Assessment of Supercritical CO2 Extraction as a Method for Plastic Waste Decontamination
Abstract
1. Introduction
2. Materials and Methods
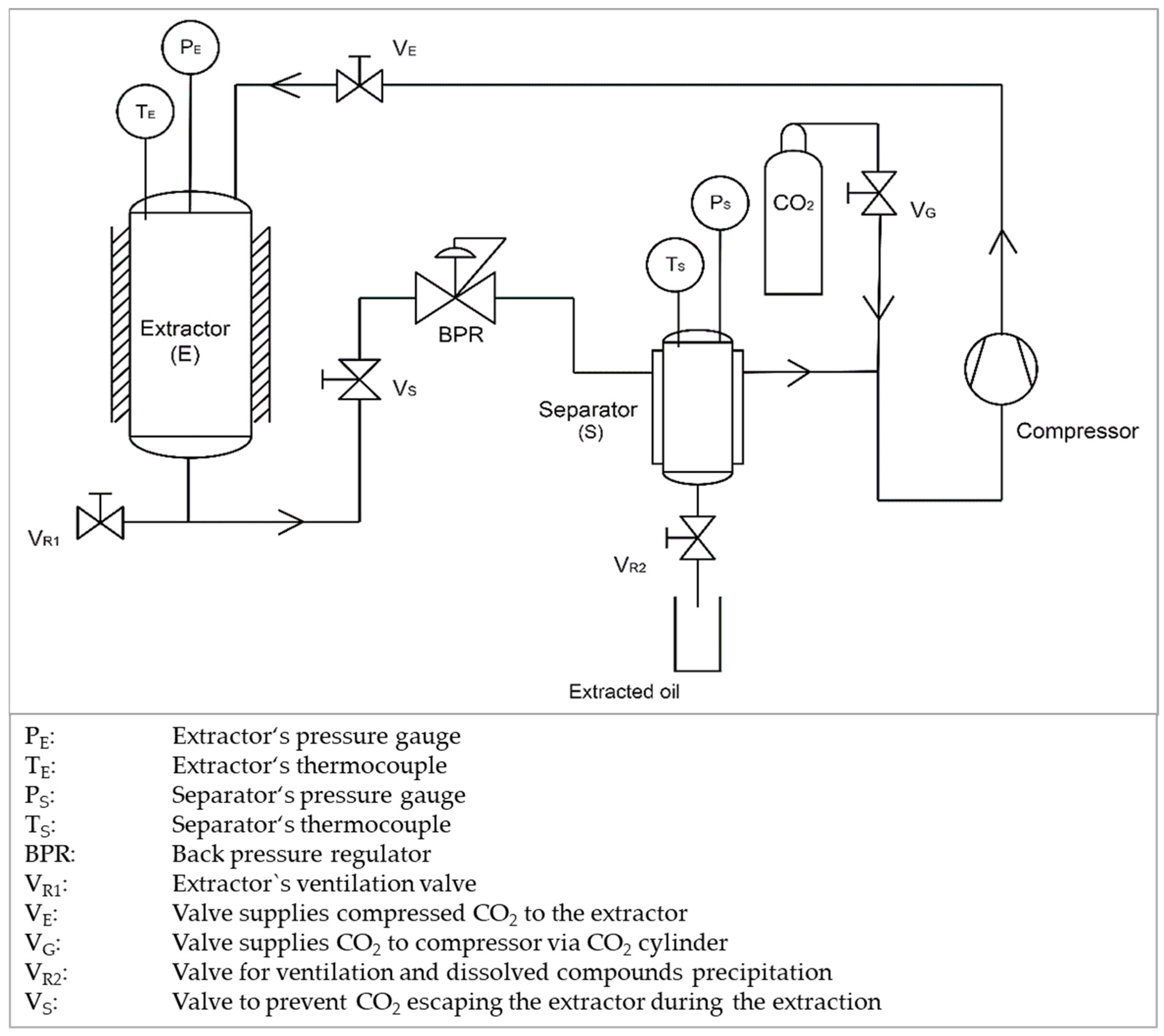
2.1. Plastics Decontamination Applying Supercritical Extraction
2.2. Plastic Specimens’ Characterization
2.2.1. Near Infrared Spectroscopy (NIR) Analysis
2.2.2. Degree of Plastic Contamination with Polycyclic Aromatic Hydrocarbons (PAHs)
- PAHs extraction by Randall hot extractionTest samples underwent preparation by extraction using Randall hot extraction. The applied extraction method was adapted from the method suggested by Geiss et al., 2018 [35]. Since the extraction temperature was not provided, a temperature slightly higher than the solvent’s boiling point was first applied and revised as needed.First, the samples were weighed in duplicates in 33 × 60 cellulose extraction sleeves (thimbles). The weight of the test samples ranged between 0.1 and 0.2 g. The reaction vessel, in which the extract is collected, was filled with 70 mL of toluene, and three to four boiling stones were added before connecting it to the condenser.Randall hot extraction consists of three main steps, immersion, rinsing and evaporation. The immersion step was conducted at 150 °C for 120 min. Subsequently, the thimble was pulled out of the sump (reaction vessel containing the extraction solvent). At this stage, the temperature was raised to 170 °C to rinse the sample for 60 min. The evaporation step was conducted at the same temperature (i.e., 170 °C) for as long as needed to reach a solution’s volume of ≤20 mL.After reaching the targeted extract volume, the heating was turned off and the reaction vessels were cooled down to room temperature. Then, 40 μL (Conc. = 40 ng mL−1) of an internal standard solution (Phenanthrene-d10 98 atom % D, 98% (CP) from Sigma-Aldrich (St. Louis, MO, USA)) was added to the solution to calculate the recovery. The extract was then filtered into a 100 mL pear-shaped flask. Finally, the extract was evaporated by a 0.7 bar nitrogen (N2) stream at room temperature. Drying was applied until the solution reached a volume of ~500 μL.
- Extract clean-upTo selectively extract polycyclic aromatic hydrocarbons (PAHs), a clean-up procedure was applied. The extract clean-up was achieved by applying solid phase extraction (SPE), by means of silica gel disposable extraction columns. The procedure of SPE consisted of column cleaning, silica bed conditioning, sample application, and elution. For silica bed conditioning, 5 mL dried n-Hexane were added to the SPE column twice. For sample elution (to separate the PAHs), 2 mL of dried CH2CL2/Hexane mixture was added to the column. This step was repeated twice in series and the eluate was collected in clean tubes. Finally, the obtained solution was concentrated to 500 μL using a 0.5 bar N2 stream.To prepare the test sample for the GC-MS analysis, the concentrated sample was transferred using a Pasteur pipette to a 1.0 mL brown glass GC-vial. The final sample volume was brought to 1.0 mL by adding n-Hexane.
- Analysis by GC-MSThe analysis of the samples was performed using a gas chromatograph HP 6890 coupled with a single ion-monitoring mass selective detector HP 5973 from Agilent Technologies Sales & Services GmbH & Co. KG (Waldbronn, Germany).Before conducting the analysis, a calibration step was conducted. The calibration was done prior to analyzing the sample set. For the calibration, different established concentrations of an external standard containing the 16 US-EPA PAHs—naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-c,d]pyrene, dibenz[a,h]anthracene, and benzo[g,h,i]perylene—were analyzed, and the respective responses were measured.
2.3. Extracted Oil Characterization
2.3.1. GC-MS Analysis of the Extracted Oil
2.3.2. Near Infrared Spectroscopy (NIR) Analysis
3. Results and Discussion
3.1. Assessment of the Extraction Behavior
3.2. Evaluation of the Samples Before and After Undergoing SCE
3.2.1. General Analysis
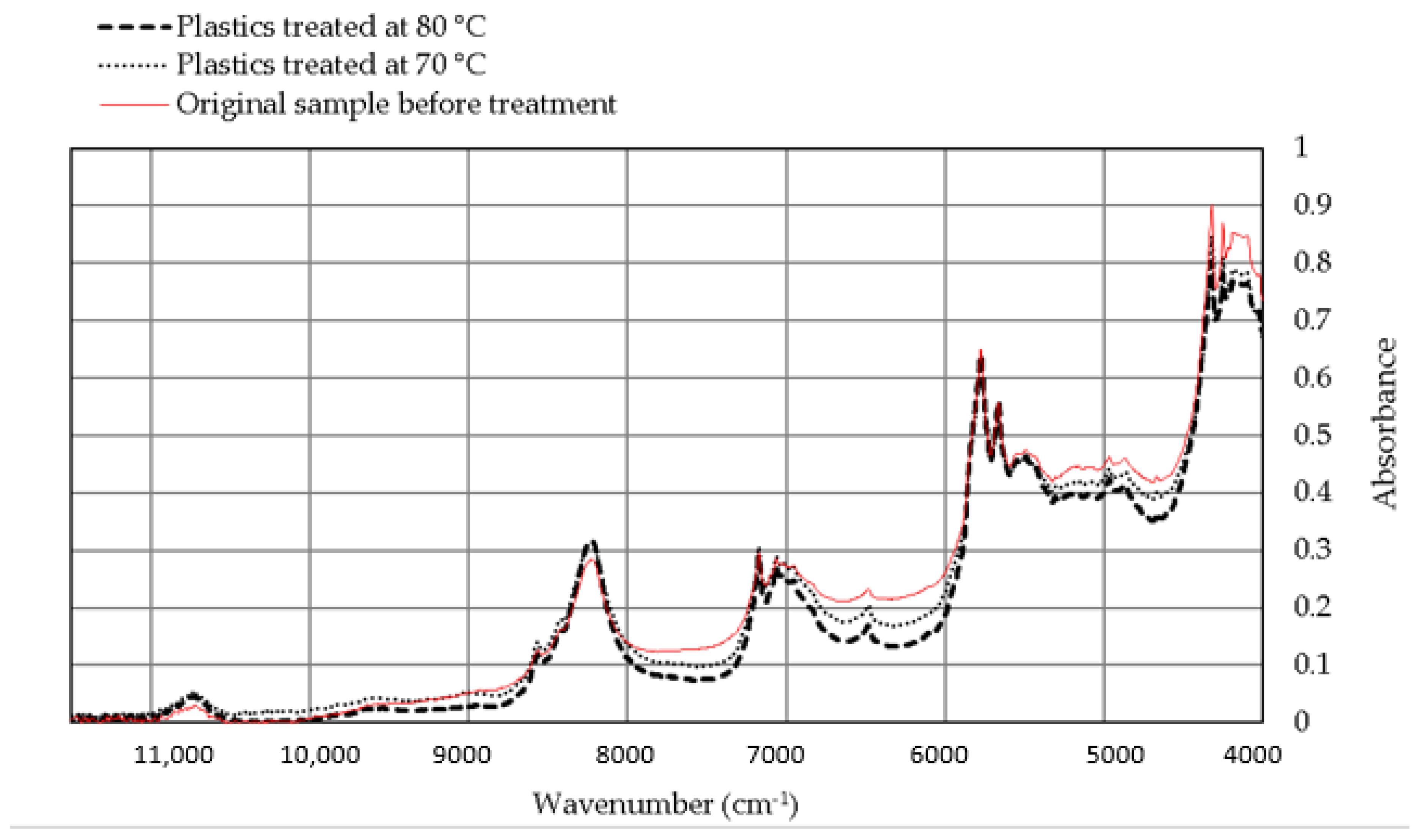
3.2.2. NIR Analysis
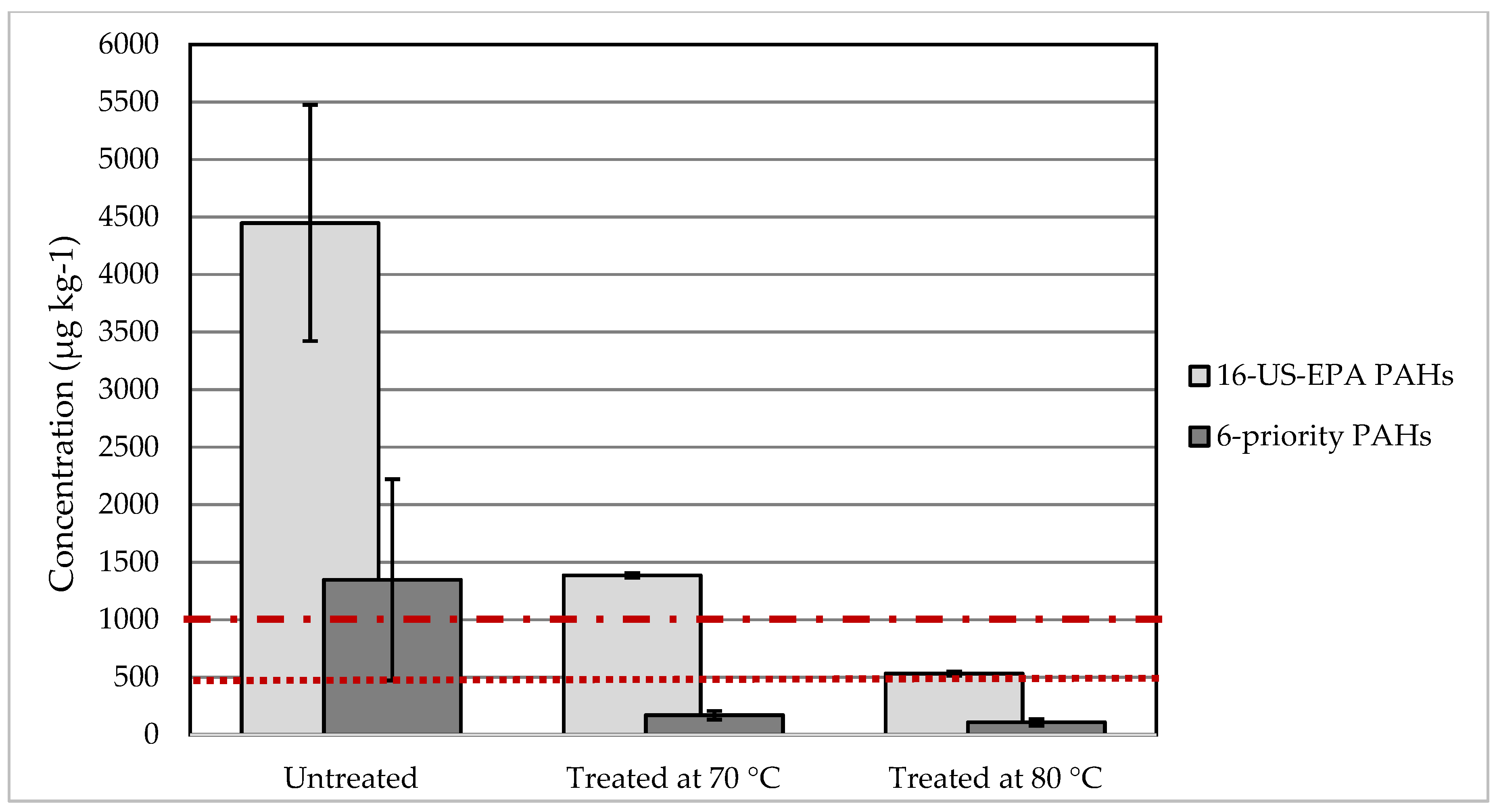
3.2.3. Efficiency of Plastics Decontamination, Focusing on the PAHs Content
3.3. Analysis of the Extracted Oil
3.3.1. Quantitative Analysis
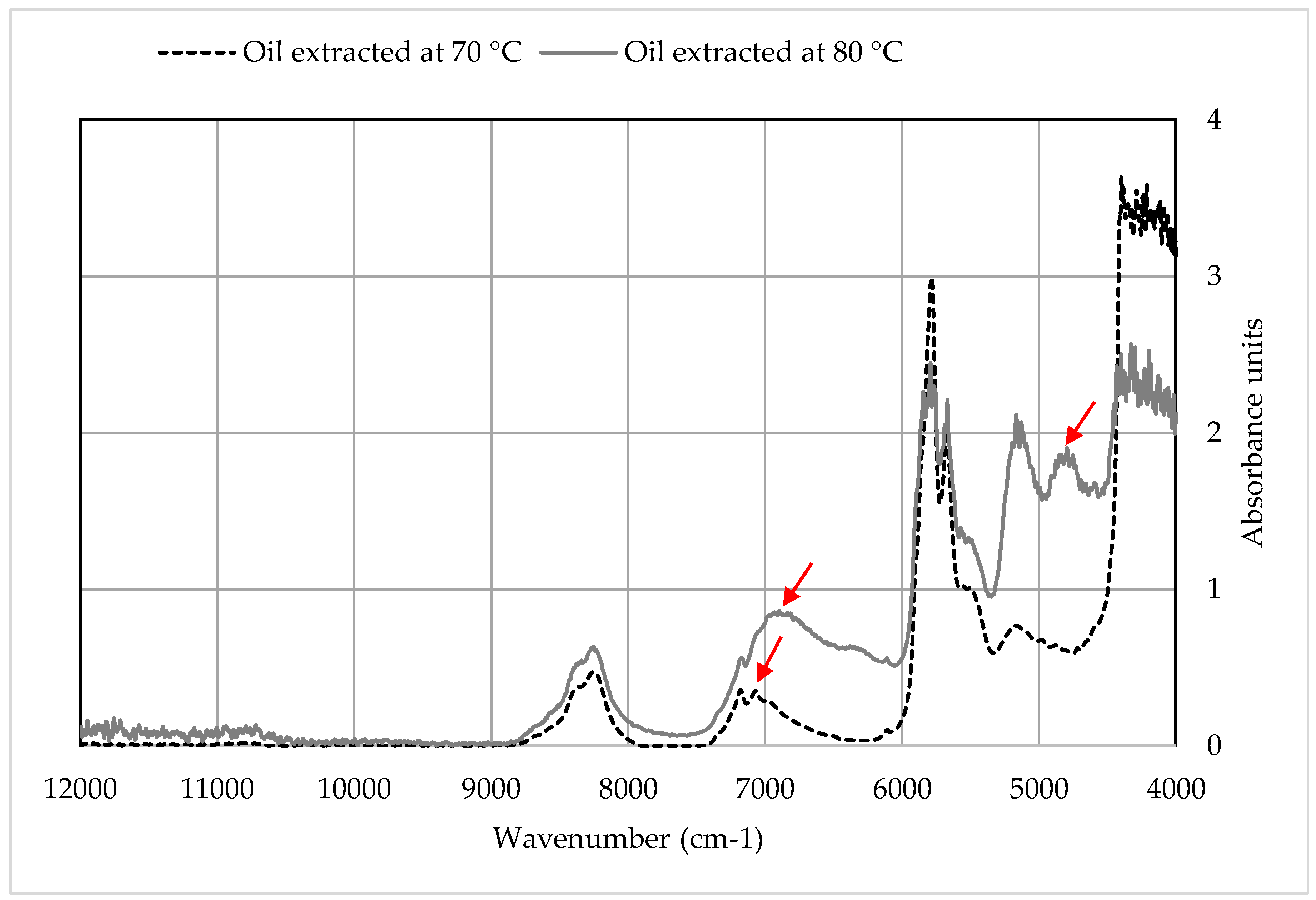
3.3.2. Qualitative Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CONVERSIO Market & Strategy. Global Plastics Flow 2018. Available online: https://www.euromap.org›Global_Plastics_Flow_Summary_Oct_2019 (accessed on 22 April 2020).
- CONVERSIO Market & Strategy. Stoffstrombild Kunststoffe in Deutschland 2017: Kurzfassung. Available online: https://www.bvse.de/images/news/Kunststoff/2018/181011_Kurzfassung_Stoffstrombild_2017.pdf (accessed on 22 April 2020).
- Fuhr, L.; Buschmann, R.; Freund, J. Plastikatlas. Daten und Fakten über eine Welt voller Kunststoff, 2nd ed.; Bund für Umwelt und Naturschutz Deutschland (BUND): Berlin, Germany, 2019; ISBN 9783869282008. [Google Scholar]
- Brooks, A.L.; Wang, S.; Jambeck, J.R. The Chinese import ban and its impact on global plastic waste trade. Sci. Adv. 2018, 4, eaat0131. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Themelis, N.J.; Sun, K.; Bourtsalas, A.C.; Huang, Q.; Zhang, Y.; Wu, Z. Current influence of China’s ban on plastic waste imports. Waste Dispos. Sustain. Energy 2019, 1, 67–78. [Google Scholar] [CrossRef]
- Plastic Waste: A European Strategy to Protect the Planet, Defend our Citizens and Empower Our Industrie; European Commission: Brussels, Belgium, 2018.
- Paket zur Kreislaufwirtschaft: Neue EU-Recyclingziele; European Parliament: Brussels, Belgium, 2017.
- Alassali, A.; Barouta, D.; Tirion, H.; Moldt, Y.; Kuchta, K. Towards a high quality recycling of plastics from waste electrical and electronic equipment through separation of contaminated fractions. J. Hazard. Mater. 2020, 387, 121741. [Google Scholar] [CrossRef] [PubMed]
- Pivnenko, K.; Jakobsen, L.G.; Eriksen, M.K.; Damgaard, A.; Astrup, T.F. Challenges in plastics recycling. Sardinia 2015-15th International Waste Management and Landfill Symposium; CISA Publisher: Padua, Italy, 2015. [Google Scholar]
- Eriksen, M.K.; Christiansen, J.D.; Daugaard, A.E.; Astrup, T.F. Closing the loop for PET, PE and PP waste from households: Influence of material properties and product design for plastic recycling. J. Waste Manag. 2019, 96, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.T.; Achten, C. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef] [PubMed]
- German Environment Agency. Polycyclic aromatic hydrocarbons. Harmful to the Environment! Toxic! Inevitable? 2016; Available online: https://www.umweltbundesamt.de/en/publikationen/polycyclic-aromatic-hydrocarbons (accessed on 13 January 2020).
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Lassen, P.; Hoffmann, L.; Thomsen, M. PAHs in Toys and Childcare Products; No. 1142011; 2012; Available online: https://www2.mst.dk/udgiv/publications/2012/01/978-87-92779-49-6.pdf (accessed on 20 April 2020).
- Wiedmer, C.; Velasco-Schön, C.; Buettner, A. Toy swords revisited: Identification of additional odour-active contaminants. J. Consum. Prot. Food Safety 2019, 14, 415–419. [Google Scholar] [CrossRef]
- Commission Regulation (EU). Evaluation, Authorization and Restriction of Chemicals (REACH) as regards polycyclic aromatic hydrocarbons. EU Patent No 1272/2013, 6 December 2013. [Google Scholar]
- European Commission. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. 2006. Available online: https://osha.europa.eu/en/legislation/directives/regulation-ec-no-1907-2006-of-the-european-parliament-and-of-the-council (accessed on 15 June 2020).
- Der Ausschuss für Produktsicherheit (AfPS). Ausschuss für Produktsicherheit (AfPS)GS-Spezifikation: Prüfung und Bewertung von Polyzyklischen Aromatischen Kohlenwasserstoffen (PAK) bei der Zuerkennung des GS-Zeichens. Spezifikation gemäß § 21 Abs. 1 Nr. 3 ProdSG- AfPS GS 2019:01 PAK. Available online: https://www.baua.de/DE/Aufgaben/Geschaeftsfuehrung-von-Ausschuessen/AfPS/pdf/AfPS-GS-2019-01-PAK.pdf?__blob=publicationFile&v=5 (accessed on 17 January 2020).
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near critical and supercritical impregnation and kinetic release of thymol in LLDPE films used for food packaging. J. Supercrit. Fluids 2014, 85, 41–48. [Google Scholar] [CrossRef]
- Said, A.B.; Guinot, C.; Ruiz, J.C.; Grandjean, A.; Dole, P.; Joly, C.; Chalamet, Y. Supercritical CO2 extraction of contaminants from polypropylene intended for food contact: Effects of contaminant molecular structure and processing parameters. J. Supercrit. Fluids 2016, 110, 22–31. [Google Scholar] [CrossRef]
- Said, A.B.; Guinot, C.; Ruiz, J.C.; Grandjean, A.; Dole, P.; Joly, C.; Chalamet, Y. Modeling of supercritical CO2 extraction of contaminants from post-consumer polypropylene: Solubilities and diffusion coefficients in swollen polymer at varying pressure and temperature conditions. Chem. Eng. Res. Des. 2017, 117, 95–109. [Google Scholar] [CrossRef]
- de San Luis, A.; Santini, C.C.; Chalamet, Y.; Dufaud, V. Removal of Volatile Organic Compounds from Bulk and Emulsion Polymers: A Comprehensive Survey of the Existing Techniques. Ind. Eng. Chem. Res. 2019, 58, 11601–11623. [Google Scholar] [CrossRef]
- Scialdone, O.; Galia, A.; Raimondi, S.; Filardo, G. Effective recovery of perfluoropolyether surfactants from PVDF and PTFE by supercritical carbon dioxide extraction. J. Supercrit. Fluids 2007, 39, 347–353. [Google Scholar] [CrossRef]
- Guerra, R.M.; Marın, M.L.; Sanchez, A.; Jimenez, A. Analysis of citrates and benzoates used in poly (vinyl chloride) by supercritical fluid extraction and gas chromatography. J. Chromatogr. A 2002, 950, 31–39. [Google Scholar] [CrossRef]
- Arias, M.; Penichet, I.; Ysambertt, F.; Bauza, R.; Zougagh, M.; Ríos, Á. Fast supercritical fluid extraction of low-and high-density polyethylene additives: Comparison with conventional reflux and automatic Soxhlet extraction. J. Supercrit. Fluids 2009, 50, 22–28. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Vasheghani-Farahani, E. Application of supercritical fluid extraction in biotechnology. Crit. Rev. Biotechnol. 2005, 25, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Raventós, M.; Duarte, S.; Alarcón, R. Application and possibilities of supercritical CO2 extraction in food processing industry: An overview. Food Sci. Technol. Int. 2002, 8, 269–284. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Hrnčič, M.K.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Rosenthal, A. Safe Design of a Continuous Supercritical Fluid Extraction System for the Treatment of Drill Cuttings. Master Thesis, The University of Guelph, Guelph, ON, Canada, 2012. [Google Scholar]
- Losowski, D. Supercritical CO2: A Green Solvent. Chem. Eng. J. 2010. Available online: https://www.chemengonline.com/supercritical-co2-a-green-solvent/?printmode=1 (accessed on 26 March 2020).
- Cunha, V.M.B.; da Silva, M.P.; da Costa, W.A.; de Oliveira, M.S.; Bezerra, F.W.F.; de Melo, A.C.; Pinto, R.H.H.; Machado, N.T.; Araujo, M.E.; de Carvalho Junior, R.N. Carbon Dioxide Use in High-Pressure Extraction Processes. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Intech Open: London, UK, 2018. [Google Scholar]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Samara, H.; Ke, L.; Ostrowski, T.V.; Ganzer, L.; Jaeger, P. Unconventional oil recovery from Al Sultani tight rock formations using supercritical CO2. J. Supercrit. Fluids 2019, 152, 104562. [Google Scholar] [CrossRef]
- Geiss, O.; Senaldi, C.; Bianchi, I.; Lucena, A.; Tirendi, S.; Barrero-Moreno, J. A fast and selective method for the determination of 8 carcinogenic polycyclic aromatic hydrocarbons in rubber and plastic materials. J. Chromatogr. A 2018, 1566, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bolmatov, D.; Brazhkin, V.V.; Fomin, Y.D.; Ryzhov, V.N.; Trachenko, K. Evidence for structural crossover in the supercritical state. J. Chem. Phys. 2013, 139, 234501. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, F.; Goodarznia, I. Supercritical extraction of phenanthrene in the crossover region. J. Chem. Eng. Data 2005, 50, 49–51. [Google Scholar] [CrossRef]
- Roberts, J.J.; Motin, J.C.; Swain, D.; Cozzolino, D. A feasibility study on the potential use of near infrared reflectance spectroscopy to analyze meat in live animals: Discrimination of muscles. J. Spectrosc. 2017, 2017. [Google Scholar] [CrossRef]
- Alassali, A.; Fiore, S.; Kuchta, K. Assessment of plastic waste materials degradation through near infrared spectroscopy. J. Waste Manag. 2018, 82, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.O.; Field, R.A.; Goldstone, M.E.; Kirk, P.W.; Lester, J.N.; Perry, R. A review of atmospheric polycyclic aromatic hydrocarbons: Sources, fate and behavior. Water Air Soil Pollut. 1991, 60, 279–300. [Google Scholar] [CrossRef]
- Nikitha, T.; Satyaprakash, M.; Vani, S.S.; Sadhana, B.; Padal, S.B. A review on polycyclic aromatic hydrocarbons: Their transport, fate and biodegradation in the environment. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1627–1639. [Google Scholar]
- ChemicalBook—Chemical Search Engine. Available online: https://www.chemicalbook.com/ProductIndex_EN.aspx (accessed on 26 March 2020).
- ChemSpider. Available online: http://www.chemspider.com/Chemical-Structure.3083038.html (accessed on 26 March 2020).
- PubChem. PubChem Compound. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 26 March 2020).

| Limits (µg kg−1) | Analyzed Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Category 1 | Category 2 | Category 3 | Original | Treated at 70 °C | Treated at 80 °C | ||
| Children under 14 | Other Products | Children under 14 | Other Products | |||||
| Benzo[a]pyrene | <200 | <200 | <500 | <500 | <1000 | 113.4 | 20.2 | 13.0 |
| Benzo[e]pyrene | <200 | <200 | <500 | <500 | <1000 | n. a. | n. a. | n. a. |
| Benzo[a]anthracene | <200 | <200 | <500 | <500 | <1000 | 266.2 | 17.6 | 14.9 |
| Benzo[b]fluoranthene | <200 | <200 | <500 | <500 | <1000 | 119.7 | 15.9 | 12.6 |
| Benzo[j]fluoranthene | <200 | <200 | <500 | <500 | <1000 | n. a. | n. a. | n. a. |
| Benzo[k]fluoranthene | <200 | <200 | <500 | <500 | <1000 | 64.6 | 11.4 | 8.4 |
| Chrysene | <200 | <200 | <500 | <500 | <1000 | 419.1 | 93.2 | 69.5 |
| Dibenzo[a,h]anthracene | <200 | <200 | <500 | <500 | <1000 | 20.3 | 10.6 | 6.0 |
| Benzo[ghi]perylene | <200 | <200 | <500 | <500 | <1000 | 178.4 | 121.7 | 51.9 |
| lndeno[1‚2,3-cd]pyrene | <200 | <200 | <500 | <500 | <1000 | 210.1 | 51.3 | 28.0 |
| Phenanthrene, Pyrene, Anthracene, Fluoranthene | <1000 Sum | <5000 Sum | <10,000 Sum | <20,000 Sum | <50,000 Sum | 2045.4 | 426.0 | 247.1 |
| Naphthalene | <1000 | <2000 | <2000 | <10,000 | <10,000 | 51.5 | 441.2 | 54.8 |
| Sum of 15 PAH | <1000 | <5000 | <10,000 | <20,000 | <50,000 | 3810.1 | 1219.6 | 512.4 |
| Retention Time (min) | Components Identification | O70 | O80 | M.W (g mole−1) | Boiling Point (°C) | GHS Hazard Statements |
|---|---|---|---|---|---|---|
| 7.51 | Ethylenhexanoic acid | 1 | 2 | 144.2 | 228 | H361d |
| 8.15 | Butoxyethoxy(ethanol) | - | 3 | 162.2 | 231 | H319 |
| 8.44 | Methoxytriethylenglycol | 3 | 1 | 164.2 | 249 | Not classified |
| 9.20 | Tridecane | - | 1 | 184.4 | 235 | H304 |
| 10.10 | Tetradecene | 1 | 1 | 196.3 | 233 | H304, H315 |
| 10.17 | Tetradecane | 1 | 1 | 198.4 | 254 | H304 |
| 10.73 | Butoxytriethylenglycol | 1 | 1 | 206.3 | 278 | H318 |
| 10.99 | Tetraethylenglycol monomethylether | 3 | - | 208.3 | 159 | H319 |
| 11.03 | Pentadecane | - | 1 | 212.4 | 271 | H304 |
| 11.106 | Di-tert. Butylphenol | 1 | 1 | 206.3 | 264 | H302, H315, H318, H319, H335, H400 and H410 |
| 11.79 | Hexadecene | 1 | 1 | 224.4 | 285 | H304 |
| 11.85 | Hexadecane | 1 | 1 | 226.4 | 287 | H304 |
| 12.61 | Heptadecane | 1 | 1 | 240.5 | 302 | H304 |
| 12.92 | Ethylene glycol monobutyl ether | 2 | - | 118.2 | 171 | H302, H311+H331, H315, H319 |
| 13.15 | Pentaethylen glycol monomethyl ether | 2 | - | 252.3 | n. a. | n. a. |
| 13.3 | Heptadecanal | 1 | 1 | 254.5 | 318 | H315 and H319 |
| 13.36 | Octadecane | 1 | 1 | 254.5 | 316 | H304 |
| 14.05 | Nonadecane | 1 | 1 | 268.5 | 330 | H304 |
| 14.15 | Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene, 2,8-dione | 1 | 2 | 276.4 | 515 | Not classified |
| 14.44 | Hexadecanoic acid | 1 | 1 | 256.4 | 352 | H315, H319, H335 and H412 |
| 14.68 | Eicosene | 1 | 1 | 280.5 | 341 | H304 |
| 15.35 | Heneicosane | 1 | 1 | 296.6 | 359 | Not classified |
| 15.71 | Octadecanoic acid | 1 | 1 | 284.5 | 383 | H315, H319, H335 and H412 |
| 15.93 | Docosene | 1 | 1 | 308.6 | 367 | H304, H315, H319 and H335 |
| 15.96 | Docosane | 1 | 1 | 310.6 | 370 | H315, H319, H335 |
| 17.83 | Diisooctyl phthalat | 1 | - | 390.6 | 370 | H360 and H413 |
| 20.06 | Octatriacontanal | 1 | 1 | 549.0 | n. a. | n. a. |
| 20.06 | pentafluoropropionate | 1 | 1 | 163.0 | 93.50 | H315, H319 and H335 |
| 22.49 | Silane | 1 | 1 | 32.1 | −112 | H220 and H280 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alassali, A.; Aboud, N.; Kuchta, K.; Jaeger, P.; Zeinolebadi, A. Assessment of Supercritical CO2 Extraction as a Method for Plastic Waste Decontamination. Polymers 2020, 12, 1347. https://doi.org/10.3390/polym12061347
Alassali A, Aboud N, Kuchta K, Jaeger P, Zeinolebadi A. Assessment of Supercritical CO2 Extraction as a Method for Plastic Waste Decontamination. Polymers. 2020; 12(6):1347. https://doi.org/10.3390/polym12061347
Chicago/Turabian StyleAlassali, Ayah, Noor Aboud, Kerstin Kuchta, Philip Jaeger, and Ahmad Zeinolebadi. 2020. "Assessment of Supercritical CO2 Extraction as a Method for Plastic Waste Decontamination" Polymers 12, no. 6: 1347. https://doi.org/10.3390/polym12061347
APA StyleAlassali, A., Aboud, N., Kuchta, K., Jaeger, P., & Zeinolebadi, A. (2020). Assessment of Supercritical CO2 Extraction as a Method for Plastic Waste Decontamination. Polymers, 12(6), 1347. https://doi.org/10.3390/polym12061347