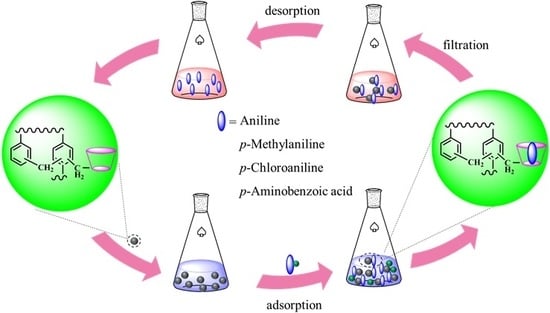
Immobilization of β-CD on a Hyper-Crosslinked Polymer for the Enhanced Removal of Amines from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
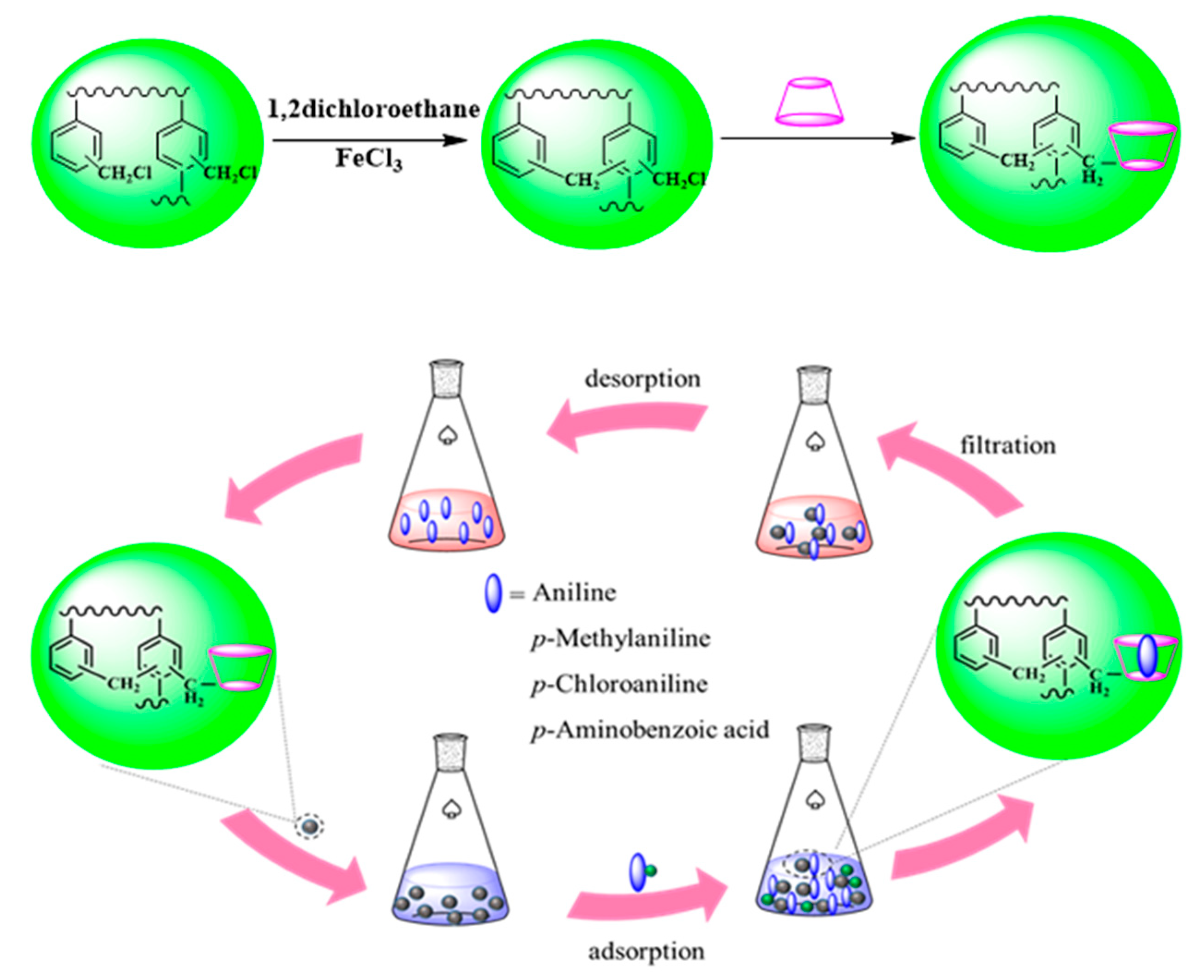
2.2. Synthesis of the CDM-HCP
2.3. Adsorbent Characterization
2.4. Adsorption Experiments
2.5. Desorption and Regeneration Experiments
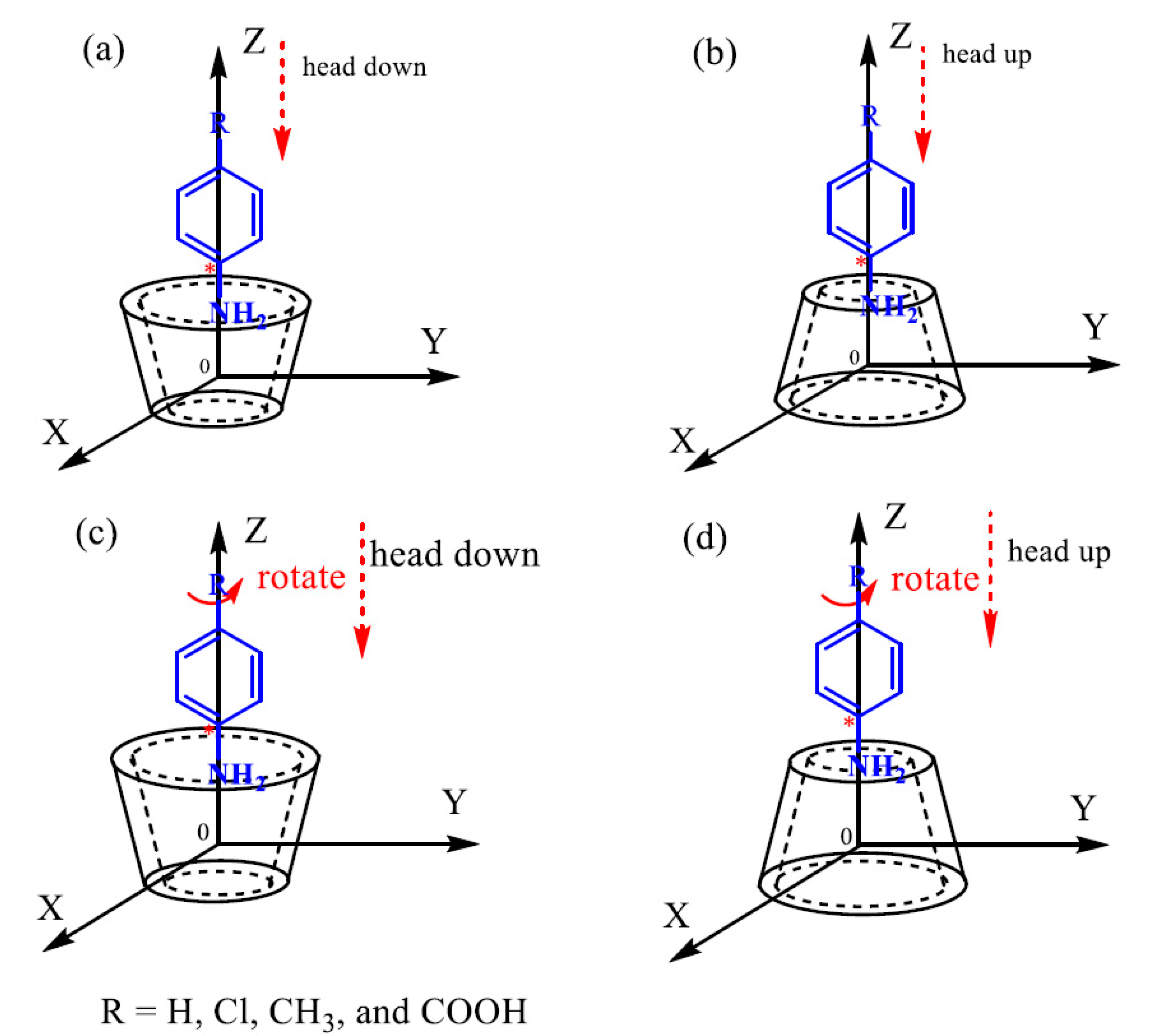
2.6. Computational Chemistry Calculations
3. Results and Discussion
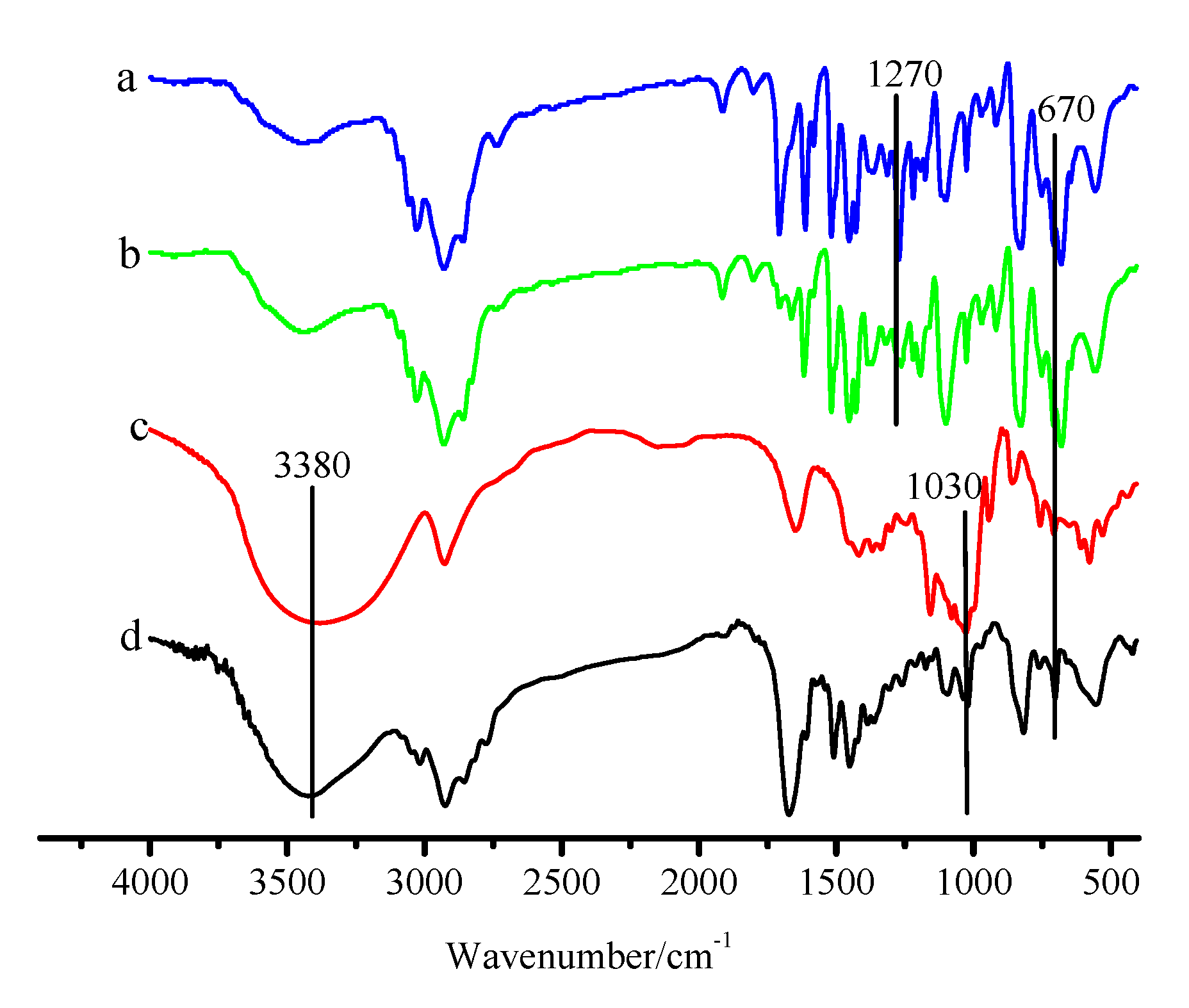
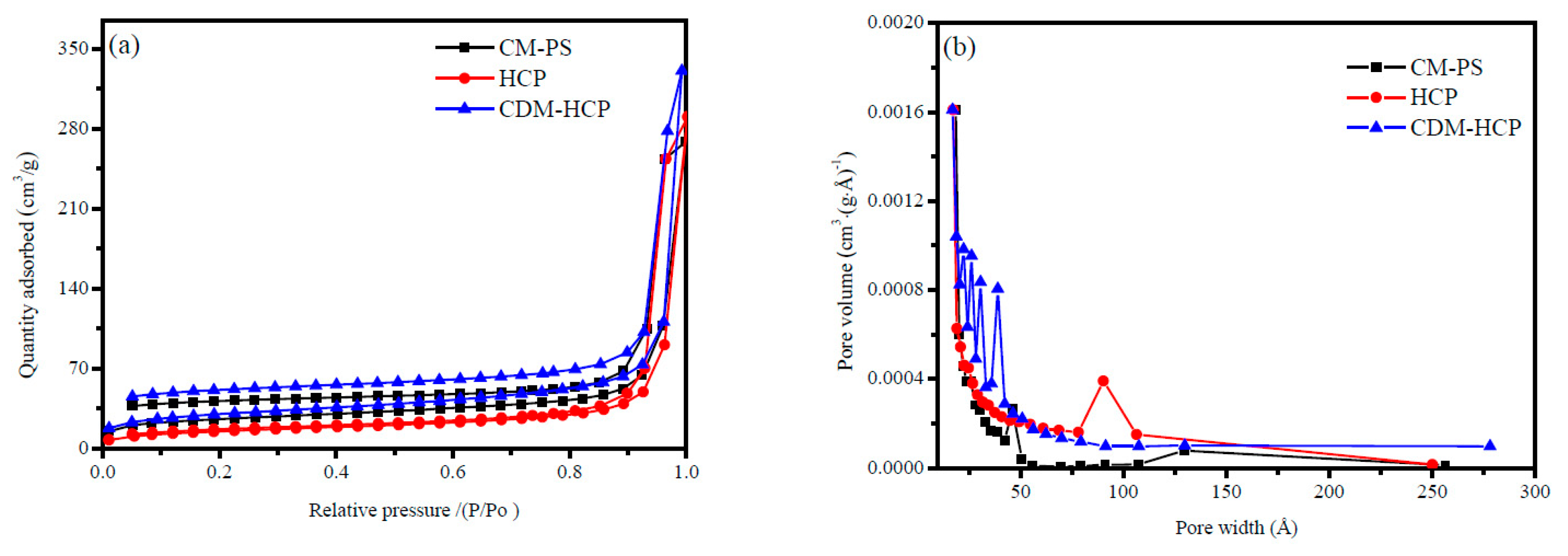
3.1. Characterization of the CDM-HCP
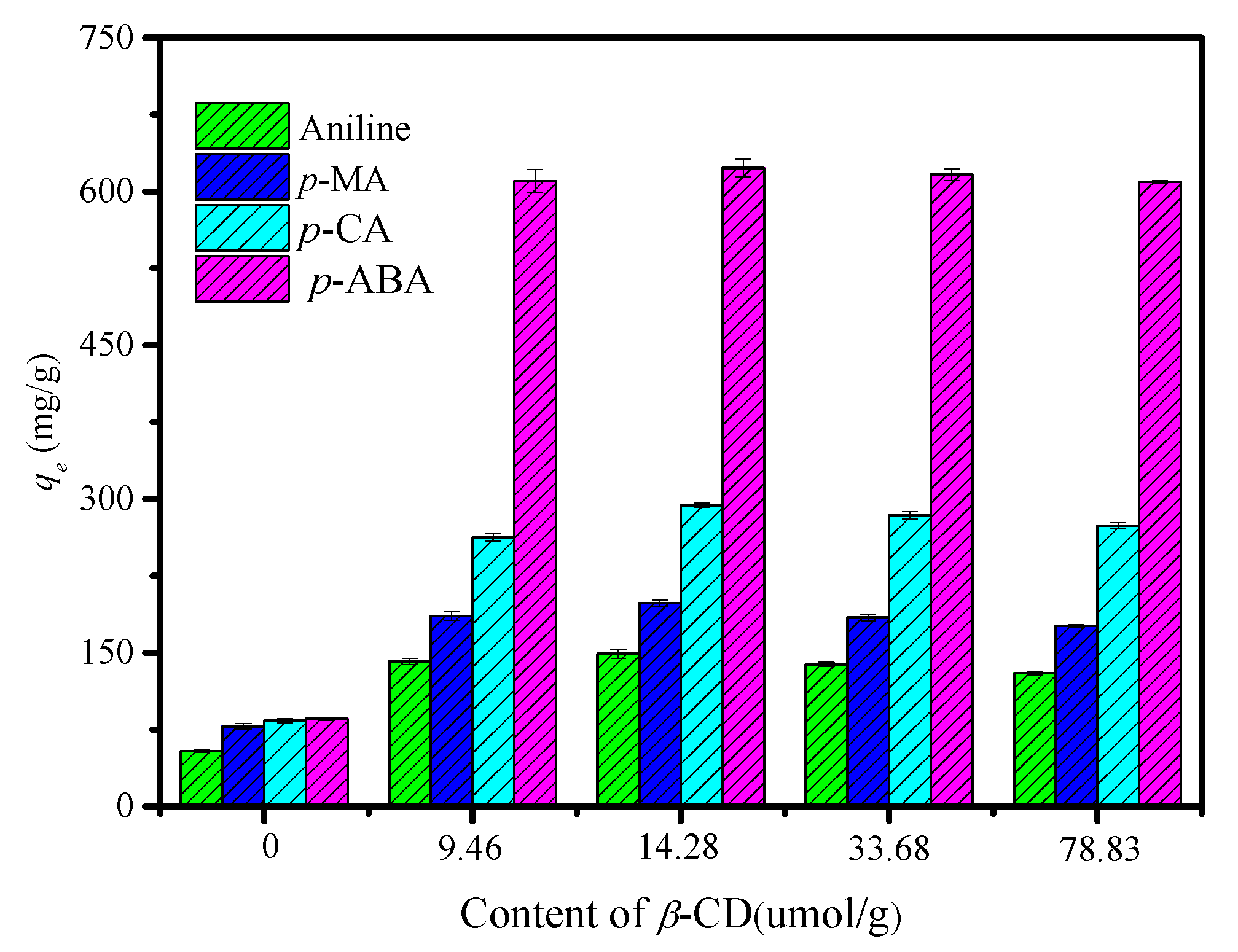
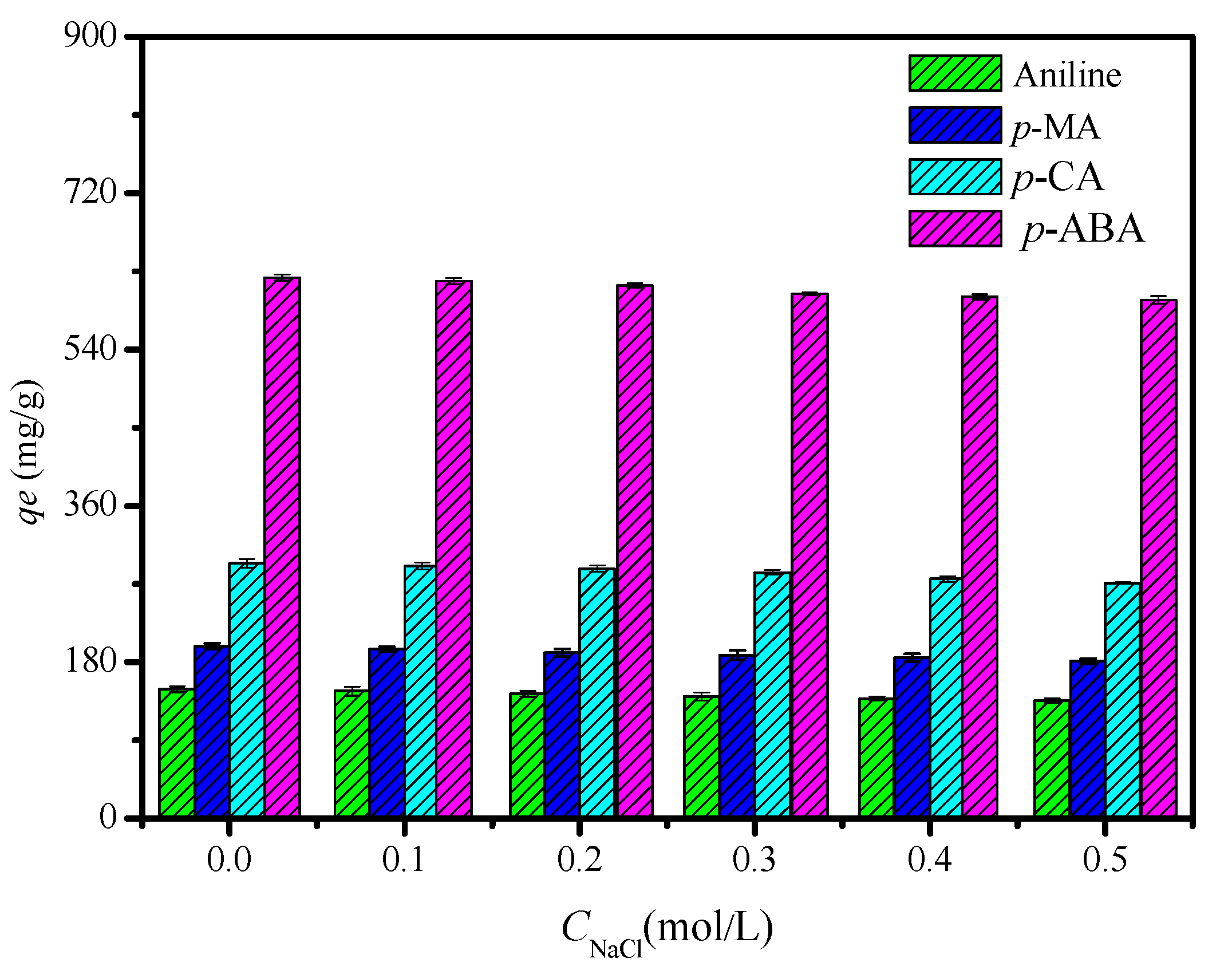
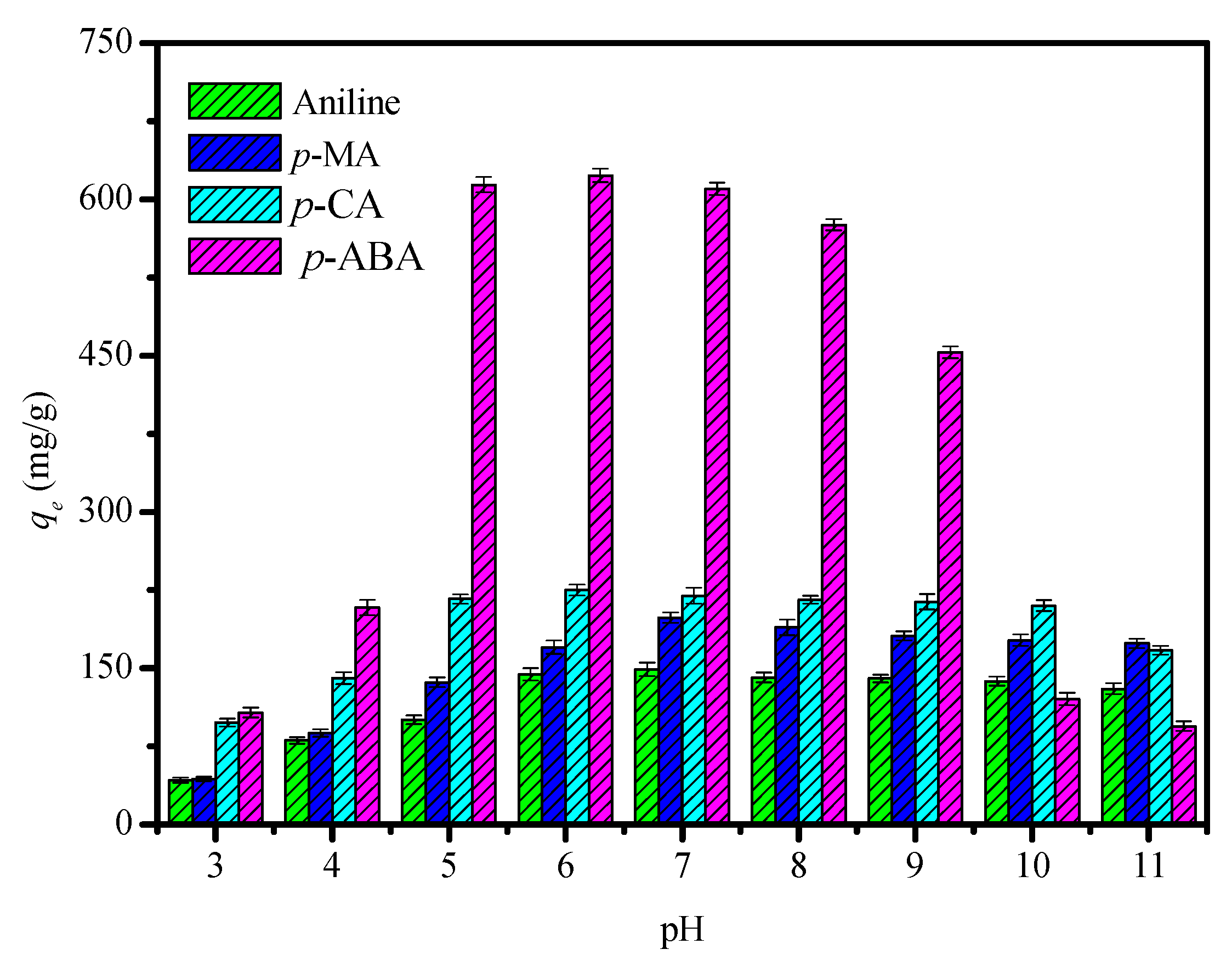
3.2. Adsorption Performance of the CDM-HCP
3.3. Adsorption Kinetics
3.4. Adsorption Isotherm
3.5. Thermodynamics of Adsorption
3.6. Regeneration Test
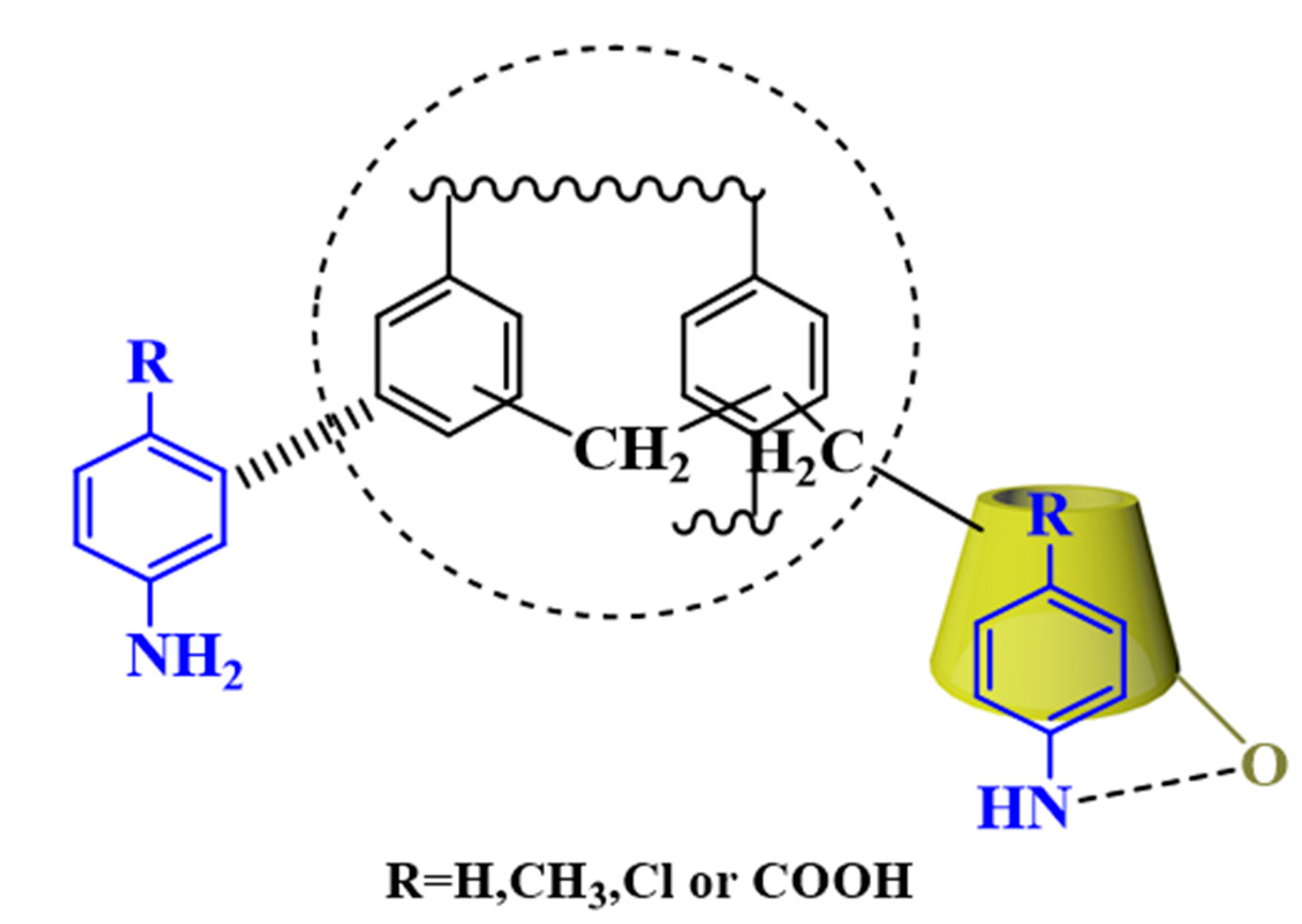
3.7. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cai, J.G.; Li, A.M.; Shi, H.Y.; Feng, Z.H.; Long, C.; Zhang, Q.X. Equilibrium and kinetic studies on the adsorption of aniline compounds from aqueous phase onto bifunctional polymeric adsorbent with sulfonic groups. Chemosphere 2005, 61, 502–509. [Google Scholar]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.Y.; Zhang, Y.Q.; Huang, S.B.; Hussain, I. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate. Chem. Eng. J. 2013, 218, 384–391. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, X.S.; Huang, S.B.; Li, H.; Tong, N.; Wen, X.Y.; Sun, C.C.; Fazal, S.; Zhang, Y.Q. Evaluation of microbial p-chloroaniline degradation in bioelectrochemical reactors in the presence of easily-biodegrading cosubstrates: Degradation efficiency and bacterial community structure. Bioresource. Technol. 2018, 270, 422–429. [Google Scholar] [CrossRef]
- Chen, C.Y.; Geng, X.H.; Huang, W.L. Adsorption of 4-chlorophenol and aniline by nanosized activated carbons. Chem. Eng. J. 2017, 327, 941–952. [Google Scholar] [CrossRef]
- Dewage, N.B.; Liyanage, A.S.; Smith, Q.; Pittman, C.U.J.; Perez, F.; Hassan, E.B.; Mohan, D.; Mlsna, T. Fast aniline and nitrobenzene remediation from water on magnetized and nonmagnetized Douglas fir biochar. Chemosphere 2019, 225, 943–953. [Google Scholar] [CrossRef]
- Jin, Q.; Hu, Z.C.; Jin, Z.F.; Qiu, L.Q.; Zhong, W.H.; Pan, Z.Y. Biodegradation of aniline in an alkaline environment by a novel strain of the halophilic bacterium, Dietzia natronolimnaea JQ-AN. Bioresour. Technol. 2012, 117, 148–154. [Google Scholar] [CrossRef]
- Ma, G.; Lu, J.L.; Meng, Q.G.; Lv, H.Q.; Shui, L.L.; Zhang, Y.G.; Jin, M.L.; Chen, Z.H.; Yuan, M.Z.; Nötzel, R.; et al. Synergistic effect of Cu-ion and WO3 nanofibers on the enhanced photocatalytic degradation of Rhodamine B and aniline solution. Appl. Surf. Sci. 2018, 451, 306–314. [Google Scholar] [CrossRef]
- Chen, C.; Jia, N.; Song, K.J.; Zheng, X.; Lan, Y.Q.; Li, Y. Sulfur-doped copper-yttrium bimetallic oxides: A novel and efficient ozonation catalyst for the degradation of aniline. Sep. Purif. Technol. 2019, 236, 116248. [Google Scholar] [CrossRef]
- Kuang, W.; Liu, Y.N.; Huang, J.H. Phenol-modified hyper-cross-linked resins with almost all micro/mesopores and their adsorption to aniline. J. Colloid Interface Sci. 2017, 487, 31–37. [Google Scholar] [CrossRef]
- Wang, X.M.; Mao, X.; Huang, J.H. Hierarchical porous hyper-cross-linked polymers modified with phenolic hydroxyl groups and their efficient adsorption of aniline from aqueous solution. Colloids Surf. A 2018, 558, 80–87. [Google Scholar] [CrossRef]
- Gan, Y.Q.; Chen, G.; Sang, Y.F.; Zhou, F.; Man, R.L.; Huang, J.H. Oxygen-rich hyper-cross-linked polymers with hierarchical porosity for aniline adsorption. Chem. Eng. J. 2019, 368, 29–36. [Google Scholar] [CrossRef]
- Shao, L.; Huang, J. N-vinylimidazole-modified hyper-cross-linked resins with controllable porosity and polarity and their efficient adsorption towards p-nitrophenol from aqueous solution. J. Colloid Interface Sci. 2017, 507, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, C.; Tao, W.H.; Li, A.M.; Zhang, Q.X. Fractal Dimensions of Macroporous and Hypercrosslinked Polymeric Adsorbents from Nitrogen Adsorption Data. J. Chem. Eng. Data 2010, 55, 3147–3150. [Google Scholar] [CrossRef]
- Zhang, W.M.; Hong, C.H.; Pan, B.C.; Xu, Z.W.; Zhang, Q.J.; Lv, L. Removal enhancement of 1-naphthol and 1-naphthylamine in single and binary aqueous phase by acid-basic interactions with polymer adsorbents. J. Hazard. Mater. 2008, 158, 293–299. [Google Scholar] [CrossRef]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Panda, S.K.; Schrader, W.; Andersson, J.T. β-Cyclodextrin as a stationary phase for the group separation of polycyclic aromatic compounds in normal-phase liquid chromatography. J. Chromatogr. A 2006, 1122, 88–96. [Google Scholar] [CrossRef]
- Li, J.Q.; Geng, S.; Wang, Y.; Lv, Y.H.; Wang, H.B.; Liu, B.G.; Liang, G.Z. The interaction mechanism of oligopeptides containing aromatic rings with β-cyclodextrin and its derivatives. Food Chem. 2019, 286, 441–448. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin-epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Li, X.M.; Zhou, M.J.; Jia, J.X.; Ma, J.T.; Jia, Q. Design of a hyper-crosslinked β-cyclodextrin porous polymer for highly efficient removal toward bisphenol a from water. Sep. Purif. Technol. 2018, 195, 130–137. [Google Scholar] [CrossRef]
- Li, H.Y.; Meng, B.; Chai, S.H.; Liu, H.L.; Dai, S. Hyper-crosslinked β-cyclodextrin porous polymer: An adsorption-facilitated molecular catalyst support for transformation of water-soluble aromatic molecules. Chem. Sci. 2016, 7, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.G.; Ahn, J.H.; Ihm, S.K. Adsorptive removal of phenolic compounds by using hypercrosslinked polystyrenic beads with bimodal pore size distribution. React. Funct. Polym. 2003, 57, 103–111. [Google Scholar] [CrossRef]
- Fischer, J.B.; Miller, J.H. Ion chromatography as an alternative to standard methods for analysis of macro-nutrients in Mehlich 1 extracts of unfertilized forest soils. Commun. Soil Sci. Plant Anal. 2004, 35, 2191–2208. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Dao, T.H.; Truong, T.T.; Nguyen, T.M.T.; Pham, T.D. Adsorption characteristic of ciprofloxacin antibiotic onto synthesized alpha alumina nanoparticles with surface modification by polyanion. J. Mol. Liq. 2020, 113150. [Google Scholar] [CrossRef]
- Pham, T.T.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2020, 110990. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chai, K.G.; Ji, H.B. Selective inclusion and separation of cinnamaldehyde and benzaldehyde by insoluble β-cyclodextrin polymer. Sep. Purif. Technol. 2011, 80, 209–216. [Google Scholar] [CrossRef]
- Yang, Z.J.; Zeng, H.; Zhou, X.T.; Ji, H.B. Mechanism into selective oxidation of cinnamaldehyde using β-cyclodextrin polymer as phase-transfer catalyst. Tetrahedron 2012, 68, 5912–5919. [Google Scholar] [CrossRef]
- Yang, Z.J.; Xiao, Z.B.; Ji, H.B. Solid inclusion complex of terpinen-4-ol/β-cyclodextrin: Kinetic release, mechanism and its antibacterial activity. Flavour. Frag. J. 2015, 30, 179–187. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, T.; Huo, J.Q.; Huang, J.H.; Liu, Y.N. Tunable porosity and polarity of polar post-cross-linked resins and selective adsorption. J. Colloid Interface Sci. 2017, 487, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Huang, X.N.; Yao, X.D.; Ji, H.B. Thiourea modified hyper-crosslinked polystyrene resin for heavy metal ions removal from aqueous solutions. J. Appl. Polym. Sci. 2018, 135, 45568. [Google Scholar] [CrossRef]
- Huang, J.H.; Zha, H.W.; Jin, X.Y.; Deng, S.G. Efficient adsorptive removal of phenol by a diethylenetriamine-modified hypercrosslinked styrene–divinylbenzene (PS) resin from aqueous solution. Chem. Eng. J. 2012, 195–196, 40–48. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, J.P.; Yao, X.D.; Rui, Z.B.; Ji, H.B. Efficient removal of BTEX from aqueous solution by β-cyclodextrin modified poly (butyl methacrylate) resin. Sep. Purif. Technol. 2016, 158, 417–421. [Google Scholar] [CrossRef]
- Lu, K.; Chai, K.G.; Liang, Q.H.; Xu, Z.J.; Li, G.Y.; Ji, H.B. Biosorption and selective separation of acetophenone and 1-phenylethanol with polysaccharide-based polymers. Chem. Eng. J. 2017, 317, 862–872. [Google Scholar] [CrossRef]
- Liu, G.L.; Wang, Y.X.; Shen, C.J.; Ju, Z.F.; Yuan, D.Q. A facile synthesis of microporous organic polymers for efficient gas storage and separation. J. Mater. Chem. A 2015, 3, 3051–3058. [Google Scholar] [CrossRef]
- Huang, J.H.; Jin, X.Y.; Mao, J.L.; Yuan, B.; Deng, R.J.; Deng, S.G. Synthesis, characterization and adsorption properties of diethylenetriamine-modified hypercrosslinked resins for efficient removal of salicylic acid from aqueous solution. J. Hazard. Mater. 2012, 217–218, 406–415. [Google Scholar] [CrossRef]
- Li, K.R.; Zhou, M.H.; Liang, L.; Jiang, L.L.; Wang, W. Ultrahigh-surface-area activated carbon aerogels derived from glucose for high-performance organic pollutants adsorption. J. Colloid Interface Sci. 2019, 546, 333–343. [Google Scholar] [CrossRef]
- Fu, Y.J.; Zu, Y.G.; Liu, W.; Hou, C.L.; Chen, L.Y.; Li, S.M.; Shi, X.G.; Tong, M.H. Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins. J. Chromatogr. A 2007, 1139, 206–213. [Google Scholar] [CrossRef]
- Wang, Y.F.; Mao, F.F.; Wei, X.L. Characterization and antioxidant activities of polysaccharides from leaves, flowers and seeds of green tea. Carbohydr. Polym. 2012, 88, 146–153. [Google Scholar] [CrossRef]
- Yu, F.; Wu, Y.Q.; Li, X.M.; Ma, J. Kinetic and thermodynamic studies of Toluene, Ethylbenzene, and m-Xylene adsorption from aqueous solutions onto KOH-activated multiwalled carbon nanotubes. J. Agric. Food Chem. 2012, 60, 12245–12253. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
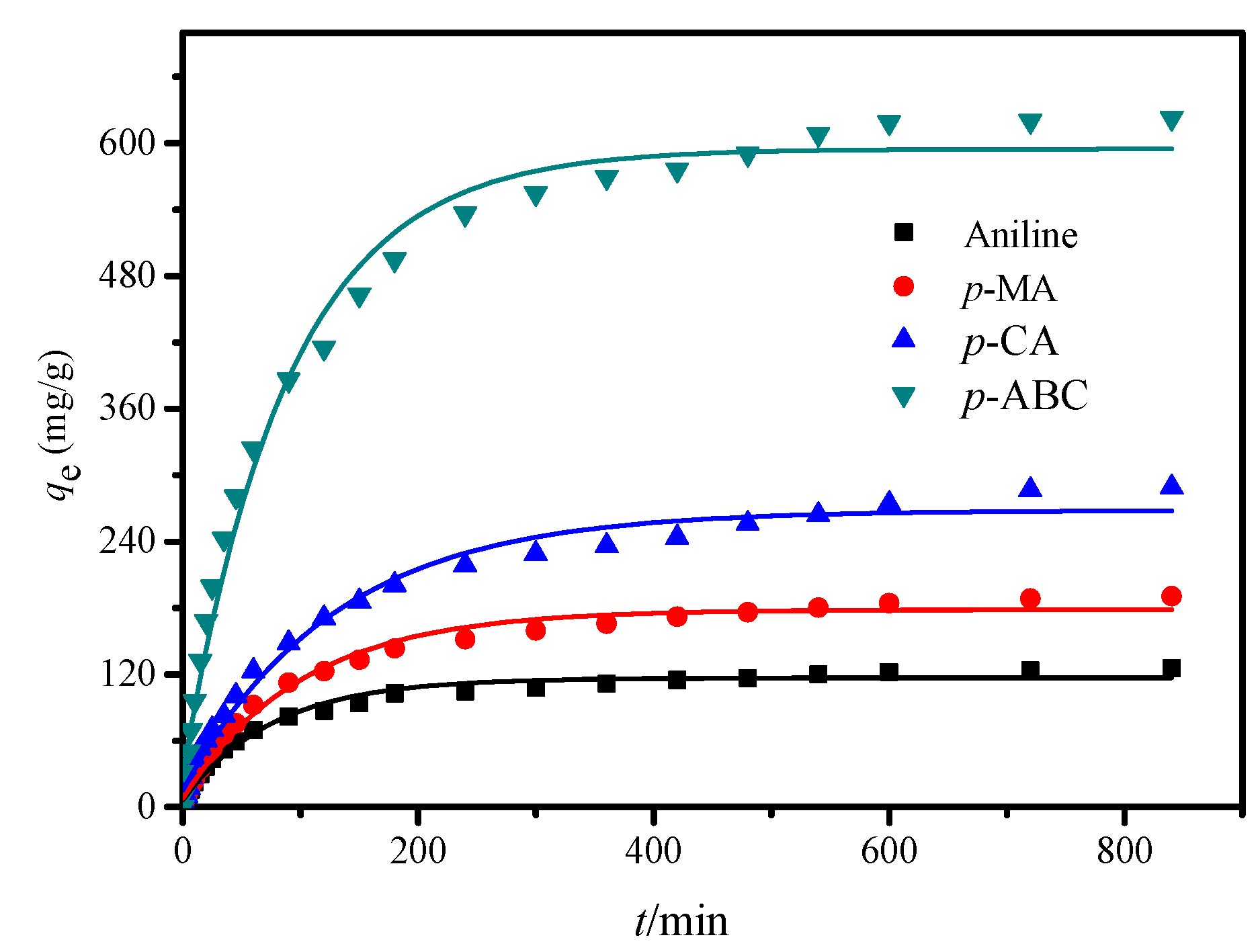
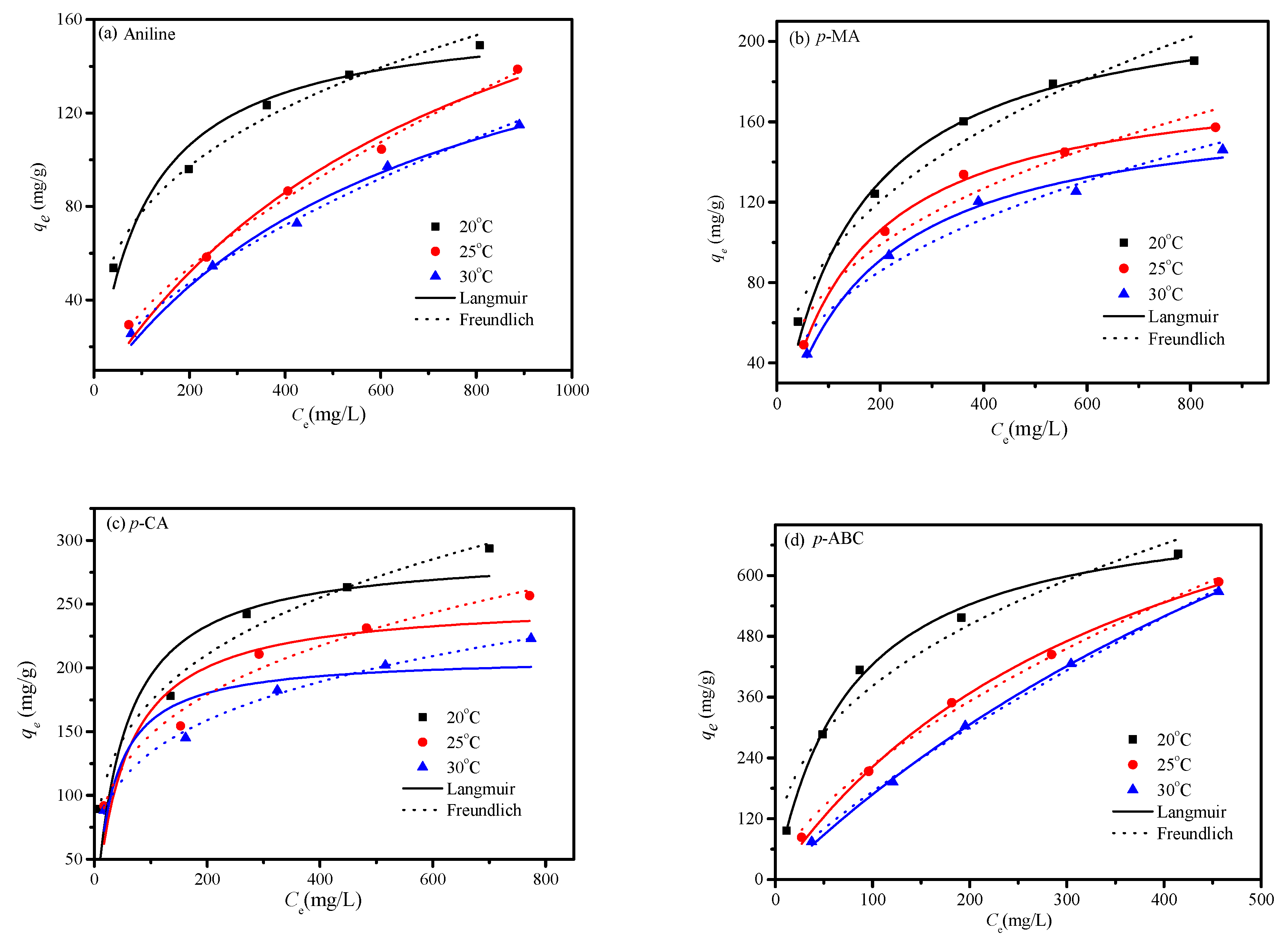
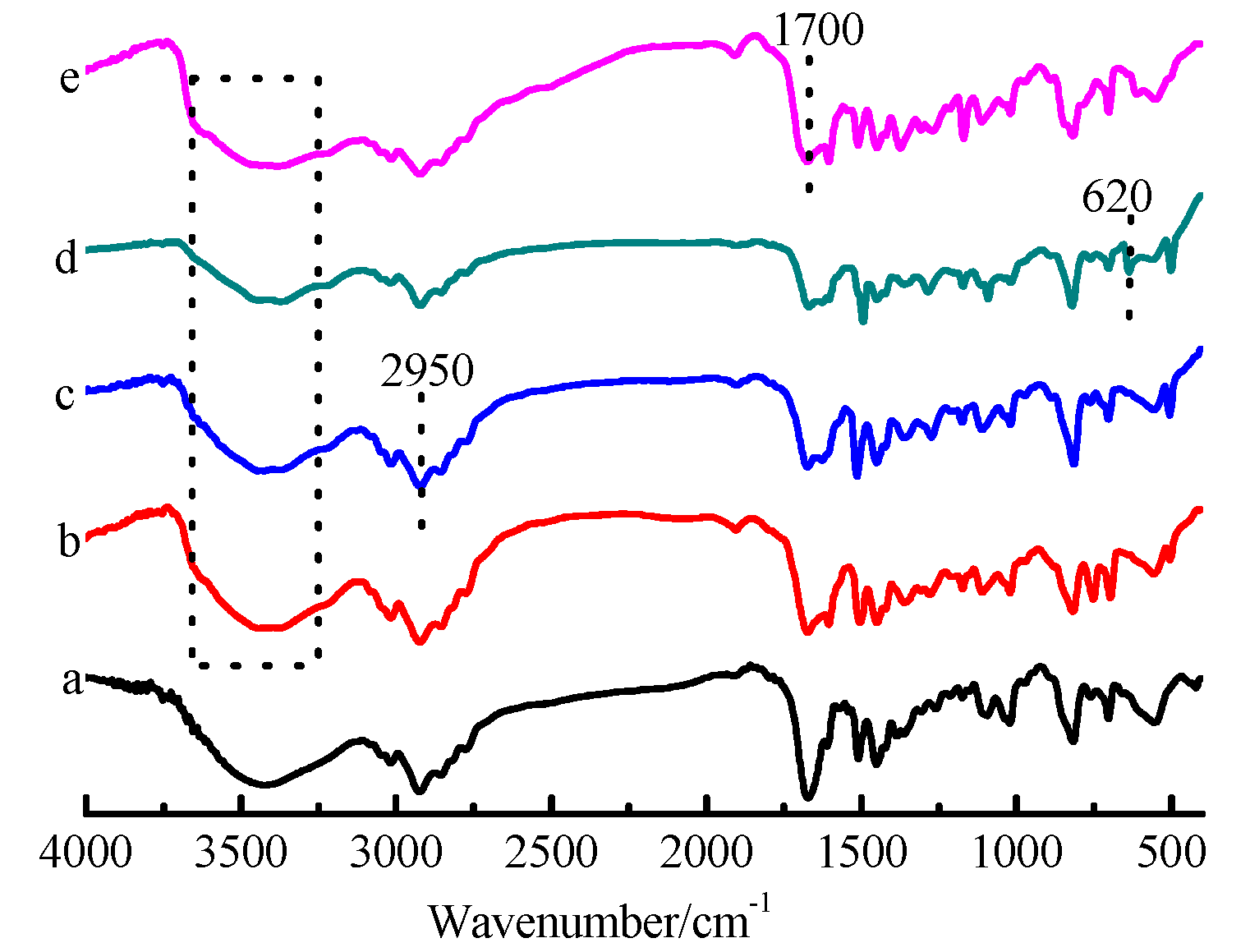
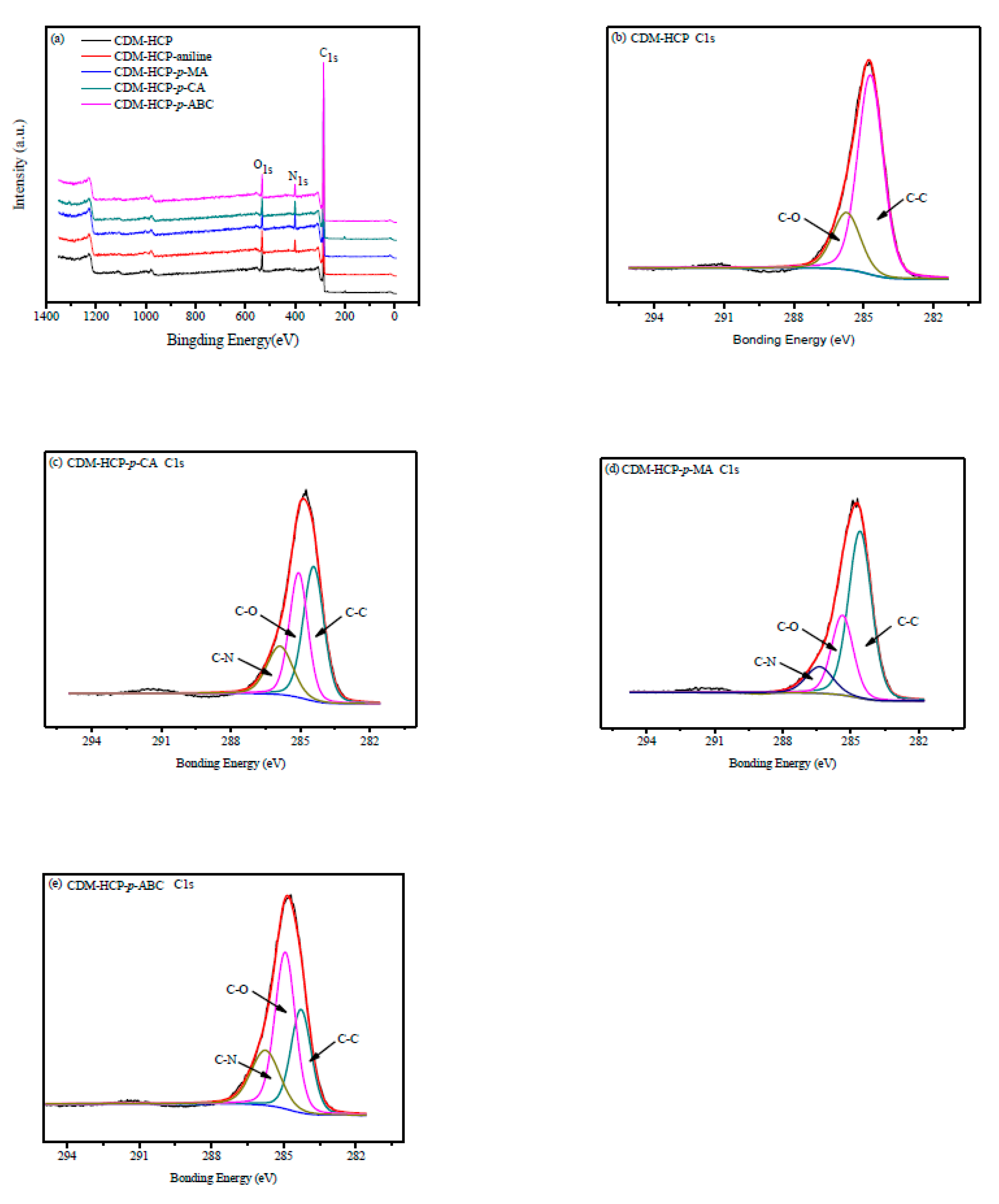
| Model | Parameter | Adsorbate | |||
|---|---|---|---|---|---|
| Aniline | p-MA | p-CA | p-ABC | ||
| this study | C0 (mg/L) | 1000 | 1000 | 1000 | 1000 |
| qe,expt (mg/g) | 148.97 | 198.45 | 293.71 | 622.91 | |
| pseudo-first-order | k1 (min−1) | 4.80 × 10−3 | 5.00 × 10−3 | 4.90 × 10−3 | 2.52 × 10−3 |
| qe,calcd (mg/g) | 142.61 | 192.59 | 291.58 | 657.69 | |
| R2 | 0.97 | 0.98 | 0.97 | 0.92 | |
| pseudo-second-order | k2 (g/(mg/min)) | 1.54 × 10−4 | 6.75 × 10−5 | 3.73 × 10−5 | 2.24 × 10−5 |
| qe,calcd (mg/g) | 130.89 | 204.50 | 309.60 | 684.93 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | |
| intraparticle diffusion | kint (g/(mg·min1/2)) | 2.18 | 4.00 | 7.02 | 13.92 |
| kd | 67.85 | 85.04 | 99.44 | 279.07 | |
| R2 | 0.92 | 0.94 | 0.96 | 0.97 | |
| Model | Temperature/°C | Parameter | Adsorbate | |||
|---|---|---|---|---|---|---|
| Aniline | p-MA | p-CA | p-ABC | |||
| Langmuir Model | 20 | KL (L/mg) | 9.32 × 10−3 | 6.84 × 10−3 | 1.99 × 10−3 | 1.29 × 10−2 |
| qmax (mg/g) | 163.25 | 226.44 | 292.50 | 753.81 | ||
| R2 | 0.968 | 0.971 | 0.824 | 0.995 | ||
| 25 | KL (L/mg) | 1.31 × 10−3 | 6.74 × 10−3 | 1.98 × 10−2 | 2.67 × 10−3 | |
| qmax (mg/g) | 152.93 | 183.54 | 254.13 | 666.40 | ||
| R2 | 0.993 | 0.996 | 0.838 | 0.998 | ||
| 30 | KL (L/mg) | 1.52 × 10−3 | 5.74 × 10−3 | 3.13 × 10−2 | 7.08 × 10−2 | |
| qmax (mg/g) | 140.25 | 171.56 | 210.18 | 579.27 | ||
| R2 | 0.996 | 0.997 | 0.848 | 0.998 | ||
| Freundlich Model | 20 | Kf (L/mg) | 1.71 | 16.62 | 48.74 | 61.42 |
| n | 3.03 | 2.70 | 3.57 | 2.50 | ||
| R2 | 0.995 | 0.994 | 0.982 | 0.945 | ||
| 25 | Kf (L/mg) | 1.95 | 14.81 | 40.98 | 11.89 | |
| n | 1.58 | 2.78 | 3.57 | 1.56 | ||
| R2 | 0.992 | 0.938 | 0.989 | 0.998 | ||
| 30 | Kf (L/mg) | 1.90 | 11.28 | 22.48 | 24.67 | |
| n | 1.64 | 2.61 | 4 | 1.26 | ||
| R2 | 0.996 | 0.959 | 0.998 | 0.997 | ||
| D–R Model | 20 | β | 4.22 × 10−4 | 2.89 × 10−4 | 1.41 × 10−5 | 3.99 × 10−5 |
| qm (mg/g) | 129.87 | 163.25 | 240.89 | 476.56 | ||
| Ea (KJ/mol) | 34.44 | 41.58 | 91.08 | 63.09 | ||
| R2 | 0.892 | 0.848 | 0.808 | 0.846 | ||
| 25 | β | 1.15 × 10−3 | 4.84 × 10−4 | 4.36 × 10−5 | 2.15 × 10−4 | |
| qm (mg/g) | 97.46 | 135.75 | 210.67 | 396.24 | ||
| Ea (KJ/mol) | 21.45 | 32.69 | 78.13 | 49.47 | ||
| R2 | 0.725 | 0.918 | 0.737 | 0.768 | ||
| 30 | β | 1.35 × 10−3 | 6.26 × 10−4 | 3.93 × 10−5 | 4.15 × 10−4 | |
| qm (mg/g) | 86.59 | 123.47 | 186.31 | 372.94 | ||
| Ea (KJ/mol) | 19.74 | 28.24 | 64.09 | 35.88 | ||
| R2 | 0.772 | 0.898 | 0.766 | 0.748 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Wu, G.; Li, Q.; Ai, H.; Yao, X.; Ji, H. Immobilization of β-CD on a Hyper-Crosslinked Polymer for the Enhanced Removal of Amines from Aqueous Solutions. Polymers 2020, 12, 1620. https://doi.org/10.3390/polym12071620
Yang Z, Wu G, Li Q, Ai H, Yao X, Ji H. Immobilization of β-CD on a Hyper-Crosslinked Polymer for the Enhanced Removal of Amines from Aqueous Solutions. Polymers. 2020; 12(7):1620. https://doi.org/10.3390/polym12071620
Chicago/Turabian StyleYang, Zujin, Guifang Wu, Qiuru Li, Hongxia Ai, Xingdong Yao, and Hongbing Ji. 2020. "Immobilization of β-CD on a Hyper-Crosslinked Polymer for the Enhanced Removal of Amines from Aqueous Solutions" Polymers 12, no. 7: 1620. https://doi.org/10.3390/polym12071620
APA StyleYang, Z., Wu, G., Li, Q., Ai, H., Yao, X., & Ji, H. (2020). Immobilization of β-CD on a Hyper-Crosslinked Polymer for the Enhanced Removal of Amines from Aqueous Solutions. Polymers, 12(7), 1620. https://doi.org/10.3390/polym12071620