | +4.60 | |
| Q1PDMAEA20-b-PNIPAM11-b-POEGA18 | 25 | 0.15 | 9 | 126 | +22.1 |
| 45 | 1.8 | 115 | 105 | +42.7 | |
| Q6PDMAEA20-b-PNIPAM11-b-POEGA18 | 25 | 0.8 | 47 | 169 | +19.2 |
| 45 | 7.3 | 429 | 137 | +25.7 | |
| SPDMAEA20-b-PNIPAM11-b-POEGA18 | 25 | 0.4 | 26 | 98 | +12.1 |
| 45 | 5.2 | 342 | 160 | +2.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giaouzi, D.; Pispas, S. Effects of Chemical Modifications on the Thermoresponsive Behavior of a PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer. Polymers 2020, 12, 1382. https://doi.org/10.3390/polym12061382
Giaouzi D, Pispas S. Effects of Chemical Modifications on the Thermoresponsive Behavior of a PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer. Polymers. 2020; 12(6):1382. https://doi.org/10.3390/polym12061382
Chicago/Turabian StyleGiaouzi, Despoina, and Stergios Pispas. 2020. "Effects of Chemical Modifications on the Thermoresponsive Behavior of a PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer" Polymers 12, no. 6: 1382. https://doi.org/10.3390/polym12061382
APA StyleGiaouzi, D., & Pispas, S. (2020). Effects of Chemical Modifications on the Thermoresponsive Behavior of a PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer. Polymers, 12(6), 1382. https://doi.org/10.3390/polym12061382







