Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Commercial poly(lactic) acid (PLA), trade name 2003D, produced by Nature Works LLC (Minnetonka, Minneapolis, MN, USA) was used. This is a commercial grade containing about 4% of D-lactic acid units that lower the melting point and the crystallization tendency, improving the processing ability. This PLA, according to the producer’s data sheet has a density of 1.24 g/cm3, a melt flow index (MFI) of 6 g/10 min (210 °C, 2.16 kg) and a nominal average molar mass of 200,000 g/mol.
- Poly(butylene succinate) (PBS), trade name BioPBS FD92PM, purchased from Mitsubishi Chemical Corporation (Tokyo, Japan). It is a copolymer of succinic acid, adipic acid and 1,4-butandiol with a melt flow index (MFI) of 4 g/10 min (190 °C, 2.16 kg) and a density of 1.24 g/cm3.
- Acetyl tributyl citrate (ATBC), a product of Tecnosintesi S.p.A (Bergamo, Italy), was used as biobased and biodegradable plasticizer. It is a colorless and odorless liquid having a density of 1.05 g/cm3 and a molecular weight of 402.5 g/mol.
- Plastistrength 550 (named PST for brevity), commercialized from Arkema (Paris, France), is a medium molecular-weight acrylic copolymer that appears as a white powder with a density of 1.17 g/cm3. It is a commercial melt strength enhancer commonly added to improve the melt processability.
- Poly(ethylene glycol) (PEG6000) provided by Sigma-Aldrich (St. Louis, MO, USA) was used, for improving the dispersion of chitin nanofibrils [48]. It is a colorless solid, with a high molecular weight of 6000 g/mol and a solubility in water of 50 mg/mL at 20 °C.
- Chitin nanofibrils (CNs) water suspension (2 wt % of concentration) was supplied by MAVI SUD (Latina, Italy). CNs represent the pure and polysaccharidic molecular portion of α-chitin obtained after elimination of the protein portion. These fibrils have an average size of 240 × 7 × 5 nanometers (nm) and a shape like thin needles [48]. The production process of chitin nano-fibrils patented by MAVI results in the formation of a stable aqueous suspension of nanofibrils containing 300 billion nano crystals per milliliter with the addition of sodium benzoate. This substance is an anti-MLD, added to the suspension to avoid the possible attack of mold and bacteria on the chitin [46].
- Two different typologies of calcium carbonate, commercialized by Omya SpA (Avenza, Italy), with different particle size distributions were used: Omyacarb 2-AV (named 2AV), Omya Smartfill 55-OM (named Smartfill). Hakuenka CC-R (named CCR) is commercialized by Shiraishi. 2AV has a micrometric particle size with a diameter value (relative to the maximum distribution curve, d98%) of 15 µm, and 38% of particles of diameter less than 2 µm. The average statistical diameter (d50%) is 2.6 µm with a specific weight of 2.7 g/cm3. Smartfill is fine ground and surface fatty acid treated calcium carbonate having 55% of particles with an average diameter <2 μm (bulk density: 1.1 g/mL). CCR is a precipitated nano-calcium carbonate coated with acids having an average particle size of 80 nm (specific weight of 2.6–2.7 g/cm3).
2.2. Characterization of Fillers
2.3. Blends Preparation and Torque Characterization
2.4. Melt Flow Rate
2.5. Thermal Characterization by Differential Scanning Calorimetry (DSC)
2.6. Tensile Test
2.7. Scanning Electron Microscopy Analysis (SEM)
2.8. Migration Tests
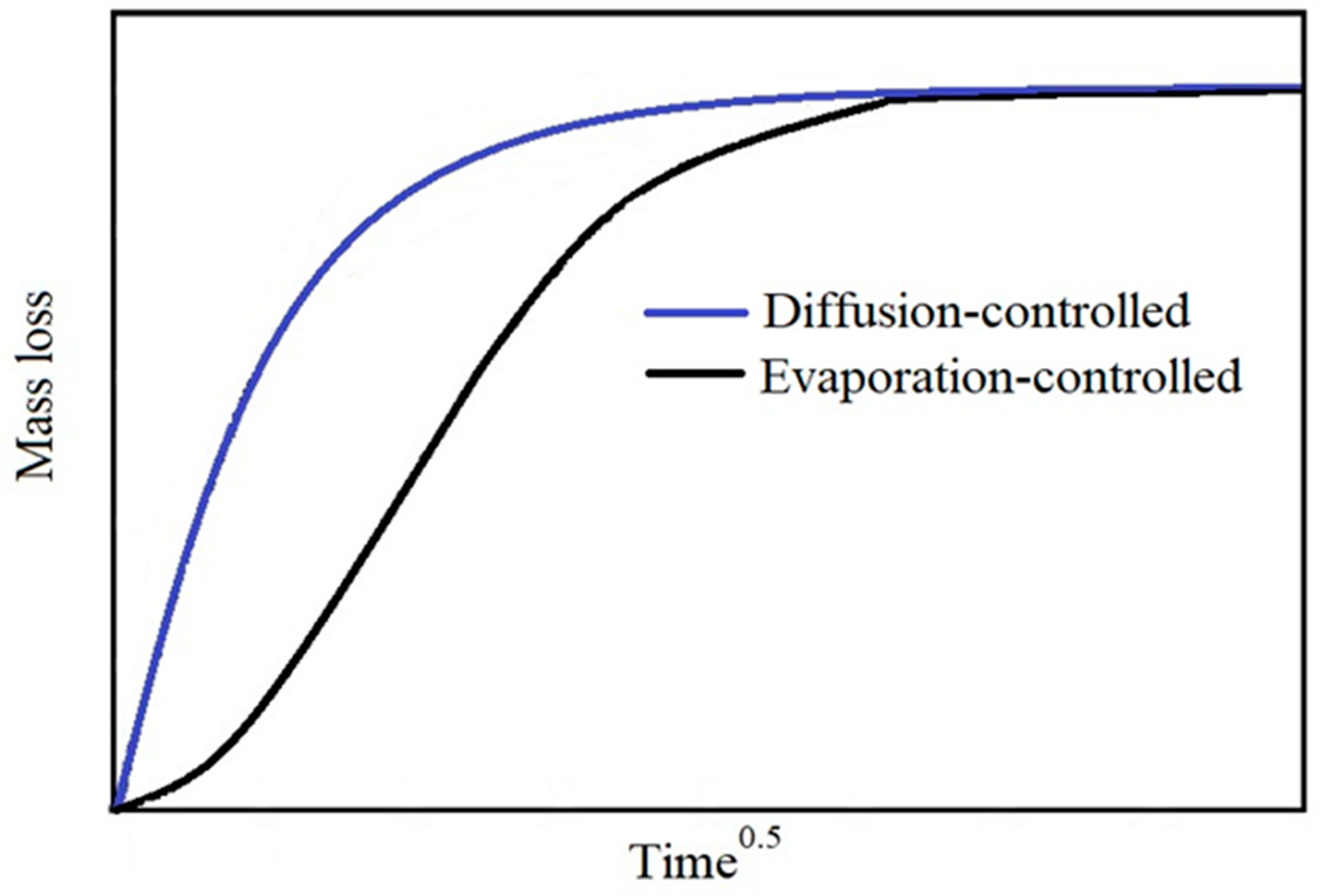
3. Theoretical Analysis
- Cx (mg/cm3) that is the concentration of the chemical species that diffuses at a distance x from the center of the sample at the time t
- C0 (mg/cm3) is the starting concentration of the chemical species that diffuses at t = 0; thus, it will coincide with the initial concentration of the plasticizer present in the sample
- D is the diffusion coefficient (cm2/s)
- h (mm) is the sample thickness
- erf is the error function (where )
4. Results and Discussion
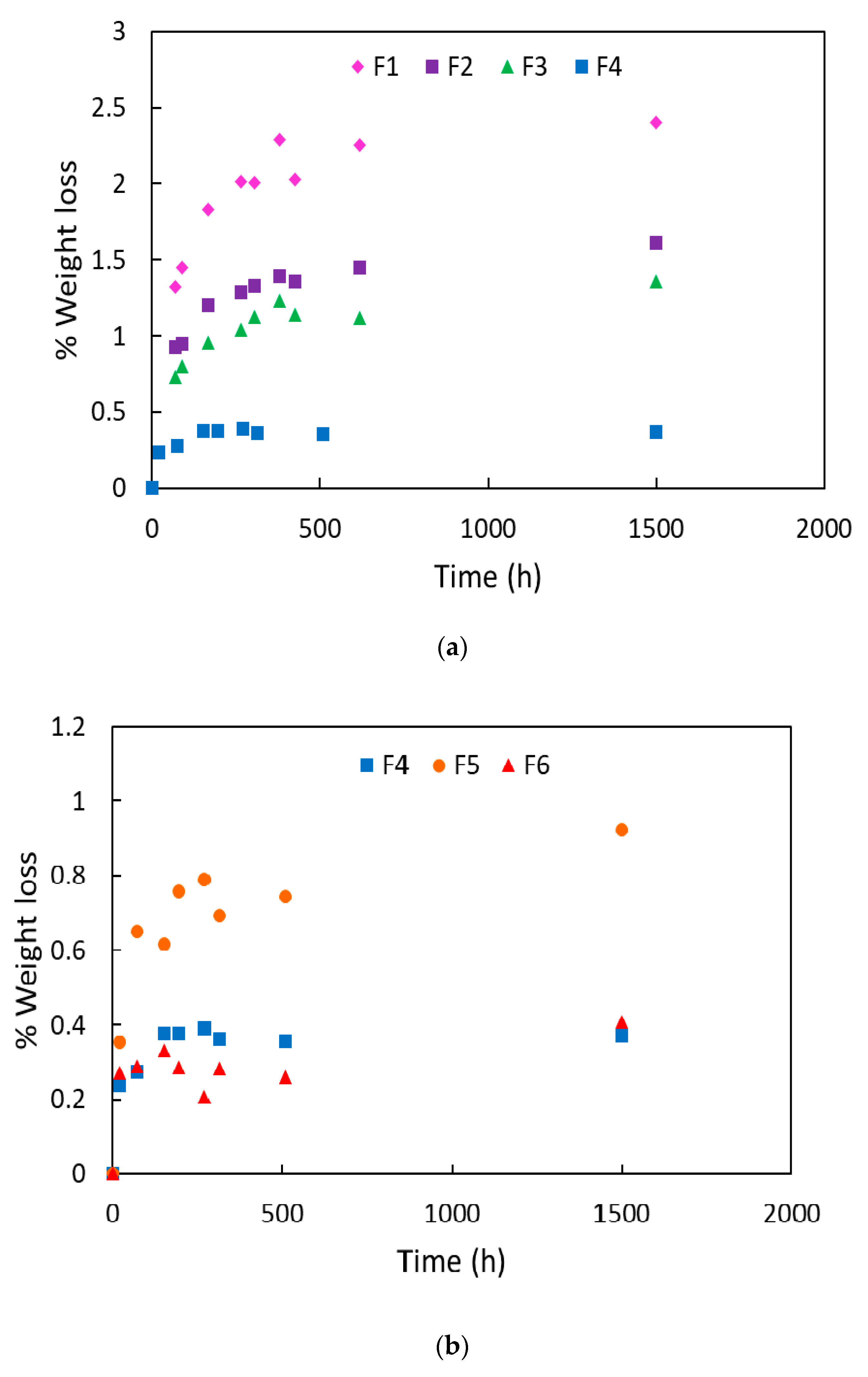
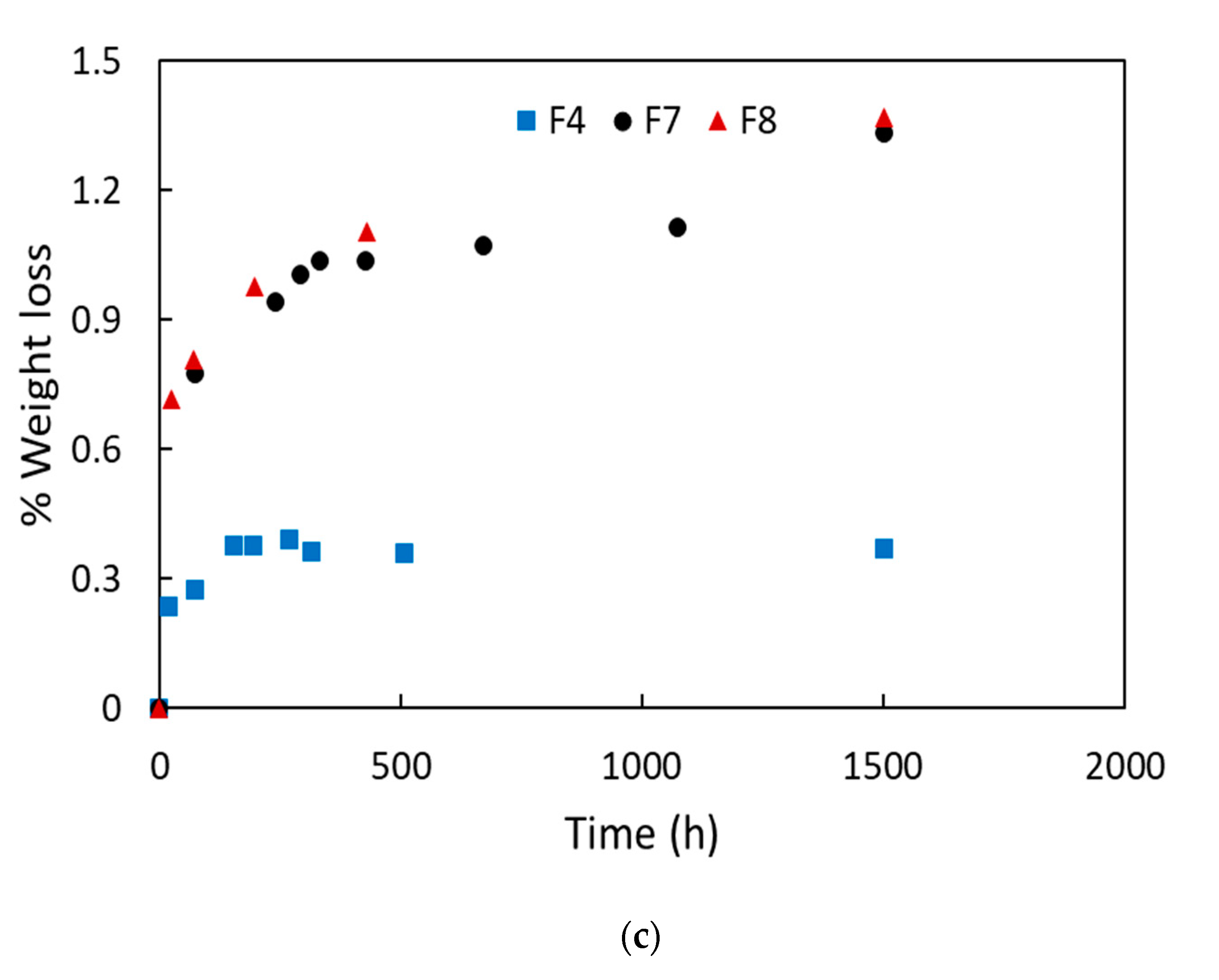
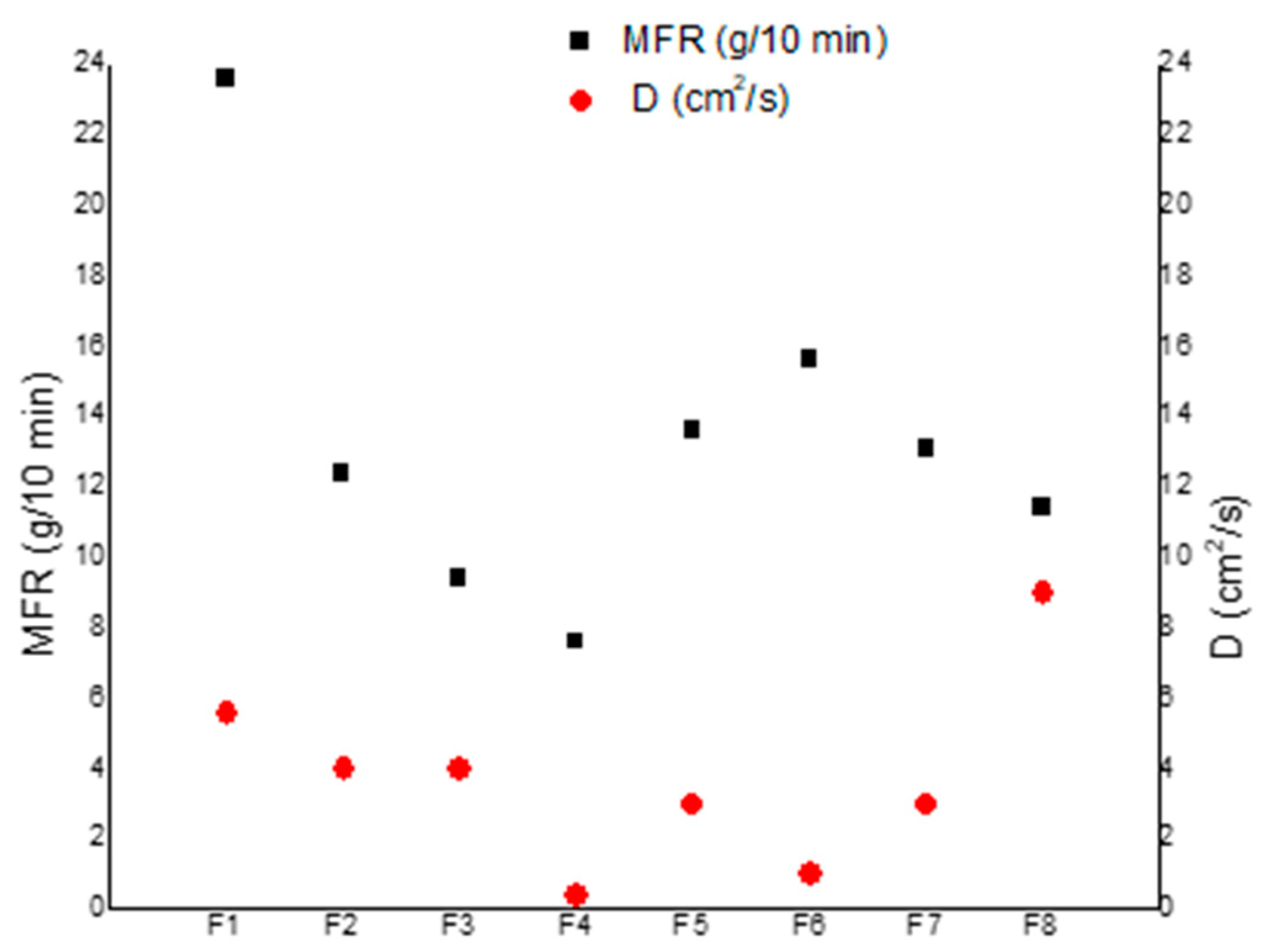
4.1. Migration Results and Determination of the Diffusion Coefficients
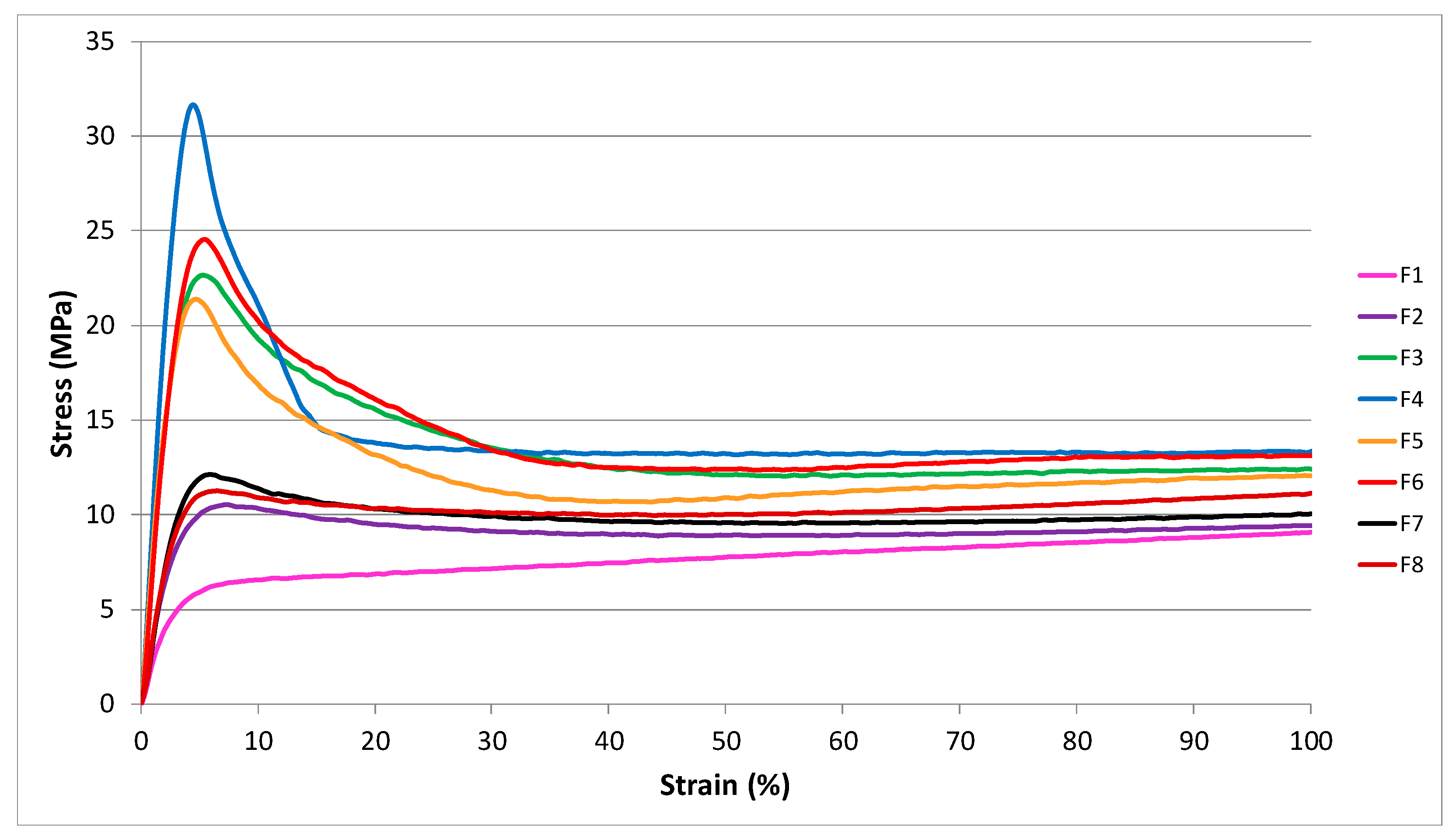
4.2. Mechanical Characterization of Blends
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, M.G.; Bharathi, P.; Akila, R.M. A comprehensive review on biopolymers. Sci. Rev. Chem. Commun. 2014, 4, 61–68. [Google Scholar]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A. Evaluation of Mechanical and Interfacial Properties of Bio-Composites Based on Poly(Lactic Acid) with Natural Cellulose Fibers. Int. J. Mol. Sci. 2019, 20, 960. [Google Scholar] [CrossRef] [PubMed]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Scatto, M.; Salmini, E.; Castiello, S.; Coltelli, M.B.; Conzatti, L.; Stagnaro, P.; Andreotti, L.; Bronco, S. Plasticized and nanofilled poly(lactic acid)-based cast films: Effect of plasticizer and organoclay on processability and final properties. J. Appl. Polym. Sci. 2013, 127, 4947–4956. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of nucleating agents on crystallinity and properties of poly(lactic acid) (PLA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Krishnan, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Toughening of Polylactic Acid: An Overview of Research Progress. Polym. Plast. Technol. Eng. 2016, 55, 1623–1652. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarría, G.; Eguiazábal, J.I. Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Jawalkar, S.S.; Aminabhavi, T.M. Molecular modeling simulations and thermodynamic approaches to investigate compatibility/incompatibility of poly(l-lactide) and poly(vinyl alcohol) blends. Polymer 2006, 47, 8061–8071. [Google Scholar] [CrossRef]
- Lipsa, R.; Tudorachi, N.; Vasile, C. Poly(vinyl alcohol)/poly(lactic acid) blends biodegradable films doped with colloidal silver. Rev. Roum. Chim. 2008, 53, 405–413. [Google Scholar]
- Bhatia, A.; Gupta, R.K.; Bhattacharya, S.N.; Choi, H.J. Compatibility of biodegradable poly(lactic acid) (PLA) and poly (butylene succinate) (PBS) blends for packaging application. Korea Aust. Rheol. J. 2007, 19, 125–131. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Wu, Q.; Ren, J. Preparation, mechanical, and thermal properties of biodegradable polyesters/poly(lactic acid) blends. J. Nanomater. 2010, 2010. [Google Scholar] [CrossRef]
- Aversa, C.; Barletta, M.; Puopolo, M.; Vesco, S. Cast extrusion of low gas permeability bioplastic sheets in PLA/PBS and PLA/PHB binary blends. Polym. Technol. Mater. 2020, 59, 231–240. [Google Scholar] [CrossRef]
- Ostrowska, J.; Sadurski, W.; Paluch, M.; Tyński, P.; Bogusz, J. The effect of poly(butylene succinate) content on the structure and thermal and mechanical properties of its blends with polylactide. Polym. Int. 2019, 68, 1271–1279. [Google Scholar] [CrossRef]
- Oguz, H.; Dogan, C.; Kara, D.; Ozen, Z.T.; Ovali, D.; Nofar, M. Development of PLA-PBAT and PLA-PBSA bio-blends: Effects of processing type and PLA crystallinity on morphology and mechanical properties. AIP Conf. Proc. 2019, 2055. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Sojoudiasli, H.; Park, C.B.; Carreau, P.J.; Heuzey, M.C.; Kamal, M.R. Mechanical and bead foaming behavior of PLA-PBAT and PLA-PBSA blends with different morphologies. Eur. Polym. J. 2017, 90, 231–244. [Google Scholar] [CrossRef]
- Quero, E.; Müller, A.J.; Signori, F.; Coltelli, M.B.; Bronco, S. Isothermal cold-crystallization of PLA/PBAT blends with and without the addition of acetyl tributyl citrate. Macromol. Chem. Phys. 2012, 213, 36–48. [Google Scholar] [CrossRef]
- Gigante, V.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rubber Toughening of Polylactic Acid (PLA) with Poly(butylene adipate-co-terephthalate) (PBAT): Mechanical Properties, Fracture Mechanics and Analysis of Ductile-to-Brittle Behavior while Varying Temperature and Test Speed. Eur. Polym. J. 2019, 115, 125–137. [Google Scholar] [CrossRef]
- Pietrosanto, A.; Scarfato, P.; Di Maio, L.; Nobile, R.M.; Incarnato, L. Evaluation of the Suitability of Poly(Lactide)/Poly(Butylene-Adipate-co-Terephthalate) Blown Films for Chilled and Frozen Food Packaging Applications. Polymers (Basel) 2020, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-F.; Linde, E.; Hedenqvist, M.S. Plasticiser loss from plastic or rubber products through diffusion and evaporation. Mater. Degrad. 2019, 3, 18. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in Vitro Toxicity and Chemical Composition of Plastic Consumer Products. Environ. Sci. Technol. 2019, 53. [Google Scholar] [CrossRef]
- Jamarani, R.; Erythropel, H.C.; Nicell, J.A.; Leask, R.L.; Marić, M. How green is your plasticizer? Polymers 2018, 10, 834. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Bosnea, L. Migration of substances from food packaging materials to foods. Crit. Rev. Food Sci. Nutr. 2004, 44, 63–76. [Google Scholar] [CrossRef]
- Balestri, E.; Menicagli, V.; Ligorini, V.; Fulignati, S.; Raspolli Galletti, A.M.; Lardicci, C. Phytotoxicity assessment of conventional and biodegradable plastic bags using seed germination test. Ecol. Indic. 2019, 102, 569–580. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Danti, S.; Trombi, L.; Morganti, P.; Donnarumma, G.; Baroni, A.; Fusco, A.; Lazzeri, A. Preparation of innovative skin compatible films to release polysaccharides for biobased beauty masks. Cosmetics 2018, 5, 70. [Google Scholar] [CrossRef]
- Hiremath, C.; Heggannavar, G.B.; Mitchell, G.R.; Kariduraganavar, M.Y. Biopolymers in Drug-Delivery Applications. In Green Polymer Composites Technology: Properties and Applications; Inamuddin, Ed.; CRC: Boca Raton, FL, USA; Taylor and Francis: Abingdon, UK, 2015; pp. 514–525. [Google Scholar]
- Xiao, H.; Lu, W.; Yeh, J. Effect of plasticizer on the crystallization behavior of poly(lactic acid). J. Appl. Polym. Sci. 2009, 113, 112–121. [Google Scholar] [CrossRef]
- Stark, T.D.; Choi, H.; Diebel, P.W. Influence of plasticizer molecular weight on plasticizer retention in PVC geomembranes. Geosynth. Int. 2005, 12, 99–110. [Google Scholar] [CrossRef]
- Higa, C.M.; Tek, A.T.; Wojtecki, R.J.; Braslau, R. Nonmigratory internal plasticization of poly(vinyl chloride) via pendant triazoles bearing alkyl or polyether esters. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2397–2411. [Google Scholar] [CrossRef]
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Vimala, K.; Raju, K.M. Surface treatment of plasticized poly(vinyl chloride) to prevent plasticizer migration. J. Appl. Polym. Sci. 2010, 115, 1589–1597. [Google Scholar] [CrossRef]
- Dressler, S. Plasma treatments. In Surface Modification Technologies: An Engineer’s Guide; Marcel Dekker: New York, NY, USA, 1989; pp. 317–419. [Google Scholar]
- Li, X.; Xiao, Y.; Wang, B.; Tang, Y.; Lu, Y.; Wang, C. Effect of Poly(1,2-propylene glycol adipate) and Nano-CacCO3 on DOP Migration and Mechanical Proprties of Flexible PVC. J. Appl. Polym. Sci. 2012, 124, 1737–1743. [Google Scholar] [CrossRef]
- Scott, M.P.; Rahman, M.; Brazel, C.S. Application of ionic liquids as low-volatility plasticizers for PMMA. Eur. Polym. J. 2003, 39, 1947–1953. [Google Scholar] [CrossRef]
- Ma, Y.; Liao, S.; Li, Q.; Guan, Q.; Jia, P.; Zhou, Y. Physical and chemical modifications of poly(vinyl chloride) materials to prevent plasticizer migration—Still on the run. React. Funct. Polym. 2020, 147, 104458. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Morreale, M.; La Mantia, F.P. The Effects of Nanoclay on the Mechanical Properties, Carvacrol Release and Degradation of a PLA/PBAT Blend. Materials 2020, 13, 983. [Google Scholar] [CrossRef]
- Argon, A.S.; Cohen, R.E. Toughenability of polymers. Polymer 2003, 44, 6013–6032. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Beatrice Coltelli, M.; Lazzeri, A. Rigid filler toughening in PLA-Calcium Carbonate composites: Effect of particle surface treatment and matrix plasticization. Eur. Polym. J. 2018, 113, 78–88. [Google Scholar] [CrossRef]
- Cioni, B.; Lazzeri, A. The Role of Interfacial Interactions in the Toughening of Precipitated Calcium Carbonate–Polypropylene Nanocomposites. Compos. Interfaces 2010, 17, 533–549. [Google Scholar] [CrossRef]
- Yang, B.; Bai, Y.; Cao, Y. Effects of inorganic nano-particles on plasticizers migration of flexible PVC. J. Appl. Polym. Sci. 2010, 115, 2178–2182. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Xiao, Y.; Li, X.H.; Wang, B. Effects of nano-particles on DOP migration and tensile properties of flexible PVC sheets. Adv. Mater. Res. 2012, 548, 119–122. [Google Scholar] [CrossRef]
- Gigante, V.; Coltelli, M.; Vannozzi, A.; Panariello, L.; Fusco, A.; Trombi, L.; Donnarumma, G.; Danti, S.; Lazzeri, A. Flat Die Extruded Biocompatible Poly(Lactic Acid). Polymers 2019, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.-B.; Gigante, V.; Vannozzi, A.; Aliotta, L.; Danti, S.; Neri, S.; Gagliardini, A.; Morganti, P.; Panariello, L.; Lazzeri, A. Poly(lactic) acid (PLA) based nano-structured functional films for personal care applications. In Proceedings of the AUTEX 2019—19th World Textile Confreence on Textiles at the Crossroads, Ghent, Belgium, 11–15 June 2019. [Google Scholar]
- Coltelli, M.B.; Gigante, V.; Panariello, L.; Morganti, P.; Cinelli, P.; Danti, S.; Lazzeri, A. Chitin nanofibrils in renewable materials for packaging and personal care applications. Adv. Mater. Lett. 2018, 10, 425–430. [Google Scholar] [CrossRef]
- Coltelli, M.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Fusco, A.; Donnarumma, G.; Lazzeri, A. Properties and Skin Compatibility of Films Based on Poly(Lactic Acid) (PLA) Bionanocomposites Incorporating Chitin Nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly(Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 1133-1:2011: Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 1; International Organization for Standardization: Geneva, Switzerland, 2011; Volume 2011, p. 24. [Google Scholar]
- Aliotta, L.; Gigante, V.; Acucella, O.; Signori, F.; Lazzeri, A. Thermal, Mechanical and Micromechanical Analysis of PLA/PBAT/POE-g-GMA Extruded Ternary Blends. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- ISO. ISO 527, Plastics, Determination of Tensile Properties. Part 2: Test Conditions for Moulding and Extrusion Plastics; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Hamdani, M.; Feigenbaum, A.; Vergnaud, J.M. Prediction of worst case migration from packaging to food using mathematical models. Food Addit. Contam. 1997, 14, 499–506. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; ISBN 0-19-853411-6. [Google Scholar]
- Vergnaud, J.M. General survey on the mass transfers taking place between a polymer and a liquid. J. Polym. Eng. 1995, 15, 57–78. [Google Scholar] [CrossRef]
- Mercer, A.; Castle, L.; Comyn, J.; Gilbert, J. Evaluation of a predictive mathematical model of di-(2-ethylhexyl) adipate plasticizer migration from PVC film into foods. Food Addit. Contam. 1990, 7, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, R.; Kontogeorgis, G.M.; Kristiansen, J.K.; Jensen, T.F. Modeling of the Migration of Glycerol Monoester Plasticizers in Highly Plasticized Poly(vinyl chloride). J. Vinyl Addit. Technol. 2009, 15, 147–158. [Google Scholar] [CrossRef]
- Till, D.E.; Reid, R.C.; Schwartz, P.S.; Sidman, K.R.; Valentine, J.R.; Whelan, R.H. Plasticizer migration from polyvinyl chloride film to solvents and foods. Food Chem. Toxicol. 1982, 20, 95–104. [Google Scholar] [CrossRef]
- Huang, J.-C.; Liu, H.; Liu, Y. Diffusion in polymers with concentration dependent diffusivity. Int. J. Polym. Mater. 2001, 49, 15–24. [Google Scholar] [CrossRef]
- Storey, R.F.; Mauritz, K.A.; Cox, B.D. Diffusion of various dialkyl phthalate plasticizers in PVC. Macromolecules 1989, 22, 289–294. [Google Scholar] [CrossRef]
- Philip, J.R. A very general class of exact solutions in concentration-dependent diffusion. Nature 1960, 185, 233. [Google Scholar] [CrossRef]
- Lundsgaard, R. Migration of Plasticisers from PVC and Other Polymers. Ph.D. Thesis, Technical University of Denmark, Kgs. Lyngby, Denmark, 2010. [Google Scholar]
- Ekelund, M.; Azhdar, B.; Gedde, U.W. Evaporative loss kinetics of di (2-ethylhexyl) phthalate (DEHP) from pristine DEHP and plasticized PVC. Polym. Degrad. Stab. 2010, 95, 1789–1793. [Google Scholar] [CrossRef]
- Smith, G.S.; Skidmore, C.B.; Howe, P.M.; Majewski, J. Diffusion, evaporation, and surface enrichment of a plasticizing additive in an annealed polymer thin film. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3258–3266. [Google Scholar] [CrossRef]
- Zhang, J.F.; Sun, X. Physical characterization of coupled poly(lactic acid)/starch/maleic anhydride blends plasticized by acetyl triethyl citrate. Macromol. Biosci. 2004, 4, 1053–1060. [Google Scholar] [CrossRef]
- Cao, Z.; Daly, M.; Clémence, L.; Geever, L.M.; Major, I.; Higginbotham, C.L.; Devine, D.M. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci. 2016, 378, 320–329. [Google Scholar] [CrossRef]
- Shi, X.; Rosa, R.; Lazzeri, A. On the coating of precipitated calcium carbonate with stearic acid in aqueous medium. Langmuir 2010, 26, 8474–8482. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A.; Suter, U.W. Surface treatment of calcite with fatty acids: Structure and properties of the organic monolayer. Chem. Mater. 2002, 14, 4408–4415. [Google Scholar] [CrossRef]
- Huang, H.; Tian, M.; Yang, J.; Li, H.; Liang, W.; Zhang, L.; Li, X. Stearic acid surface modifying Mg(OH)2: Mechanism and its effect on properties of ethylene vinyl acetate/Mg(OH)2 composites. J. Appl. Polym. Sci. 2008, 107, 3325–3331. [Google Scholar] [CrossRef]
- Rezaei Gomari, K.A.; Denoyel, R.; Hamouda, A.A. Wettability of calcite and mica modified by different long-chain fatty acids (C18 acids). J. Colloid Interface Sci. 2006, 297, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Jiménez, A.; González-Martínez, C.; Chiralt, A. Influence of plasticizers on thermal properties and crystallization behaviour of poly(lactic acid) films obtained by compression moulding. Polym. Int. 2016, 65, 970–978. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Nowicki, M.; Vuković-Kwiatkowska, I.; Nowakowska, S. Crosslinked blends of poly(lactic acid) and polyacrylates: AFM, DSC and XRD studies. J. Polym. Res. 2013, 20. [Google Scholar] [CrossRef]
- Broers, L.; van Dongen, S.; de Goederen, V.; Ton, M.; Spaen, J.; Boeriu, C.; Schroën, K. Addition of Chitin Nanoparticles improves Polylactic Acid Film Properties. Nanotechnol. Adv. Mater. Sci. 2018, 1, 1–8. [Google Scholar]
- Chremos, A.; Jeong, C.; Douglas, J.F. Influence of polymer architectures on diffusion in unentangled polymer melts. Soft Matter 2017, 13, 5778–5784. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.B.; Bronco, S. Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]





| Blends Name | PLA (wt %) | PBS (wt %) | ATBC (wt %) | PST (wt %) | CaCO3 (wt %) | PEG6000 (wt %) | NC (wt %) |
|---|---|---|---|---|---|---|---|
| F1 | 63 | 17 | 20 | - | - | - | - |
| F2 | 62 | 16 | 20 | 2 | - | - | - |
| F3 | 59 | 15 | 20 | 2 | 4 (2AV) | - | - |
| F4 | 59 | 15 | 17 | 2 | 7 (2AV) | - | - |
| F5 | 59 | 15 | 17 | 2 | 7 (Smartfill) | - | - |
| F6 | 59 | 15 | 17 | 2 | 7 (CCR) | - | - |
| F7 | 61 | 15 | 18 | 2 | - | 2 | 2 |
| F8 | 57.5 | 14.5 | 15 | 2 | 7 (2AV) | 2 | 2 |
| Blends Name | Weight Loss (wt %) | Lost ATBC (wt %) |
|---|---|---|
| F1 | 2.40 ± 0.31 | 12.01 ± 1.53 |
| F2 | 1.61 ± 0.01 | 8.05 ± 0.08 |
| F3 | 1.36 ± 0.19 | 6.78 ± 0.95 |
| F4 | 0.39 ± 0.15 | 2.29 ± 0.08 |
| F5 | 0.92 ± 0.14 | 5.44 ± 0.83 |
| F6 | 0.22 ± 0.07 | 1.31 ± 0.43 |
| F7 | 1.33 ± 0.43 | 7.40 ± 2.38 |
| F8 | 1.37 ± 0.16 | 9.12 ± 1.06 |
| Samples | Langmuir Area (m2/g) | BET (m2/g) | Total Pore Volume (cm3/g) | Number of Particles Per Gram |
|---|---|---|---|---|
| 2AV | 4.3094 | 2.7126 | 0.0013 | 1.3 × 1012 |
| Smartfill | 6.1180 | 3.7659 | 0.0018 | 3.5 × 1012 |
| CCR | 32.1068 | 18.4610 | 0.0085 | 4.0 × 1014 |
| NC | 61.7235 | 39.1443 | 0.0194 | 4.1 × 1013 |
| Blends | Tg (°C) | Tcc (°C) | ΔHCC (J/g) | Tm (°C) | ΔHm (J/g) | XC (%) |
|---|---|---|---|---|---|---|
| F1 | 43.6 | 96.4 | 3.05 | 143.7 | 18.84 | 27 |
| F2 | 31.7 | 86.7 | 12.97 | 145.3 | 19.94 | 12 |
| F3 | 30.3 | 85.4 | 13.30 | 144.8 | 19.19 | 11 |
| F4 | 44 | 92.9 | 17.56 | 145.9 | 20.44 | 5 |
| F5 | 36.2 | 90 | 16.93 | 146.5 | 18.6 | 3 |
| F6 | 44.1 | 93.3 | 18.37 | 147.9 | 19.92 | 3 |
| F7 | 46.4 | 87.5 | 19.31 | 143.4 | 20.57 | 2 |
| F8 | 43.5 | 85.7 | 11.97 | 145.5 | 18.82 | 13 |
| Blends | Tg (°C) | Tcc (°C) | ΔHCC (J/g) | Tm (°C) | ΔHm (J/g) | XC (%) |
|---|---|---|---|---|---|---|
| F1start | 43.6 | 96.4 | 3 | 143.7 | 18.8 | 27 |
| F1finish | 42.4 | - | - | 145.6 | 23.5 | 40 |
| F2start | 42.7 | 86.7 | 12.9 | 145.3 | 19.9 | 12 |
| F2finish | 43.5 | - | - | 146.8 | 21.8 | 38 |
| F4start | 44 | 92.9 | 17.5 | 145.9 | 20.4 | 5 |
| F4finish | 43.8 | - | - | 147.4 | 21.9 | 40 |
| F7start | 46.4 | 87.5 | 19.31 | 143.4 | 20.57 | 2 |
| F7finish | 48.8 | - | - | 149.1 | 21.84 | 38.5 |
| Blends | D (cm2/s) Equation (7) | D (cm2/s) Equation (6) Weight Averaged |
|---|---|---|
| F1 | 5.6 × 10−12 | 1.3 × 10−10 |
| F2 | 4 × 10−12 | 1.2 × 10−10 |
| F3 | 4 × 10−12 | 1.1 × 10−10 |
| F4 | 4 × 10−13 | 8.2 × 10−11 |
| F5 | 3 × 10−12 | 9.8 × 10−11 |
| F6 | 1 × 10−12 | 8.3 × 10−11 |
| F7 | 3 × 10−12 | 9.7 × 10−11 |
| F8 | 9 × 10−12 | 1.3 × 10−10 |
| Blends | Torque (N∙cm) | MVR (cm3/10 min) | MFR (g/10 min) |
|---|---|---|---|
| F1 | 67.8 ± 5.4 | 22.5 ± 2.0 | 23.6 ± 2.1 |
| F2 | 72.8 ± 6.0 | 11.8 ± 0.9 | 12.4 ± 0.9 |
| F3 | 73.4 ± 9.8 | 8.7 ± 0.6 | 9.4 ± 0.6 |
| F4 | 83.4 ± 4.0 | 8.4 ± 2.7 | 7.6 ± 2.4 |
| F5 | 74.1 ± 5.4 | 12.0 ± 1.9 | 13.6 ± 2.2 |
| F6 | 66.4 ± 5.9 | 14.1 ± 1.9 | 15.6 ± 2.1 |
| F7 | 63.3 ± 3.6 | 11.8 ± 1.1 | 13.1 ± 1.3 |
| F8 | 73.5 ± 5.2 | 10.0 ± 1.3 | 11.4 ± 1.5 |
| Blends | σb (MPa) | εb (%) | σy (MPa) |
|---|---|---|---|
| F1 | 31.8 ± 1.4 | 572.7 ± 20.7 | - |
| F2 | 33.0 ± 1.2 | 554.2 ± 12.3 | 10.2 ± 0.7 |
| F3 | 32.5 ± 1.6 | 543.7 ± 29.8 | 23.3 ± 1.9 |
| F4 | 29.3 ± 3.4 | 512.5 ± 13.8 | 31.1 ± 2.2 |
| F5 | 29.0 ± 1.2 | 491.4 ± 25.9 | 20.7 ± 2.4 |
| F6 | 28.4 ± 1.8 | 507.6 ± 13.9 | 24.5 ± 1.7 |
| F7 | 25.5 ± 1.3 | 421.9 ± 25.1 | 11.6 ± 0.7 |
| F8 | 25.5 ± 1.0 | 400.1 ± 21.9 | 10.8 ± 1.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.-B.; Lazzeri, A. Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films. Polymers 2020, 12, 1366. https://doi.org/10.3390/polym12061366
Aliotta L, Vannozzi A, Panariello L, Gigante V, Coltelli M-B, Lazzeri A. Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films. Polymers. 2020; 12(6):1366. https://doi.org/10.3390/polym12061366
Chicago/Turabian StyleAliotta, Laura, Alessandro Vannozzi, Luca Panariello, Vito Gigante, Maria-Beatrice Coltelli, and Andrea Lazzeri. 2020. "Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films" Polymers 12, no. 6: 1366. https://doi.org/10.3390/polym12061366
APA StyleAliotta, L., Vannozzi, A., Panariello, L., Gigante, V., Coltelli, M.-B., & Lazzeri, A. (2020). Sustainable Micro and Nano Additives for Controlling the Migration of a Biobased Plasticizer from PLA-Based Flexible Films. Polymers, 12(6), 1366. https://doi.org/10.3390/polym12061366










