Self-Assembly of Amphiphilic Linear−Dendritic Carbosilane Block Surfactant for Waterborne Polyurethane Coating
Abstract
1. Introduction

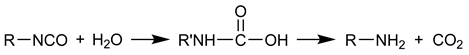

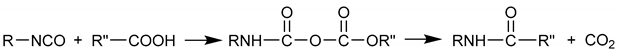
2. Experimental
2.1. Materials and Instruments
2.2. Preparation of LDBS
2.3. Preparation of Waterborne Polyurethane Coatings
3. Results and Discussion
3.1. Mechanisms of LDBS Self-Assembly to Form Physically Isolated Interface
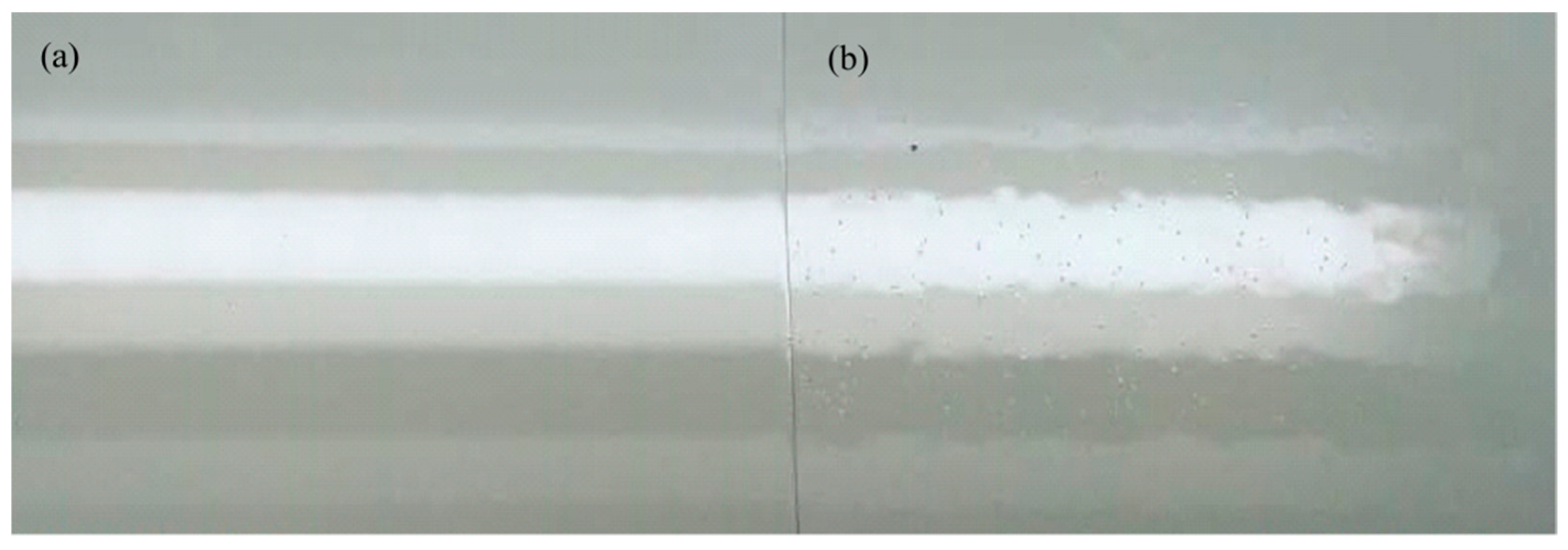
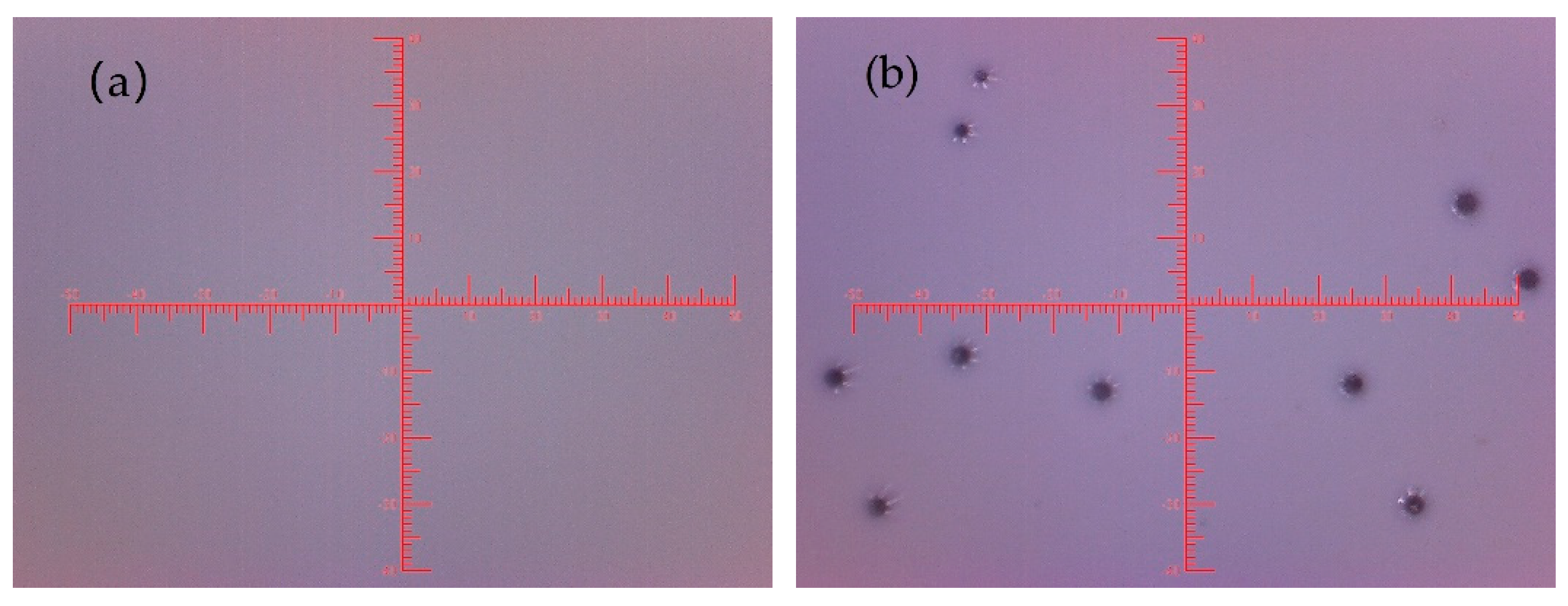
3.2. Evaluation of the BFFT
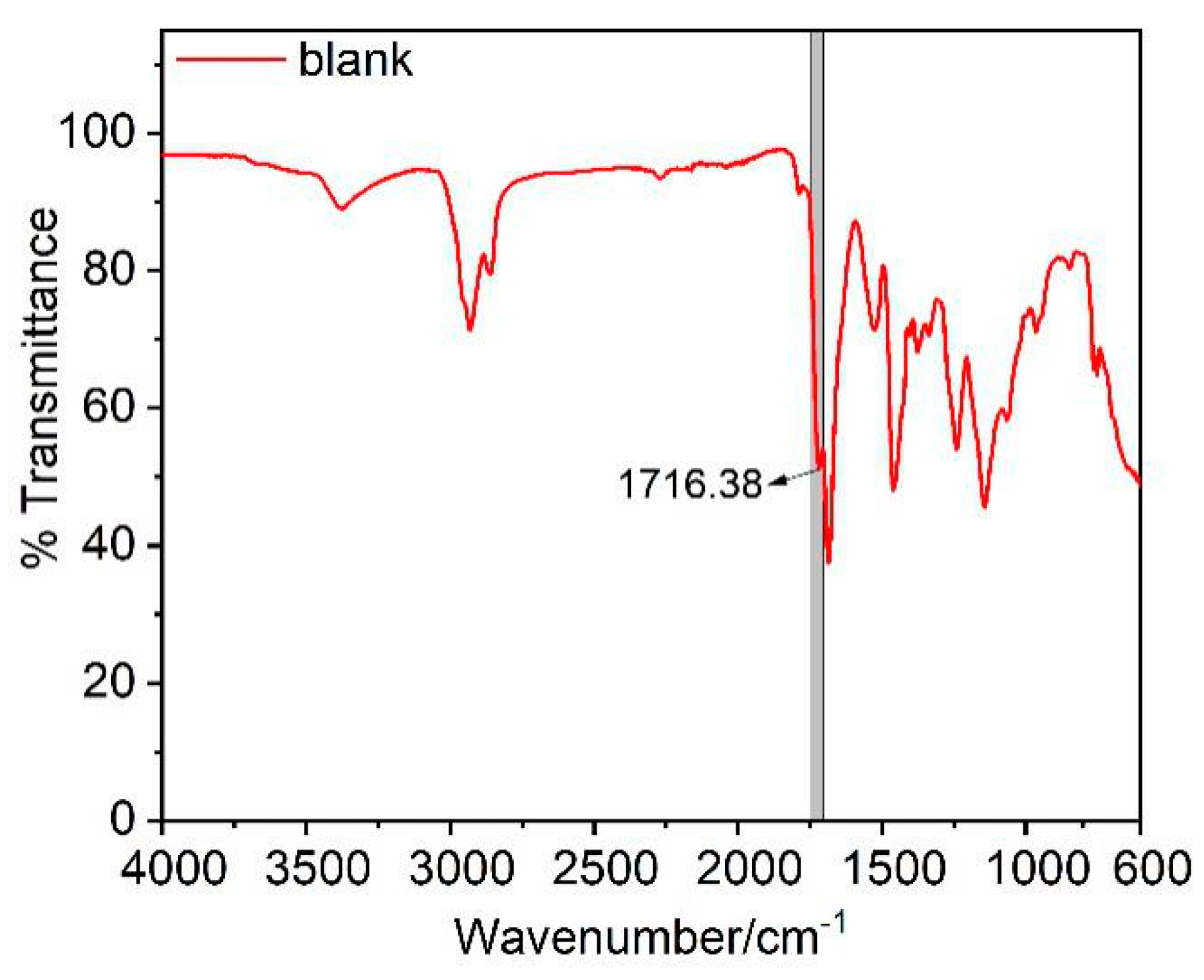
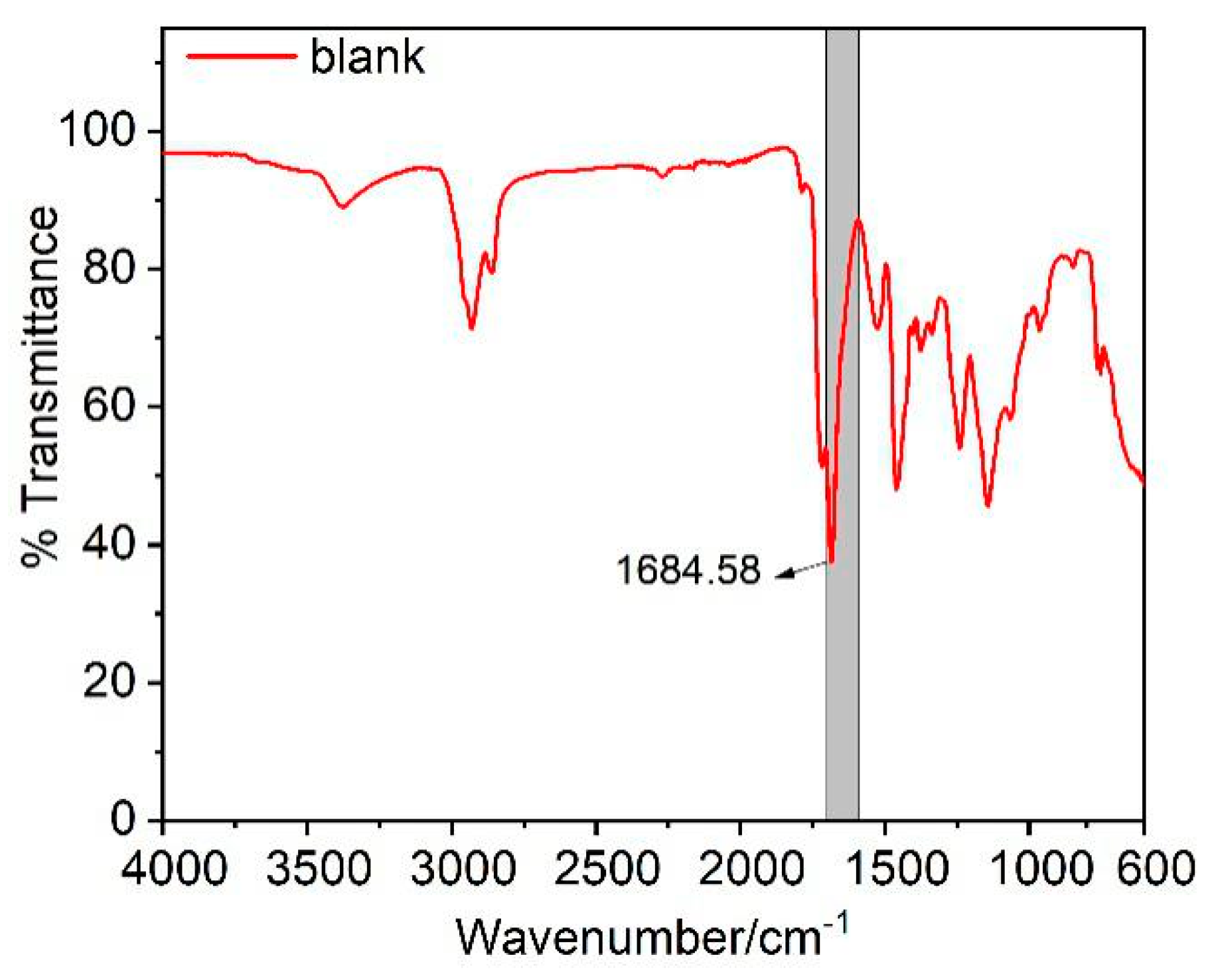
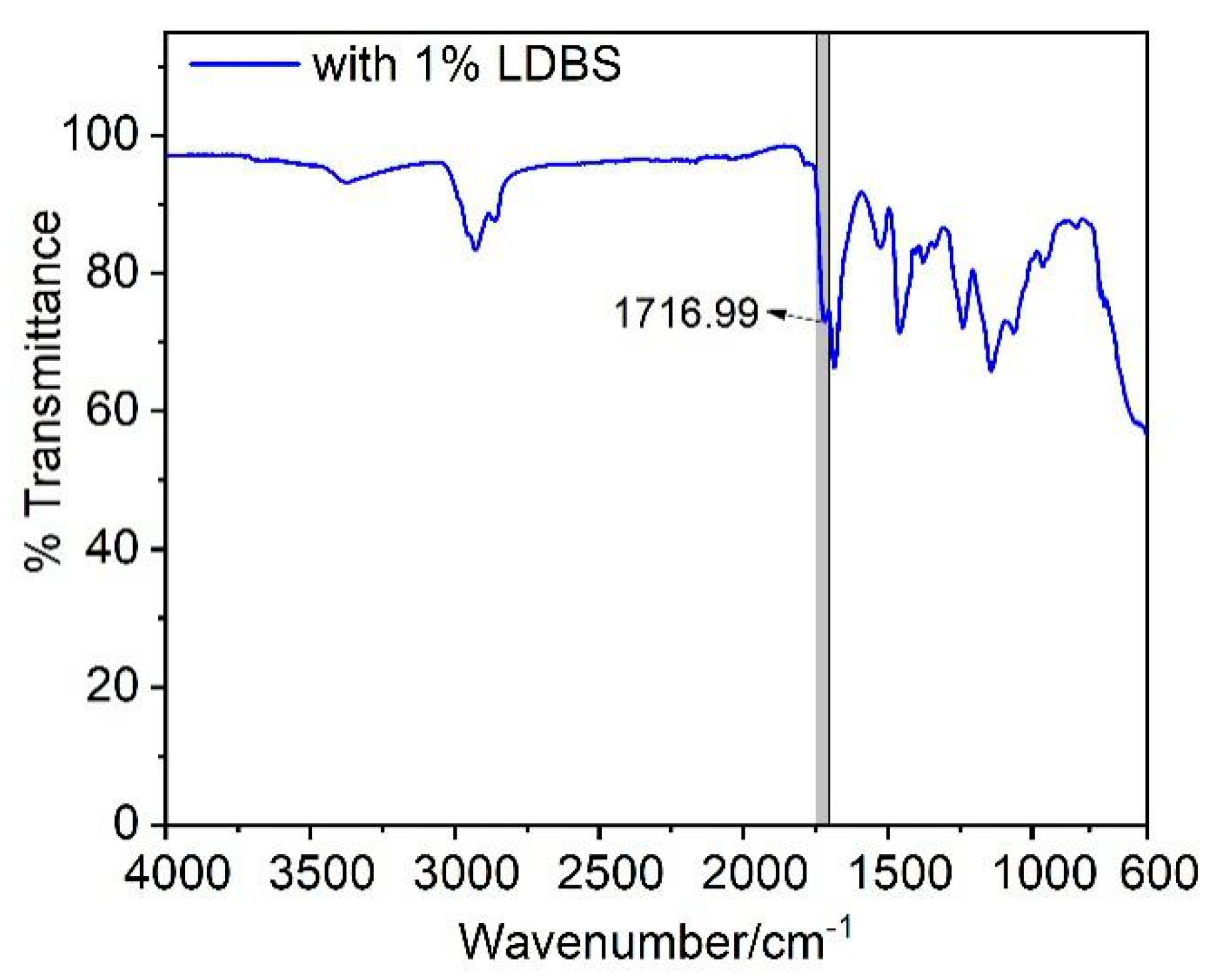
3.3. FT-IR Spectroscopy Analysis
3.4. Industrial Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dwight, G.W. Failure Analysis of Paints and Coatings; John Wiley and Sons Ltd Press: Chichester, UK, 2009. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, T.; Sun, X.; Guo, L.; He, L.; Ye, J.; Li, X. Achievement of Both Mechanical Properties and Intrinsic Self-Healing under Body Temperature in Polyurethane Elastomers: A Synthesis Strategy from Waterborne Polymers. Polymers 2020, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wu, Y.; Zhang, S.; Zhang, H.; Zhao, S.; Zhang, J.; Fei, B. Mechanical and Thermal Properties of Waterborne Polyurethane Coating Modified through One-Step Cellulose Nanocrystals/Graphene Materials Sols Method. Coatings 2020, 10, 40. [Google Scholar] [CrossRef]
- Hsu, Y.-T.; Wang, W.-H.; Hung, W.-H. Evaluating the Properties of a Coating Material with Polycaprolactone-Degradable Fluorinated Silicon-Containing Waterborne Polyurethane. Sustainability 2020, 12, 3745. [Google Scholar] [CrossRef]
- Kong, L.; Xu, D.; He, Z.; Wang, F.; Gui, S.; Fan, J.; Pan, X.; Dai, X.; Dong, X.; Liu, B. Nanocellulose-Reinforced Polyurethane for Waterborne Wood Coating. Molecules 2019, 24, 3151. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ye, X.; Lu, L.; Qian, Y.; Wang, L.; Bi, Y.; Wang, Z.; Cai, Z. Preparation of Cross-Linkable Waterborne Polyurethane-Acrylate Coating Films with Multifunctional Properties. Coatings 2020, 10, 65. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Thorley, M. Organic Coatings: Science and Technology. Carbohydr. Polym. 2000, 41, 427. [Google Scholar] [CrossRef]
- Noble, K.L. Waterborne polyurethanes. Prog. Org. Coat. 1997, 32, 131–136. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, J.C. Waterborne polyurethanes and their properties. J. Polym. Sci. Part A 1996, 34, 1095–1104. [Google Scholar] [CrossRef]
- Wicks, Z.W.; Wicks, D.A.; Rosthauser, J.W. Two package waterborne urethane systems. Prog. Org. Coat. 2002, 44, 161–183. [Google Scholar] [CrossRef]
- Chen, C.; Wei, S.; Xiang, B.; Wang, B.; Wang, Y.; Liang, Y.; Yuan, Y. Synthesis of Silane Functionalized Graphene Oxide and Its Application in Anti-Corrosion Waterborne Polyurethane Composite Coatings. Coatings 2019, 9, 587. [Google Scholar] [CrossRef]
- Fuensanta, M.; Khoshnood, A.; Rodriguezllansola, F.; Martinmartinez, J.M. New Waterborne Polyurethane-Urea Synthesized with Ether-Carbonate Copolymer and Amino-Alcohol Chain Extenders with Tailored Pressure-Sensitive Adhesion Properties. Materials 2020, 13, 627. [Google Scholar] [CrossRef]
- Abushammala, H. On the Para/Ortho Reactivity of Isocyanate Groups during the Carbamation of Cellulose Nanocrystals Using 2,4-Toluene Diisocyanate. Polymers 2019, 11, 1164. [Google Scholar] [CrossRef]
- Zhu, D. Study on the Application of Waterborne Two Conponent Polyurethane Construction Coating; Shanghai Jiaotong University: Shanghai, China, 2012. [Google Scholar]
- Liu, H.; Bi, Z.; Wan, Z.; Wang, X.; Wan, Y.; Guo, X.; Cai, Z. Preparation and Performance Optimization of Two-Component Waterborne Polyurethane Locomotive Coating. Coatings 2019, 10, 4. [Google Scholar] [CrossRef]
- Yu, X.; Lang, M.; Zhang, W.; Li, S.; Zhang, M.; Yu, X. An Empirical Study on the Comprehensive Optimization Method of a Train Diagram of the China High Speed Railway Express. Sustainability 2019, 11, 2141. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, Q.; Zhu, S.; Pang, R.; Shan, W. Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating. e-Polymers 2019, 19, 444–452. [Google Scholar] [CrossRef]
- Liu, J.; Xu, K.; Zhang, N.; Lu, C.; Zhang, Q.; Yu, L.; Feng, F.; Li, X. In Situ Incorporation of Diamino Silane Group into Waterborne Polyurethane for Enhancing Surface Hydrophobicity of Coating. Molecules 2019, 24, 1667. [Google Scholar] [CrossRef]
- Rui, W.; Zhao, J.; Zhi, W.; Min, Z.; Jian, Z.; Ji, L. Improving BFFT of waterborne polyurethane coating by building encapsulated polyisocyanate emulsion with hydrophobic inter-facial agent. Abs. Pap. Am. Chem. Soc. 2019, 05, 257–258. [Google Scholar]
- Yin, X.; Luo, Y.; Zhang, J. Synthesis and Characterization of Halogen-Free Flame Retardant Two-Component Waterborne Polyurethane by Different Modification. Ind. Eng. Chem. Res. 2017, 56, 1791–1802. [Google Scholar] [CrossRef]
- Mo, Q.; Li, W.; Yang, H.; Gu, F.; Chen, Q.; Yang, R. Water resistance and corrosion protection properties of waterborne polyurethane coating enhanced by montmorillonite modified with Ce3+. Prog. Org. Coat. 2019, 136, 105213. [Google Scholar] [CrossRef]
- Bai, T.; Lv, L.; Du, W.; Fang, W.; Wang, Y. Improving the Tribological and Anticorrosion Performance of Waterborne Polyurethane Coating by the Synergistic Effect between Modified Graphene Oxide and Polytetrafluoroethylene. Nanomaterials 2020, 10, 137. [Google Scholar] [CrossRef]
- Hou, J.; Ma, Y.; Zhang, Z.; Yang, X.; Huang, M.; Chai, C. The Relationship between Solid Content and Particle Size Ratio of Waterborne Polyurethane. Coatings 2019, 9, 401. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Mu, C.; Lin, W. A waterborne polyurethane coating functionalized by isobornyl with enhanced antibacterial adhesion and hydrophobic property. Eur. Polym. J. 2018, 108, 498–506. [Google Scholar] [CrossRef]
- Patel, R.H.; Kapatel, P.M. Studies on the effect of the size of waterborne polyurethane nanoparticles on properties and performance of coatings. Int. J. Polym. Anal. Charact. 2019, 24, 1–9. [Google Scholar] [CrossRef]
- Chang, Y.; Kwon, Y.C.; Lee, S.C.; Kim, C. Amphiphilic linear PEO-dendritic carbosilane block copolymers. Macromolecules 2000, 33, 4496–4500. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, H.M.; Wang, F. Synthesis and Surface Properties of Amphiphilic Linear-Dendritic Carbosilane Block Copolymers. Fine Chem. 2015, 32, 505–509. [Google Scholar]
- Sambasivam, A.; Sangwai, A.V.; Sureshkumar, R. Self-Assembly of Nanoparticle-Surfactant Complexes with Rodlike Micelles: A Molecular Dynamics Study. Langmuir 2016, 32, 1214–1219. [Google Scholar] [CrossRef]
- Hongbao, C. Study on Hydrogen Bond in Polyurethane Urea by FT-IR. Chem. Propellants Polym. Mater. 2009, 7, 60–63. [Google Scholar]
- Yang, P.; Li, T.; Li, J.; Zhu, X.; Xia, Y. Kinetics and Mechanism of Carbamate Reaction of 4-Hydroxybenzyl Alcohol with Phenyl Isocyanate. Prog. React. Kinetics Mechan. 2010, 35, 93–104. [Google Scholar] [CrossRef]
- Yang, P.F.; De Han, Y.; Li, T.; Li, J.Y. Effects of solvent polarity on the reaction of phenol with tolylene-2,4-diisocyanate. J. Appl. Polym. Sci. 2012, 123, 580–584. [Google Scholar] [CrossRef]
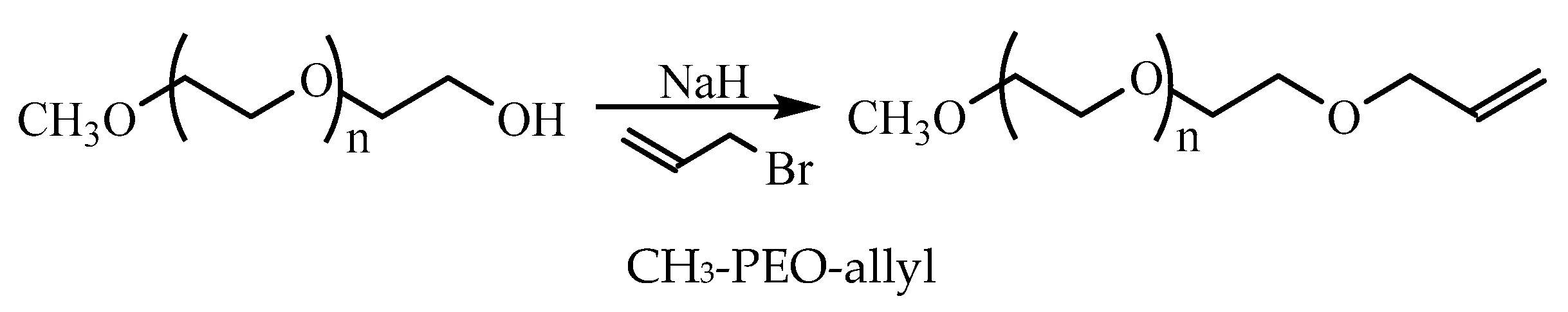
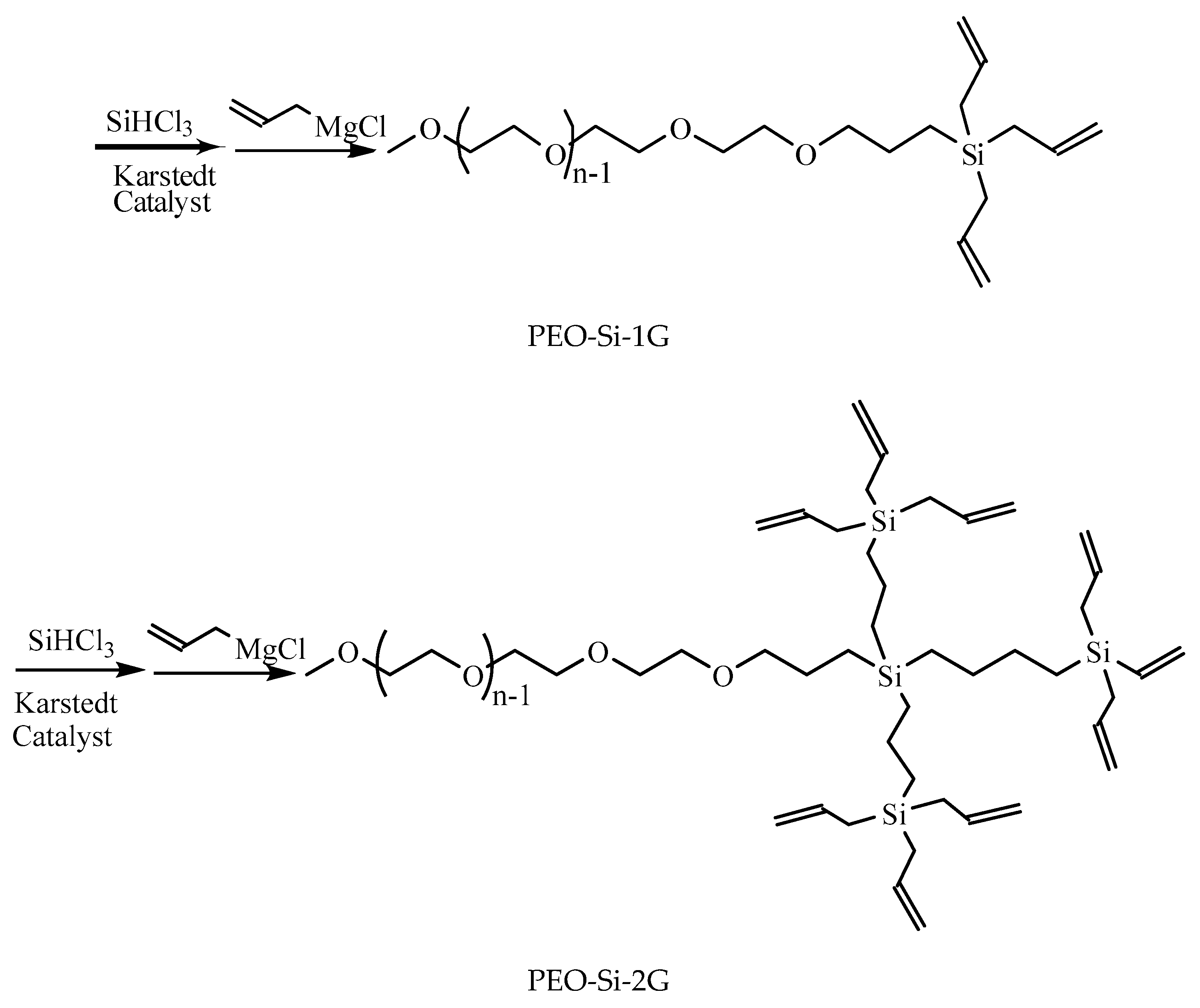





| No. of End Groups | Mn a | wt.% b | CMC c | |
|---|---|---|---|---|
| PEO-Si-1G | 3 | 1009 | 19.3 | 82.6 |
| PEO-Si-2G | 9 | 1488 | 49.6 | 2.3 |
| Function | Material | wt. % |
|---|---|---|
| Hydroxyl polyacrylate dispersion | Macrynal VSM 6299 w/42 WA | 49.6 |
| Pigment | Kronos 2160 | 27.4 |
| Wetting agent | Surfynol 104 BC | 0.9 |
| Dispersing agent | Additol VXW 6208 | 3.1 |
| Thickener | Borchigel 0620 | 0.2 |
| Leveling agent | Additol VXW 5929 | 0.6 |
| Defoamer | Byk 011 | 1.4 |
| Deionized water | 4.0 | |
| Disperse 15 min, grind 30 min on the bead-mill | ||
| Thickener | Borchigel 0620 | 0.2 |
| Solvent | Texanol | 0.9 |
| Deionized water | 11.7 | |
| Total | 100.0 | |
| Function | Material | wt. % |
|---|---|---|
| Curing agent | Bayhydur XP 2655 | 75.0 |
| Solvent | Dowanol PGDA | 25.0 |
| Surfactant | LDBS | 0.0–1.5 |
| Total | 100.0 |
| Coatings Formulation | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| LDBS% | 0 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 1.5 |
| BFFT (μm) | 80.4 | 92.1 | 104.6 | 117.3 | 121.2 | 114.2 | 107.2 |
| Coatings Formulation | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| LDBS% | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 |
| BFFT (μm) | 113.6 | 119.5 | 121.7 | 117.2 | 117.3 |
| Sample | Carbamate Carbonyl | Urea Carbonyl | X(%) | ||
|---|---|---|---|---|---|
| Absorption Peak/cm−1 | So/Peak Area | Absorption Peak/cm−1 | Sn/Peak Area | ||
| Blank | 1716.38 | 399.60 | 1684.58 | 729.58 | 35.38 |
| 1.0%LDBS | 1716.99 | 249.63 | 1684.63 | 322.03 | 43.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Li, C.; Jiang, Z.; Wang, Z. Self-Assembly of Amphiphilic Linear−Dendritic Carbosilane Block Surfactant for Waterborne Polyurethane Coating. Polymers 2020, 12, 1318. https://doi.org/10.3390/polym12061318
Wang R, Li C, Jiang Z, Wang Z. Self-Assembly of Amphiphilic Linear−Dendritic Carbosilane Block Surfactant for Waterborne Polyurethane Coating. Polymers. 2020; 12(6):1318. https://doi.org/10.3390/polym12061318
Chicago/Turabian StyleWang, Ruitao, Chunxiang Li, Zhaohua Jiang, and Zhijiang Wang. 2020. "Self-Assembly of Amphiphilic Linear−Dendritic Carbosilane Block Surfactant for Waterborne Polyurethane Coating" Polymers 12, no. 6: 1318. https://doi.org/10.3390/polym12061318
APA StyleWang, R., Li, C., Jiang, Z., & Wang, Z. (2020). Self-Assembly of Amphiphilic Linear−Dendritic Carbosilane Block Surfactant for Waterborne Polyurethane Coating. Polymers, 12(6), 1318. https://doi.org/10.3390/polym12061318





