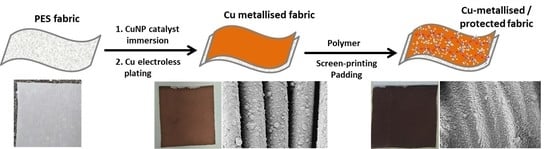
The Efficacy of Polymer Coatings for the Protection of Electroless Copper Plated Polyester Fabric
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Selective Metallisation of Fabric by Electroless Copper Plating
2.3. Application of Polymer Protective Coatings
2.4. Washing
2.5. Analytical Methods
2.5.1. Characterization of Polymers’ Coated Textiles
2.5.2. Analysis of Conductive Textiles
3. Results and Discussion
3.1. Characterization of Polymers’ Coatings
3.1.1. Add-on, Thickness and Air Permeability
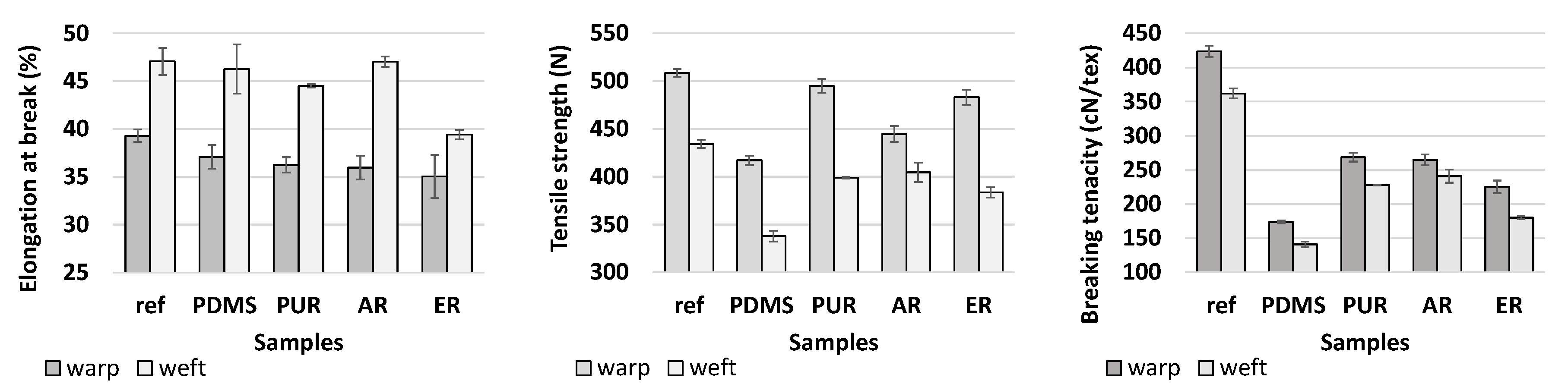
3.1.2. Mechanical Features
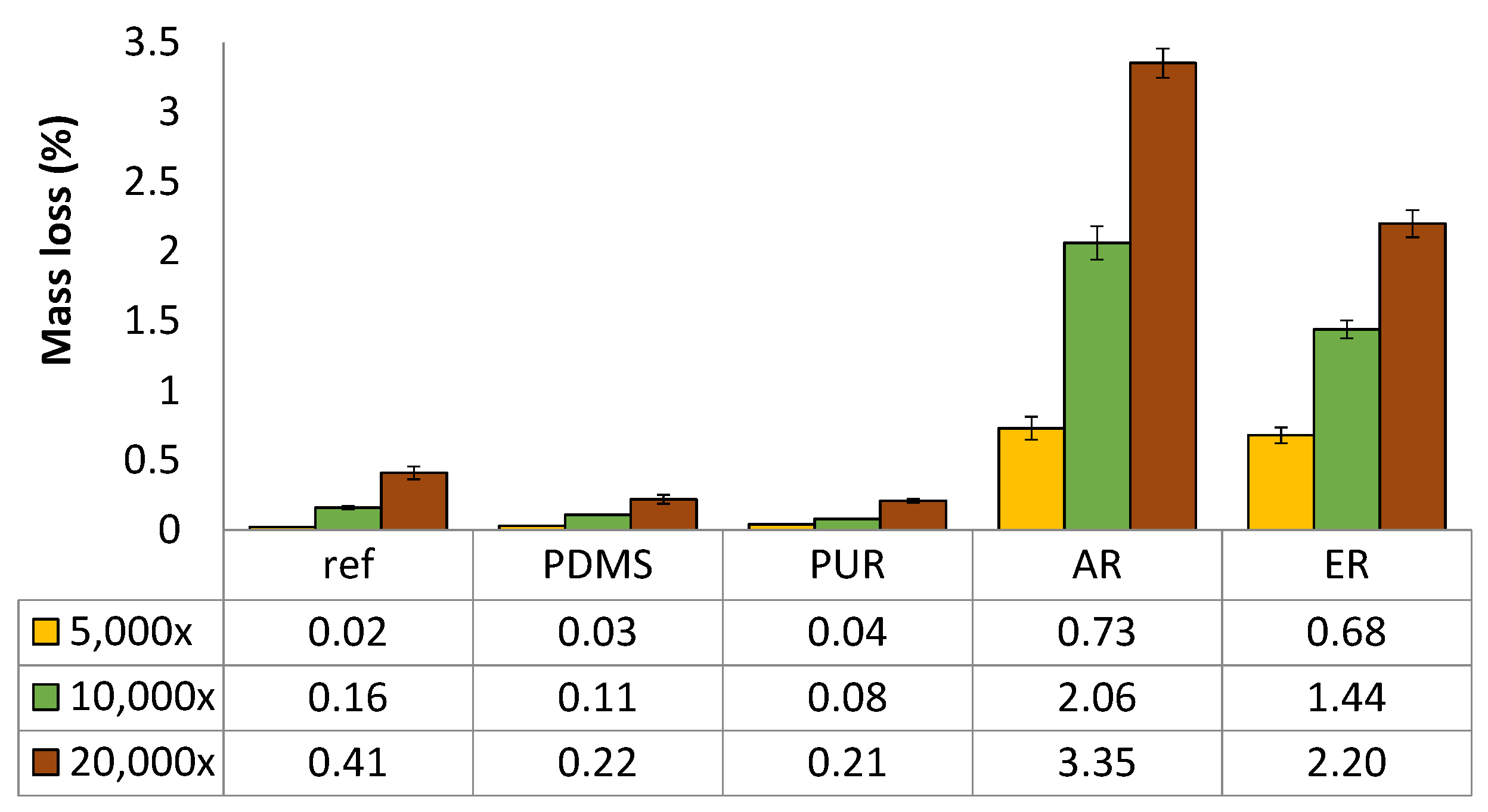
3.1.3. Abrasion Resistance
3.1.4. UV Photo-Stability
3.2. Characterisation of Cu Metallised/Polymer Coated PES
3.2.1. Electrical Resistivity
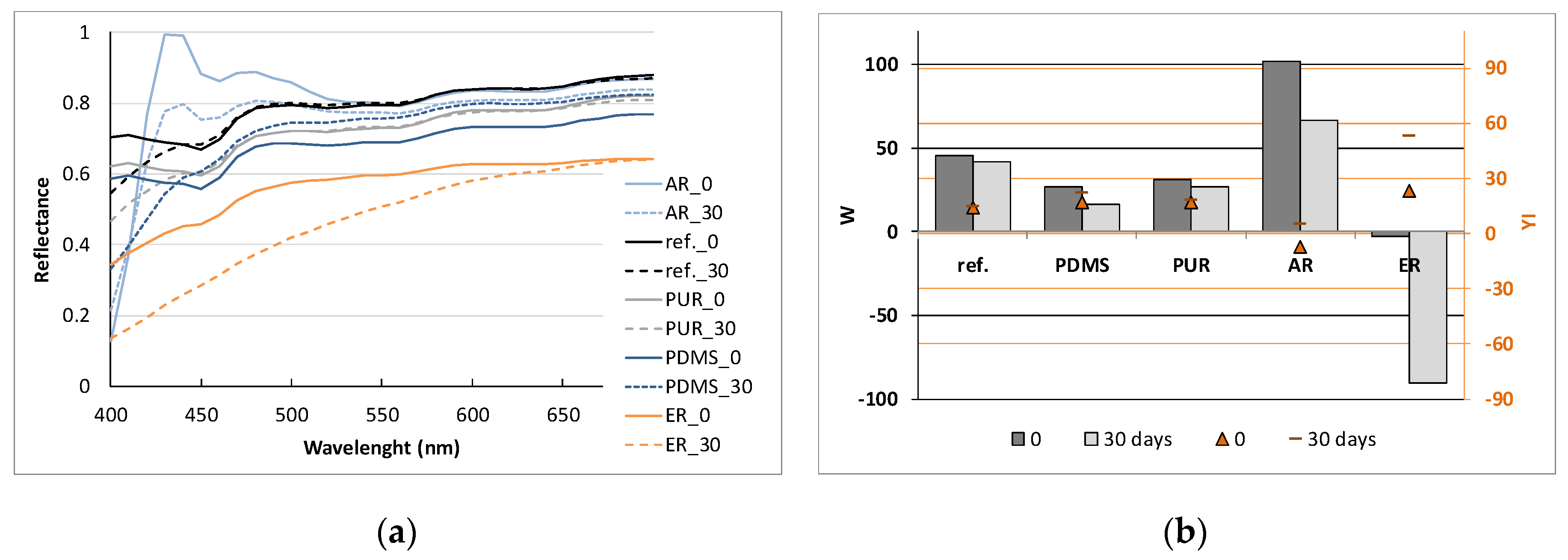
3.2.2. Colour Evaluation of Samples
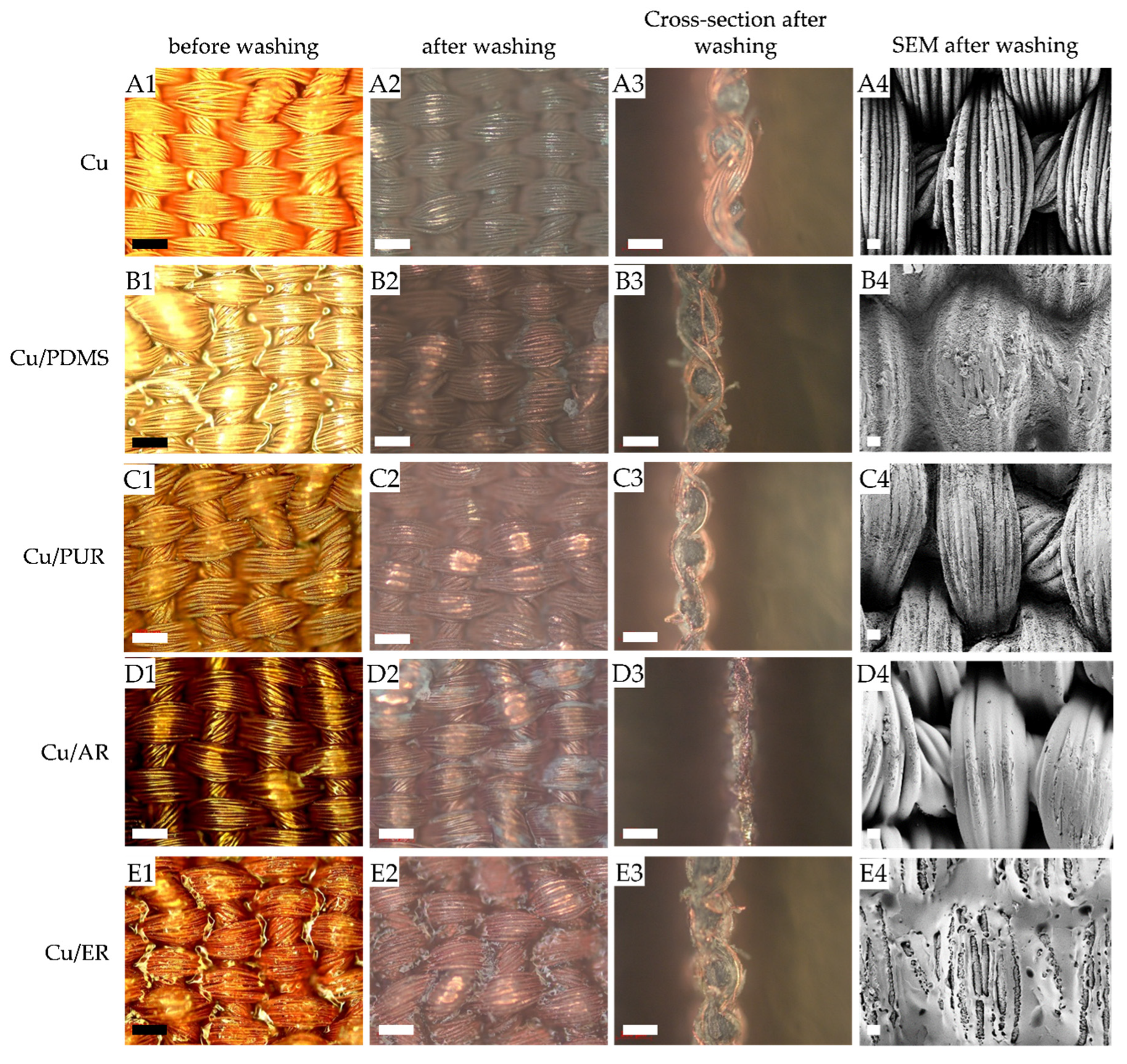
3.2.3. Surface Observation by OM and SEM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Govaert, F.; Vanneste, M. Preparation and Application of Conductive Textile Coatings Filled with Honeycomb Structured Carbon Nanotubes. J. Nanomater. 2014, 2014, 6–12. [Google Scholar] [CrossRef]
- Ghosh, S. Electroless copper deposition: A critical review. Thin Solid Film. 2019, 669, 641–658. [Google Scholar] [CrossRef]
- Wills, K.A.; Krzyzak, K.; Bush, J.; Ashayer-Soltani, R.; Graves, J.E.; Hunt, C.; Cobley, A.J. Additive process for patterned metallized conductive tracks on cotton with applications in smart textiles. J. Text. Inst. 2018, 109, 268–277. [Google Scholar] [CrossRef]
- Graves, J.E.; Sugden, M.; Litchfield, R.E.; Hutt, D.A.; Mason, T.J.; Cobley, A.J. Ultrasound assisted dispersal of a copper nanopowder for electroless copper activation. Ultrason. Sonochemistry 2016, 29, 428–438. [Google Scholar] [CrossRef]
- Litchfield, R.E.; Graves, J.E.; Sugden, M.A.; Hutt, D.E.; Cobley, A. Functionalised copper nanoparticles as catalysts for electroless plating. In Proceedings of the 2014 IEEE 16th Electronic Packaging Technology Conference, Singapore, 3–5 December 2014; pp. 235–240. [Google Scholar]
- Paniwynk, L.; Cobley, A. Ultrasonic Surface Modification of Electronics Materials. Phys. Procedia 2010, 3, 1103–1108. [Google Scholar] [CrossRef][Green Version]
- Al-Maqdasi, Z.; Hajlane, A.; Renbi, A.; Ouarga, A.; Chouhan, S.S.; Joffe, R. Conductive Regenerated Cellulose Fibers by Electroless Plating. Fibres 2019, 7, 38. [Google Scholar] [CrossRef]
- Cui, X.; Hutt, D.A.; Scurr, D.J.; Conway, P.P. The evolution of Pd/Sn catalytic surfaces in electroless copper deposition. J. Electrochem. Soc. 2011, 158, D172–D177. [Google Scholar] [CrossRef]
- Liu, X.; Guo, R.; Shi, Y.; Deng, L.; Li, Y. Durable, Washable, and Flexible Conductive PET Fabrics Designed by Fiber Interfacial Molecular Engineering. Macromol. Mater. Eng. 2016, 301, 1383–1389. [Google Scholar] [CrossRef]
- Shahariar, H.; Kim, I.; Soewardiman, H.; Jur, J.S. Inkjet Printing of Reactive Silver Ink on Textiles. ACS Appl. Mater. Interfaces 2019, 11, 6208–6216. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Y.; Liu, X. Polymer interface molecular engineering for E-textiles. Polymers 2018, 10, 573. [Google Scholar] [CrossRef]
- Cho, J.; Moon, J.; Jeong, K.; Cho, G. Application of PU-sealing into Cu/Ni electroless plated polyester fabrics for e-textiles. Fibers Polym. 2007, 8, 330–334. [Google Scholar] [CrossRef]
- Baldissera, A.F.; Ferreira, C.A. Coatings based on electronic conducting polymers for corrosion protection of metals. Prog. Org. Coat. 2012, 75, 241–247. [Google Scholar] [CrossRef]
- Kazani, I.; Hertleer, C.; De Mey, G.; Schwarz, A.; Guxho, G.; Van Langenhove, L. Electrical Conductive Textiles Obtained by Screen Printing. Fibres Text. East. Eur. 2012, 20, 57–63. [Google Scholar]
- Yang, K.; Torah, R.; Wei, Y.; Beeby, S.; Tudor, J. Waterproof and durable screen printed silver conductive tracks on textiles. Text. Res. J. 2013, 83, 2023–2031. [Google Scholar] [CrossRef]
- Paul, G.; Torah, R.; Beeby, S.; Tudor, J. The development of screen printed conductive networks on textiles for biopotential monitoring applications. Sens. Actuators A Phys. 2014, 206, 35–41. [Google Scholar] [CrossRef]
- Jiang, S.; Miao, D.; Li, A.; Guo, R.; Shang, S. Adhesion and Durability of Cu Film on Polyester Fabric Prepared by Finishing Treatment with Polyester-polyurethane and Aqueous Acrylate. Fibers Polym. 2016, 17, 1397–1402. [Google Scholar] [CrossRef]
- Fakin, D.; Stana-Kleinschek, K.; Kurečič, M.; Ojstršek, A. Effects of nanoTiO2-SiO2 on the hidrophilicity/dyeability of polyester fabric and photostability of disperse dyes under UV irradiation. Surf. Coat. Technol. 2014, 253, 185–193. [Google Scholar] [CrossRef]
- Kara, S.; Yeşilpinar, S.; Akşit, A. Permeability properties and abrasion resistance of coated polypropylene fabrics. Fibres Text. 2018, 2, 40–47. [Google Scholar]
- Moiz, A.; Vijayan, A.; Padhye, R.; Wang, X. Chemical and water protective surface on cotton fabric by pad-knife-pad coating of WPU-PDMS-TMS. Cellulose 2016, 23, 3377–3388. [Google Scholar] [CrossRef]
- Lee, S.; Park, C.H. Conductivity, superhydrophobicity and mechanical properties of cotton fabric treated with polypyrrole by in-situ polymerization using the binary oxidants ammonium Peroxodisulfate and ferric chloride. Text. Res. J. 2019, 89, 2376–2394. [Google Scholar] [CrossRef]
- Bulut, Y.; Suülar, V. Effects of process parameters on mechanical properties of coated fabrics. Int. J. Cloth. Sci. Technol. 2011, 23, 205–221. [Google Scholar] [CrossRef]
- Somogyi Škoc, M.; Pezelj, E. Abrasion Resistance of High. Performance Fabrics. In Abrasion Resistance of Materials; Marcin, A., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Textor, T.; Derksen, L.; Bahners, T.; Gutmann, J.S.; Mayer-Gall, T. Abrasion resistance of textiles: Gaining insight into the damaging mechanisms of different test procedures. J. Eng. Fibers Fabr. 2019, 14, 1558925019829481. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Nachman, M. The Abrasive Wear Resistance of the Segmented Linear Polyurethane Elastomers Based on a Variety of Polyols as Soft Segments. Polymers 2017, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Israeli, Y.; Lacoste, J.; Cavezzad, J.; Lemaire, J. Photooxidation of polydimethylsiloxane oils and resins. IV-effect of phenyl groups. Polym. Degrad. Stab. 1995, 47, 357–362. [Google Scholar] [CrossRef]
- Tokarska, M.; Frydrysiak, M.; Zięba, J. Electrical properties of flat textile material as inhomegeneous and anisotropic structure. J. Mater. Sci. Mater. Electron. 2013, 24, 5061–5068. [Google Scholar] [CrossRef]

| Polymer | Colour | Transparency | Viscosity (m Pa/s) | Cure Time | Pot Life (h) | Dielectric Strength (kV/mm) | |
|---|---|---|---|---|---|---|---|
| At 25 °C (h) | At 100 °C (min) | ||||||
| Polydimethylsiloxane (PDMS) | clear | high | 3500 | 48 | 35 | up to 2 | 19 |
| Polyurethane resin (PUR) | colourless | high | 30 | 4 | 20 | up to 2 | 75 |
| Modified acrylate resin (AR) | fluorescent | high | low | - | 5 | 1 | 60 |
| Epoxy resin (ER) | yellowish | high | 1500 | 72 | 45 | 1.5 | 45 |
| Sample | Process Employed | Drying | Add-on (%) | Thickness (mm) | Air Permeability (L/m2 s) | |
|---|---|---|---|---|---|---|
| T (°C) | Time (min) | |||||
| reference | - | - | - | - | 0.289 | 228.4 ± 9.5 |
| PDMS | Screen-printing | 100 | 25 | 39.26 | 0.323 | 47.8 ± 2.4 |
| PUR | Padding | 100 | 20 | 25.38 | 0.314 | 215.4 ± 13.6 |
| AR | Padding | 100 | 5 | 28.23 | 0.318 | 194.5 ± 12.2 |
| ER | Screen-printing | 100 | 45 | 42.18 | 0.346 | ˂ 5 |
| Electrical Resistivity [Ω] | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu Metallized | Cu Metallized/Polymer Coated | Washing Cycles | ||||||||||||||||
| 5 | 10 | 20 | 30 | |||||||||||||||
| 1 | 2 | 3 | Polymer | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| 0.4 | 0.2 | 0.1 | - | 20.5 | 37.4 | 35.5 | 46.2 k | 1 M | 68.7 k | - | - | - | - | - | - | |||
| 0.8 | 0.4 | 0.3 | PDMS | 1.2 | 0.5 | 1.8 | 1.5 | 0.6 | 2.0 | 1.8 | 0.7 | 2.5 | 1.8 | 1.3 | 3.4 | 5.8 | 1.5 | 55 |
| 0.2 | 0.2 | 0.9 | PUR | 2.5 | 2.3 | 2.9 | 7.5 | 3.5 | 6.9 | 12.7 | 5.8 | 14 | 17.2 | 12.2 | 16 | 22 | 26 | 15.5 |
| 0.2 | 0.2 | 1.2 | AR | 1.7 | 0.2 | 1.5 | 2.5 | 0.4 | 5.8 | 2.8 | 0.8 | 6.1 | 2.8 | 0.8 | 6.5 | 2.9 | 0.9 | 12.2 |
| 0.3 | 0.6 | 1.5 | ER | 3.7 | 7.1 | 7.5 | 7.8 | 5.9 | 2.7 | 11.8 k | 17.4 k | 65.3 k | - | - | - | - | - | - |
| CIE L*a*b* Values | CIE Colour Differences | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu Metallized | Cu Metallized/Polymer Coated | Washing Cycles | ||||||||||||||||
| 5 | 15 | 30 | ||||||||||||||||
| L* | a* | b* | Polymer | L* | a* | b* | dL* | da* | db* | dE* | dL* | da* | db* | dE* | dL* | da* | db* | dE* |
| 42.98 | 14.88 | 13.01 | - | −8.50 | −7.44 | −7.35 | 13.47 | −8.26 | −6.96 | −6.02 | 12.37 | |||||||
| 43.29 | 14.82 | 12.91 | PDMS | 32.67 | 8.11 | 7.48 | 0.53 | −1.58 | −0.77 | 1.84 | −0.47 | −2.90 | −1.16 | 3.16 | 0.13 | −4.00 | −1.06 | 4.13 |
| 43.22 | 15.17 | 13.13 | PUR | 33.70 | 8.35 | 7.96 | 1.97 | −0.42 | −1.33 | 2.41 | 2.06 | −0.62 | −0.48 | 2.21 | 3.63 | −2.01 | −1.50 | 3.30 |
| 44.66 | 14.21 | 15.49 | AR | 36.25 | 9.82 | −0.19 | 0.97 | −0.56 | 0.57 | 1.25 | −0.05 | −0.88 | 1.68 | 1.90 | 0.52 | −1.68 | −2.79 | 4.41 |
| 44.02 | 14.47 | 12.66 | ER | 35.52 | 7.91 | 4.24 | 0.44 | 0.04 | 1.97 | 2.02 | 1.17 | −1.12 | 2.49 | 2.97 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojstršek, A.; Virant, N.; Fox, D.; Krishnan, L.; Cobley, A. The Efficacy of Polymer Coatings for the Protection of Electroless Copper Plated Polyester Fabric. Polymers 2020, 12, 1277. https://doi.org/10.3390/polym12061277
Ojstršek A, Virant N, Fox D, Krishnan L, Cobley A. The Efficacy of Polymer Coatings for the Protection of Electroless Copper Plated Polyester Fabric. Polymers. 2020; 12(6):1277. https://doi.org/10.3390/polym12061277
Chicago/Turabian StyleOjstršek, Alenka, Natalija Virant, Daryl Fox, Latha Krishnan, and Andrew Cobley. 2020. "The Efficacy of Polymer Coatings for the Protection of Electroless Copper Plated Polyester Fabric" Polymers 12, no. 6: 1277. https://doi.org/10.3390/polym12061277
APA StyleOjstršek, A., Virant, N., Fox, D., Krishnan, L., & Cobley, A. (2020). The Efficacy of Polymer Coatings for the Protection of Electroless Copper Plated Polyester Fabric. Polymers, 12(6), 1277. https://doi.org/10.3390/polym12061277