Synthesis and Characterization of a 2,3-Dialkoxynaphthalene-Based Conjugated Copolymer via Direct Arylation Polymerization (DAP) for Organic Electronics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Theoretical Calculations
2.4. OPV Devices Fabrication
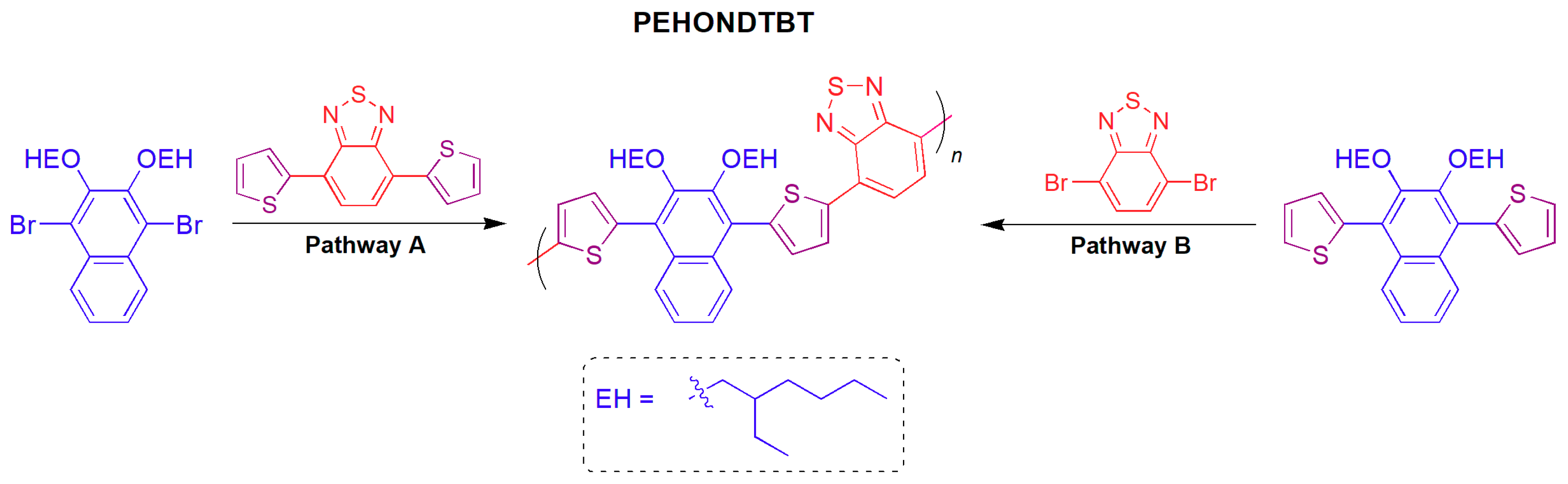
2.5. General Procedure for the Synthesis of PEHONDTBT via DAP
3. Results and Discussion
3.1. Synthesis and Characterization of PEHONDTBT
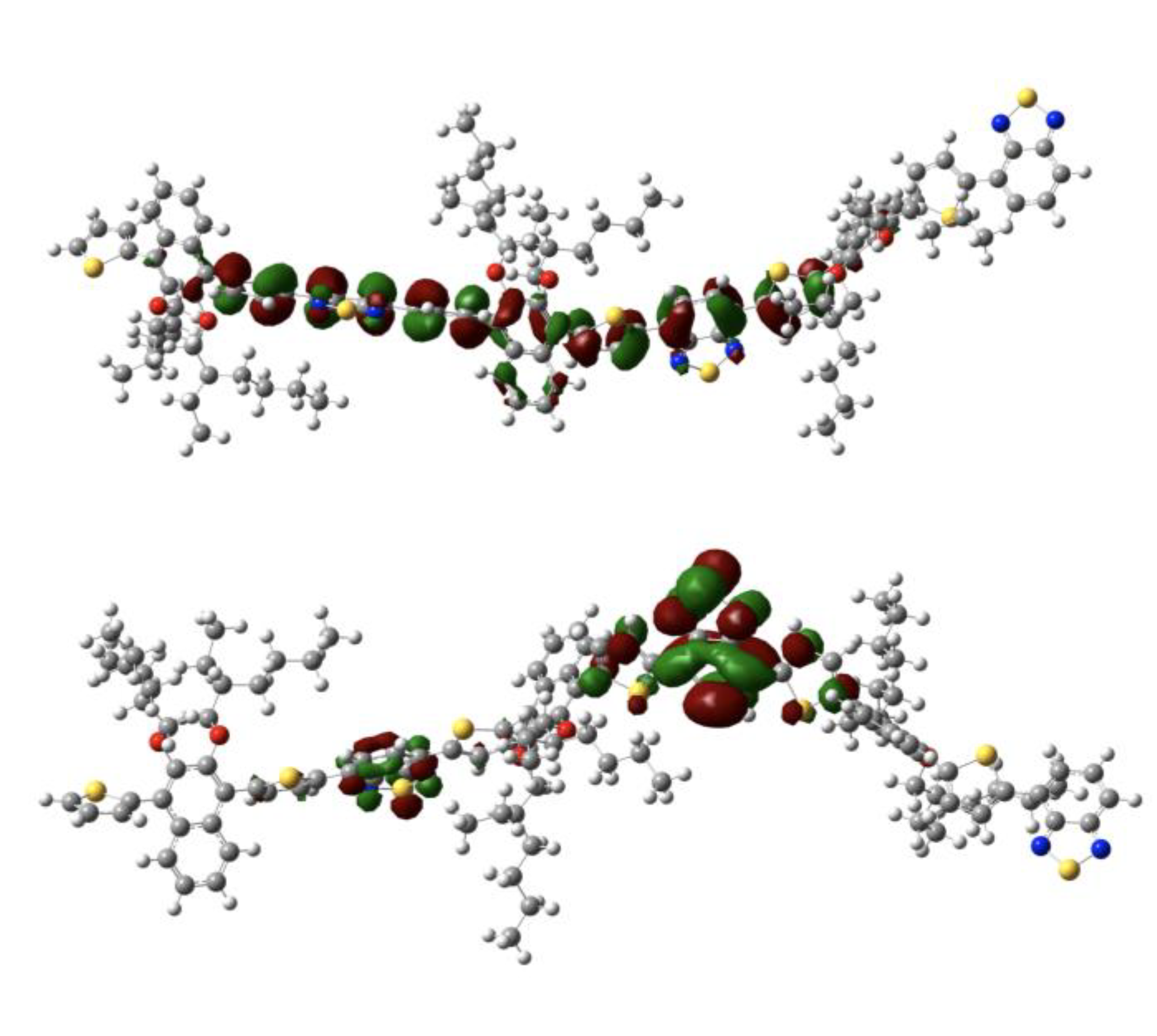
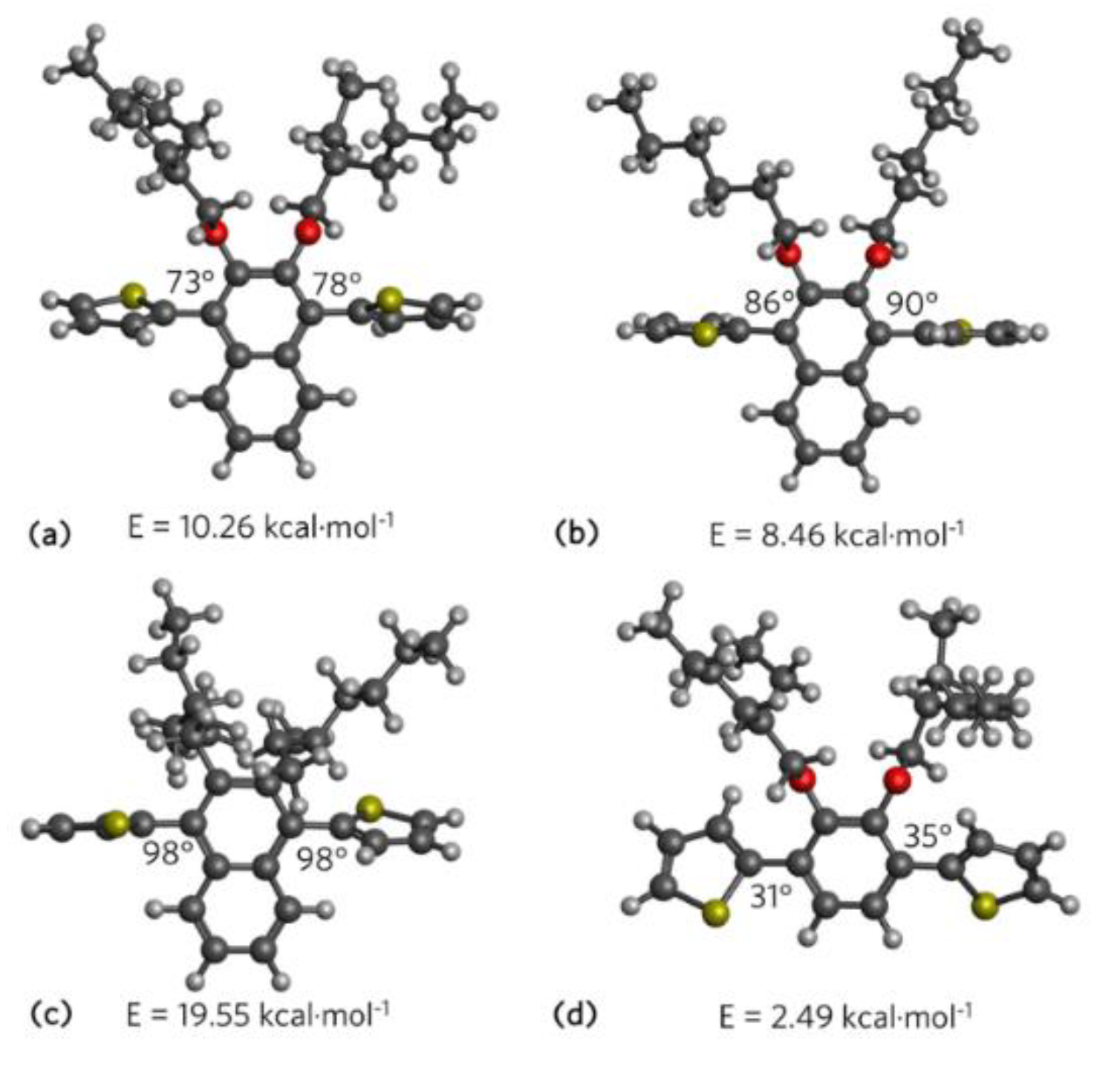
3.2. Theoretical Calculations
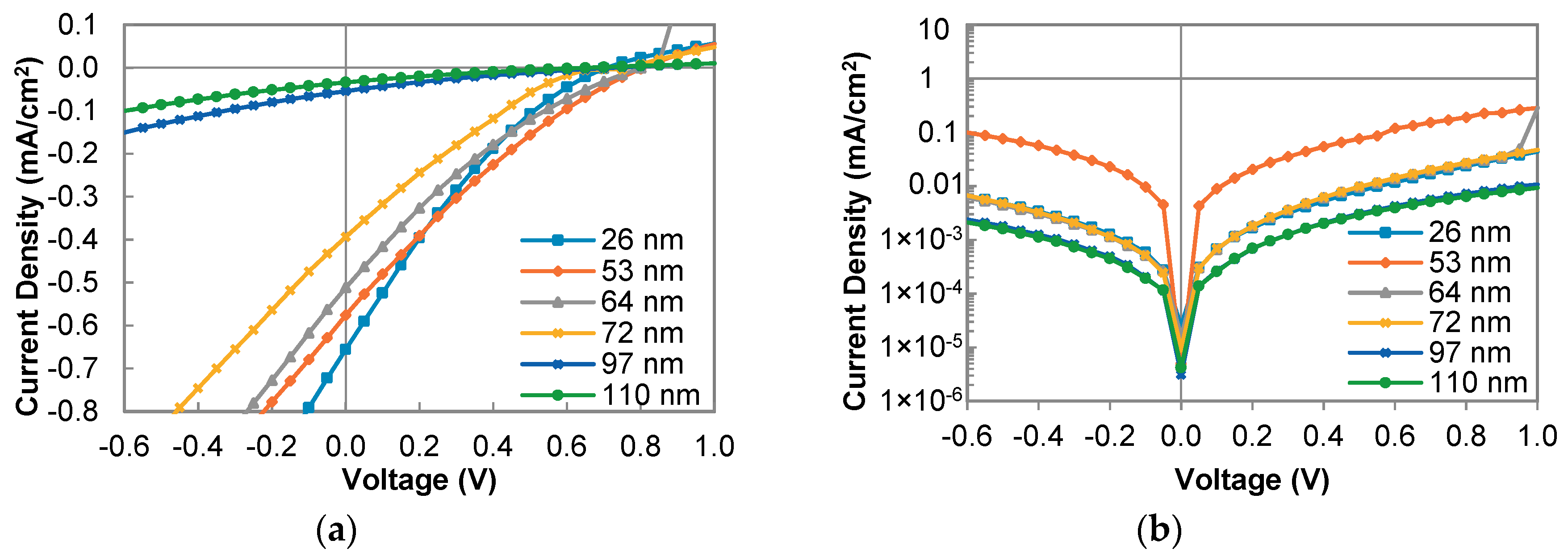
3.3. Photovoltaic Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Chueh, C.-C.; Jen, A.K.Y. Recent advances in molecular design of functional conjugated polymers for high-performance polymer solar cells. Prog. Polym. Sci. 2019, 99, 101175. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Zhan, C.; Yu, G.; Lu, Y.; Wang, L.; Wujcik, E.; Wei, S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C 2017, 5, 1569–1585. [Google Scholar] [CrossRef]
- Ho, S.; Liu, S.; Chen, Y.; So, F. Review of recent progress in multilayer solution-processed organic light-emitting diodes. J. Photonics Energy 2015, 5, 057611. [Google Scholar] [CrossRef]
- Surya, S.G.; Raval, H.N.; Ahmad, R.; Sonar, P.; Salama, K.N.; Rao, V.R. Organic field effect transistors (OFETs) in environmental sensing and health monitoring: A review. TrAC Trends Anal. Chem. 2019, 111, 27–36. [Google Scholar] [CrossRef]
- Liao, C.; Yan, F. Organic Semiconductors in Organic Thin-Film Transistor-Based Chemical and Biological Sensors. Polym. Rev. 2013, 53, 352–406. [Google Scholar] [CrossRef]
- Bhosale, M.E.; Chae, S.; Kim, J.M.; Choi, J.-Y. Organic small molecules and polymers as an electrode material for rechargeable lithium ion batteries. J. Mater. Chem. A 2018, 6, 19885–19911. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Haupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef]
- Borges-González, J.; Kousseff, C.J.; Nielsen, C.B. Organic semiconductors for biological sensing. J. Mater. Chem. C 2019, 7, 1111–1130. [Google Scholar] [CrossRef]
- Wang, J.; Lv, F.; Liu, L.; Ma, Y.; Wang, S. Strategies to design conjugated polymer based materials for biological sensing and imaging. Coord. Chem. Rev. 2018, 354, 135–154. [Google Scholar] [CrossRef]
- Leclerc, M.; Brassard, S.; Beaupré, S. Direct (hetero) arylation polymerization: Toward defect-free conjugated polymers. Polym. J. 2019, 52, 13–20. [Google Scholar] [CrossRef]
- Blaskovits, J.T.; Leclerc, M. CH Activation as a Shortcut to Conjugated Polymer Synthesis. Macromol. Rapid Commun. 2019, 40, e1800512. [Google Scholar] [CrossRef] [PubMed]
- Gobalasingham, N.S.; Thompson, B.C. Direct arylation polymerization: A guide to optimal conditions for effective conjugated polymers. Prog. Polym. Sci. 2018, 83, 135–201. [Google Scholar] [CrossRef]
- Yu, S.; Liu, F.; Yu, J.; Zhang, S.; Cabanetos, C.; Gao, Y.; Huang, W. Eco-friendly direct (hetero)-arylation polymerization: Scope and limitation. J. Mater. Chem. C 2017, 5, 29–40. [Google Scholar] [CrossRef]
- Leclerc, M.; Morin, J.-F. Synthetic Methods for Conjugated Polymers and Carbon Materials; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Bura, T.; Blaskovits, J.T.; Leclerc, M. Direct (Hetero) arylation Polymerization: Trends and Perspectives. J. Am. Chem. Soc. 2016, 138, 10056–10071. [Google Scholar] [CrossRef]
- Wakioka, M.; Ozawa, F. Highly Efficient Catalysts for Direct Arylation Polymerization (DArP). Asian J. Org. Chem. 2018, 7, 1206–1216. [Google Scholar] [CrossRef]
- Nakabayashi, K. Direct arylation polycondensation as conjugated polymer synthesis methodology. Polym. J. 2018, 50, 475–483. [Google Scholar] [CrossRef]
- Bohra, H.; Wang, M. Direct C–H arylation: A “Greener” approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A 2017, 5, 11550–11571. [Google Scholar] [CrossRef]
- Kowalski, S.; Allard, S.; Zilberberg, K.; Riedl, T.; Scherf, U. Direct arylation polycondensation as simplified alternative for the synthesis of conjugated (co)polymers. Prog. Polym. Sci. 2013, 38, 1805–1814. [Google Scholar] [CrossRef]
- Blaskovits, J.T.; Johnson, P.A.; Leclerc, M. Mechanistic Origin of β-Defect Formation in Thiophene-Based Polymers Prepared by Direct (Hetero) arylation. Macromolecules 2018, 51, 8100–8113. [Google Scholar] [CrossRef]
- Bura, T.; Beaupre, S.; Legare, M.A.; Quinn, J.; Rochette, E.; Blaskovits, J.T.; Fontaine, F.G.; Pron, A.; Li, Y.; Leclerc, M. Direct heteroarylation polymerization: Guidelines for defect-free conjugated polymers. Chem. Sci. 2017, 8, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Suraru, S.-L.; Lee, J.A.; Luscombe, C.K. C–H Arylation in the Synthesis of π-Conjugated Polymers. ACS Macro Lett. 2016, 5, 724–729. [Google Scholar] [CrossRef]
- Kowalski, S.; Allard, S.; Scherf, U. Scope and Limitations of a Direct Arylation Polycondensation Scheme in the Synthesis of PCPDTBT-Type Copolymers. Macromol. Rapid Commun. 2015, 36, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Leem, G.; Cekli, S.; Schanze, K.S. Conjugated Polyelectrolyte-Sensitized TiO2 Solar Cells: Effects of Chain Length and Aggregation on Efficiency. ACS Appl. Mater. Interfaces 2015, 7, 16601–16608. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef]
- Wang, K.; Chen, H.; Wei, X.; Bohra, H.; He, F.; Wang, M. Over 7% photovoltaic efficiency of a semicrystalline donor-acceptor polymer synthesized via direct arylation polymerization. Dyes Pigment. 2018, 158, 183–187. [Google Scholar] [CrossRef]
- Livi, F.; Zawacka, N.K.; Angmo, D.; Jørgensen, M.; Krebs, F.C.; Bundgaard, E. Influence of Side Chain Position on the Electrical Properties of Organic Solar Cells Based on Dithienylbenzothiadiazole-alt-phenylene Conjugated Polymers. Macromolecules 2015, 48, 3481–3492. [Google Scholar] [CrossRef]
- Operamolla, A.; Colella, S.; Musio, R.; Loiudice, A.; Hassan Omar, O.; Melcarne, G.; Mazzeo, M.; Gigli, G.; Farinola, G.M.; Babudri, F. Low band gap poly(1,4-arylene-2,5-thienylene)s with benzothiadiazole units: Synthesis, characterization and application in polymer solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 3490–3503. [Google Scholar] [CrossRef]
- Carlé, J.E.; Andreasen, J.W.; Jørgensen, M.; Krebs, F.C. Low band gap polymers based on 1,4-dialkoxybenzene, thiophene, bithiophene donors and the benzothiadiazole acceptor. Sol. Energy Mater. Sol. Cells 2010, 94, 774–780. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Yang, M.C.; Higashihara, T.; Chen, W.-C.; Ueda, M. Synthesis and characterization of poly(2,6-dialkoxy-1,5-naphthylene)s with low dielectric constants. Polym. J. 2018, 50, 277–280. [Google Scholar] [CrossRef]
- Carlé, J.E.; Jørgensen, M.; Krebs, F.C. Polymers for organic photovoltaics based on 1,5-bis(2-hexyldecyloxy)-naphthalene, thiophene, and benzothiadiazole. J. Photonics Energy 2011, 1, 011111. [Google Scholar] [CrossRef]
- Kwon, J.H.; An, J.-Y.; Jang, H.; Choi, S.; Chung, D.S.; Lee, M.-J.; Cha, H.-J.; Park, J.-H.; Park, C.-E.; Kim, Y.-H. Development of a new conjugated polymer containing dialkoxynaphthalene for efficient polymer solar cells and organic thin film transistors. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1119–1128. [Google Scholar] [CrossRef]
- Kim, S.-O.; Chung, D.S.; Cha, H.; Hwang, M.C.; Park, J.-W.; Kim, Y.-H.; Park, C.E.; Kwon, S.-K. Efficient polymer solar cells based on dialkoxynaphthalene and benzo [c] [1,2,5] thiadiazole: A new approach for simple donor–acceptor pair. Sol. Energy Mater. Sol. Cells 2011, 95, 1678–1685. [Google Scholar] [CrossRef]
- Chung, D.S.; Park, J.W.; Kim, S.-O.; Heo, K.; Park, C.E.; Ree, M.; Kim, Y.-H.; Kwon, S.-K. Alternating Copolymers Containing Bithiophene and Dialkoxynaphthalene for the Applications to Field Effect Transistor and Photovoltaic Cell: Performance and Stability. Chem. Mater. 2009, 21, 5499–5507. [Google Scholar] [CrossRef]
- Segura, J.L.; Martín, N.; Hanack, M. Oligo-2,6-naphthylenevinylenes—New Building Blocks for the Preparation of Photoluminescent Polymeric Materials. Eur. J. Org. Chem. 1999, 1999, 643–651. [Google Scholar] [CrossRef]
- Samanta, S.K.; Kumar, G.S.; Ghorai, U.K.; Scherf, U.; Acharya, S.; Bhattacharya, S. Synthesis of High Molecular Weight 1,4-Polynaphthalene for Solution-Processed True Color Blue Light Emitting Diode. Macromolecules 2018, 51, 8324–8329. [Google Scholar] [CrossRef]
- Mori, T.; Kijima, M. Synthesis and optical properties of polynaphthalene derivatives. Opt. Mater. 2007, 30, 545–552. [Google Scholar] [CrossRef]
- Pankow, R.M.; Munteanu, J.D.; Thompson, B.C. Influence of the aryl spacer in 2,5-dialkoxyphenylene and diaryl substituted thieno [3,4-c] pyrrole-4,6-dione copolymers. J. Mater. Chem. C 2018, 6, 5992–5998. [Google Scholar] [CrossRef]
- Pankow, R.M.; Ye, L.; Gobalasingham, N.S.; Salami, N.; Samal, S.; Thompson, B.C. Investigation of green and sustainable solvents for direct arylation polymerization (DArP). Polym. Chem. 2018, 9, 3885–3892. [Google Scholar] [CrossRef]
- Roy, C.; Bura, T.; Beaupré, S.; Légaré, M.-A.; Sun, J.-P.; Hill, I.G.; Leclerc, M. Fluorinated Thiophene-Based Synthons: Polymerization of 1,4-Dialkoxybenzene and Fluorinated Dithieno-2,1,3-benzothiadiazole by Direct Heteroarylation. Macromolecules 2017, 50, 4658–4667. [Google Scholar] [CrossRef]
- Livi, F.; Gobalasingham, N.S.; Thompson, B.C.; Bundgaard, E. Analysis of diverse direct arylation polymerization (DArP) conditions toward the efficient synthesis of polymers converging with stille polymers in organic solar cells. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2907–2918. [Google Scholar] [CrossRef]
- Wakioka, M.; Morita, H.; Ichihara, N.; Saito, M.; Osaka, I.; Ozawa, F. Mixed-Ligand Approach to Palladium-Catalyzed Direct Arylation Polymerization: Synthesis of Donor–Acceptor Polymers Containing Unsubstituted Bithiophene Units. Macromolecules 2019, 53, 158–164. [Google Scholar] [CrossRef]
- Aldrich, T.J.; Dudnik, A.S.; Eastham, N.D.; Manley, E.F.; Chen, L.X.; Chang, R.P.H.; Melkonyan, F.S.; Facchetti, A.; Marks, T.J. Suppressing Defect Formation Pathways in the Direct C–H Arylation Polymerization of Photovoltaic Copolymers. Macromolecules 2018, 51, 9140–9155. [Google Scholar] [CrossRef]
- Robitaille, A.; Jenekhe, S.A.; Leclerc, M. Poly(naphthalene diimide-alt-bithiophene) Prepared by Direct (Hetero)arylation Polymerization for Efficient All-Polymer Solar Cells. Chem. Mater. 2018, 30, 5353–5361. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, M.; Lu, Y.; Sun, Y.; Li, C.; Yang, X.; Zhang, M.; Chen, Y. A Direct C–H Coupling Method for Preparing π-Conjugated Functional Polymers with High Regioregularity. Macromolecules 2018, 51, 379–388. [Google Scholar] [CrossRef]
- Ye, L.; Pankow, R.M.; Schmitt, A.; Thompson, B.C. Synthesis of conjugated polymers using aryl-bromides via Cu-catalyzed direct arylation polymerization (Cu-DArP). Polym. Chem. 2019, 10, 6545–6550. [Google Scholar] [CrossRef]
- Lombeck, F.; Marx, F.; Strassel, K.; Kunz, S.; Lienert, C.; Komber, H.; Friend, R.; Sommer, M. To branch or not to branch: C–H selectivity of thiophene-based donor–acceptor–donor monomers in direct arylation polycondensation exemplified by PCDTBT. Polym. Chem. 2017, 8, 4738–4745. [Google Scholar] [CrossRef]
- Nübling, F.; Komber, H.; Sommer, M. All-Conjugated, All-Crystalline Donor–Acceptor Block Copolymers P3HT-b-PNDIT2 via Direct Arylation Polycondensation. Macromolecules 2017, 50, 1909–1918. [Google Scholar] [CrossRef]
- Morin, P.-O.; Bura, T.; Sun, B.; Gorelsky, S.I.; Li, Y.; Leclerc, M. Conjugated Polymers à la Carte from Time-Controlled Direct (Hetero)Arylation Polymerization. ACS Macro Lett. 2014, 4, 21–24. [Google Scholar] [CrossRef]
- Grenier, F.; Goudreau, K.; Leclerc, M. Robust Direct (Hetero)arylation Polymerization in Biphasic Conditions. J. Am. Chem. Soc. 2017, 139, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Jessop, I.A.; Diaz, F.R.; Terraza, C.A.; Tundidor-Camba, A.; Leiva, A.; Cattin, L.; Bernede, J.C. PANI Branches onto Donor-Acceptor Copolymers: Synthesis, Characterization and Electroluminescent Properties of New 2D-Materials. Polymers 2018, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Michinobu, T. Benzothiadiazole and its π-extended, heteroannulated derivatives: Useful acceptor building blocks for high-performance donor–acceptor polymers in organic electronics. J. Mater. Chem. C 2016, 4, 6200–6214. [Google Scholar] [CrossRef]
- Kowalski, S.; Allard, S.; Scherf, U. Synthesis of Poly(4,4-dialkyl-cyclopenta [2,1-b:3,4-b′] dithiophene-alt-2,1,3-benzothiadiazole) (PCPDTBT) in a Direct Arylation Scheme. ACS Macro Lett. 2012, 1, 465–468. [Google Scholar] [CrossRef]
- Keller, T.; Gahlmann, T.; Riedl, T.; Scherf, U. Direct Arylation Polycondensation (DAP) Synthesis of Alternating Quaterthiophene-Benzothiadiazole Copolymers for Organic Solar Cell Applications. Chempluschem 2019, 84, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.C.; Kim, Y.-G.; Reynolds, J.R. Spectral Broadening in MEH-PPV: PCBM-Based Photovoltaic Devices via Blending with a Narrow Band Gap Cyanovinylene–Dioxythiophene Polymer. Macromolecules 2005, 38, 5359–5362. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density—Functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Lapointe, D.; Fagnou, K. Analysis of the palladium-catalyzed (aromatic) C-H bond metalation-deprotonation mechanism spanning the entire spectrum of arenes. J. Org. Chem. 2012, 77, 658–668. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M. Synthesis of donor–acceptor conjugated polymers based on benzo [1¨C-b:4,5-b′] dithiophene and 2,1,3-benzothiadiazole via direct arylation polycondensation: Towards efficient C–H activation in nonpolar solvents. Polym. Chem. 2014, 5, 5784–5792. [Google Scholar] [CrossRef]
- Xu, Z.; Du, H.; Yin, M.; Wang, B.; Zhao, J.; Xie, Y. Benzothiadiazole, hexylthiophen and alkoxy benzene based solution processable copolymer: Effect of the electron withdrawing substituents (fluorine atoms) on electrochemical, optical and electrochromic properties. Org. Electron. 2018, 61, 1–9. [Google Scholar] [CrossRef]
- Jessop, I.A.; Bustos, M.; Hidalgo, D.; Terraza, C.A.; Tundidor-Camba, A.; Pardo, M.A.; Fuentealba, D.; Hssein, M.; Bernède, C. Synthesis of 2H-benzotriazole based donor-acceptor polymers bearing carbazole derivative as pendant groups: Optical, electronical and photovoltaic properties. Int. J. Electrochem. Sci. 2016, 11, 9822–9838. [Google Scholar] [CrossRef]
- Menke, S.M.; Holmes, R.J. Exciton diffusion in organic photovoltaic cells. Energy Environ. Sci. 2014, 7, 499–512. [Google Scholar] [CrossRef]
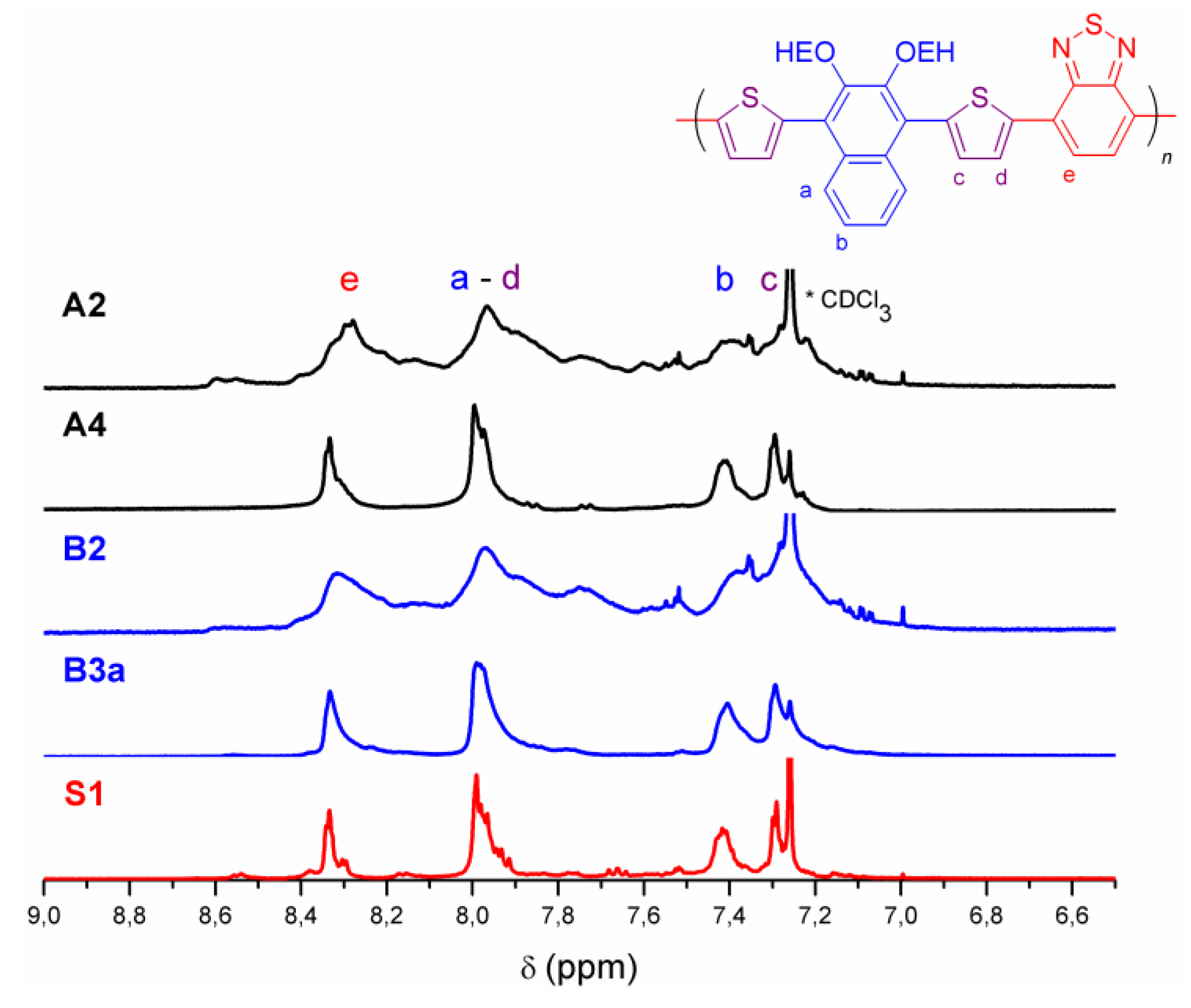

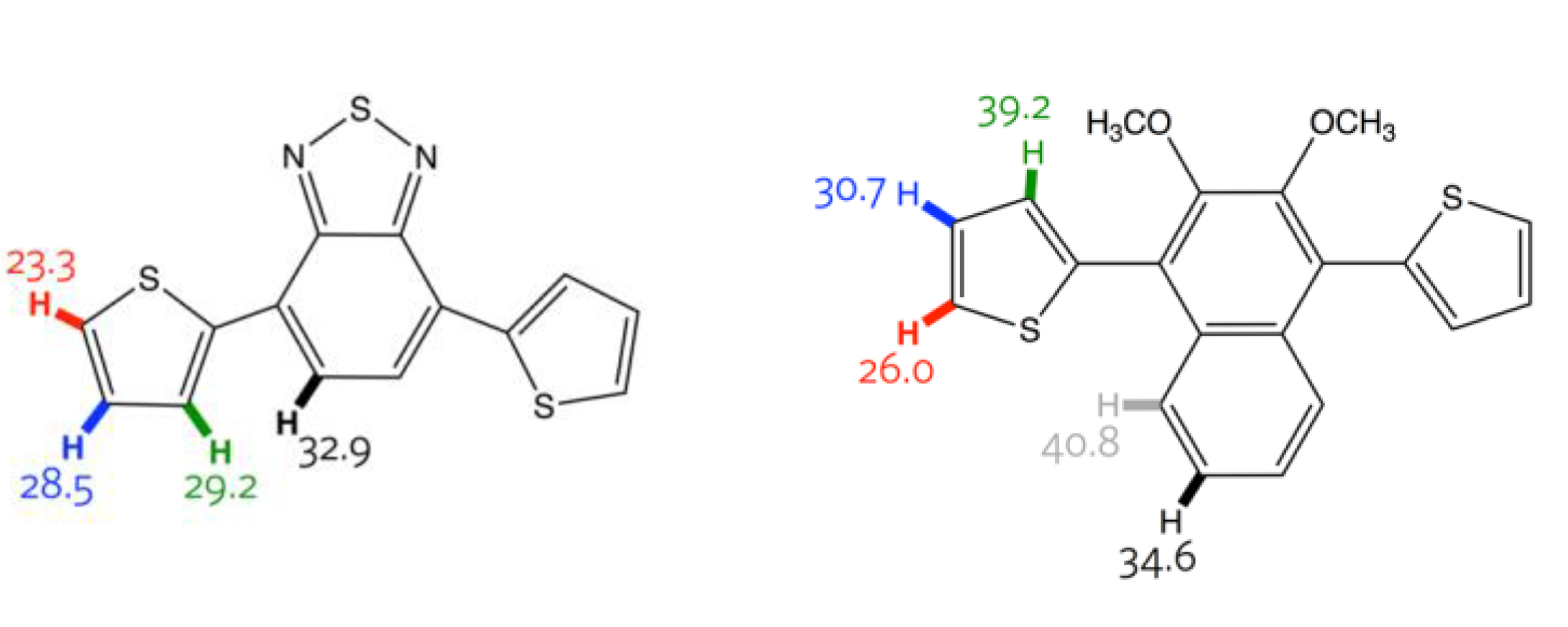

| Entry | Catalyst (%) | Solvent | Time (h) | Yield (%) 3 | Mw (kDa) | Mn (kDa) | PDI | λmaxabs (nm) 6 |
|---|---|---|---|---|---|---|---|---|
| A1 | Pd2(dba)3•CHCl3 (2) | THF | 0.5 | - 4 | - | - | − | - |
| A2 | Pd(OAc)2 (5) | THF | 2.0 | 20 5 | 37 | 20 | 1.9 | 489 |
| A3 | Pd(OAc)2 (5) | DMAc | 24 | - 4 | - | - | - | - |
| A4 | Pd(OAc)2 (5) | Toluene | 21 | 54 | 4.1 | 2.5 | 1.6 | 484 |
| B1 | Pd(OAc)2 (5) | THF | 1.5 | 13 5 | 110 | 19 | 5.8 | 488 |
| B2 | Pd2(dba)3•CHCl3 (2) | THF | 2.0 | 33 5 | 83 | 24 | 3.5 | 488 |
| B3a | Pd2(dba)3•CHCl3 (2) | Toluene | 3.0 | 72 | 72 | 30 | 2.4 | 482 |
| B3b | Pd2(dba)3•CHCl3 (2) | Toluene | 3.0 | 79 | 127 | 49 | 2.6 | 481 |
| B3c | Pd2(dba)3•CHCl3 (2) | Toluene | 5.0 | 74 | 105 | 43 | 2.4 | 480 |
| B4 | Pd(Herrmann) (2) | Toluene | 5.0 | 69 | 78 | 20 | 3.9 | 482 |
| B5 | Pd(OAc)2 (2) | Toluene | 3.0 | 64 | 42 | 18 | 2.3 | 482 |
| B61 | Pd2(dba)3•CHCl3 (2) | Toluene | 5.0 | 67 | 67 | 34 | 2.0 | 482 |
| B72 | Pd2(dba)3•CHCl3 (2) | Toluene | 5.0 | 38 | 19 | 14 | 1.4 | 481 |
| S1 (SPC) | Pd[PPh3]4 (4) | Toluene | 48 | 18 | 9.0 | 6.0 | 1.5 | 478 |
| Entry | TDT5% (°C) 1 | λmaxsol (nm) 2 | Egopt sol (eV) 3 | λmaxfilm (nm) 2 | Egopt Film (eV) 3 | EHOMO (eV) 4 | ELUMO (eV) 5 |
|---|---|---|---|---|---|---|---|
| B3a | 381 | 482 | 2.24 | 498 | 2.15 | −5.31 | −3.17 |
| B3b | 381 | 482 | 2.24 | 496 | 2.15 | −5.35 | −3.20 |
| B3c | 381 | 482 | 2.24 | 496 | 2.15 | −5.47 | −3.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jessop, I.A.; Chong, A.; Graffo, L.; Camarada, M.B.; Espinoza, C.; Angel, F.A.; Saldías, C.; Tundidor-Camba, A.; Terraza, C.A. Synthesis and Characterization of a 2,3-Dialkoxynaphthalene-Based Conjugated Copolymer via Direct Arylation Polymerization (DAP) for Organic Electronics. Polymers 2020, 12, 1377. https://doi.org/10.3390/polym12061377
Jessop IA, Chong A, Graffo L, Camarada MB, Espinoza C, Angel FA, Saldías C, Tundidor-Camba A, Terraza CA. Synthesis and Characterization of a 2,3-Dialkoxynaphthalene-Based Conjugated Copolymer via Direct Arylation Polymerization (DAP) for Organic Electronics. Polymers. 2020; 12(6):1377. https://doi.org/10.3390/polym12061377
Chicago/Turabian StyleJessop, Ignacio A., Aylin Chong, Linda Graffo, María B. Camarada, Catalina Espinoza, Felipe A. Angel, Cesar Saldías, Alain Tundidor-Camba, and Claudio A. Terraza. 2020. "Synthesis and Characterization of a 2,3-Dialkoxynaphthalene-Based Conjugated Copolymer via Direct Arylation Polymerization (DAP) for Organic Electronics" Polymers 12, no. 6: 1377. https://doi.org/10.3390/polym12061377
APA StyleJessop, I. A., Chong, A., Graffo, L., Camarada, M. B., Espinoza, C., Angel, F. A., Saldías, C., Tundidor-Camba, A., & Terraza, C. A. (2020). Synthesis and Characterization of a 2,3-Dialkoxynaphthalene-Based Conjugated Copolymer via Direct Arylation Polymerization (DAP) for Organic Electronics. Polymers, 12(6), 1377. https://doi.org/10.3390/polym12061377






