Tuning the Properties of Furandicarboxylic Acid-Based Polyesters with Copolymerization: A Review
Abstract
1. Introduction
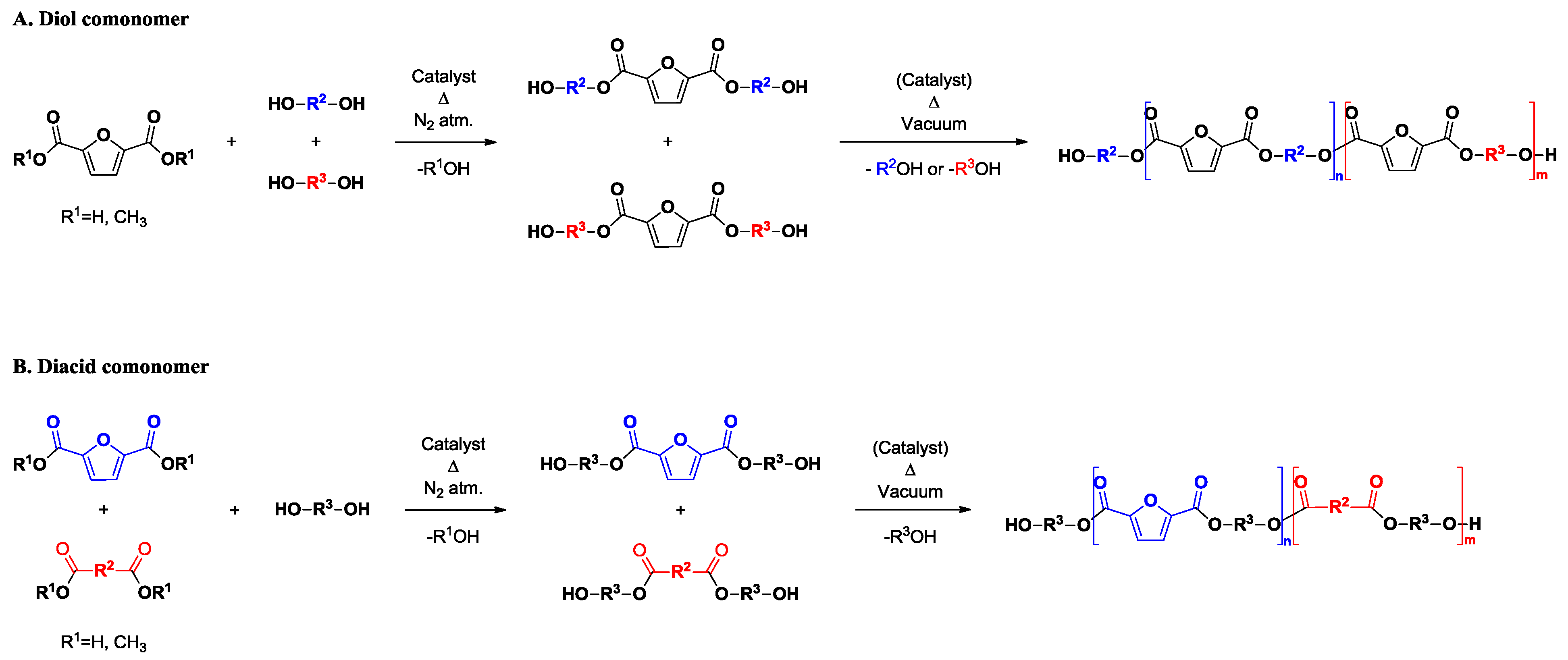
2. Synthesis, Molecular Weight, and Randomness of FDCA-Based Copolyesters
2.1. Melt Polycondensation
2.2. Ring-Opening Polymerization (ROP)
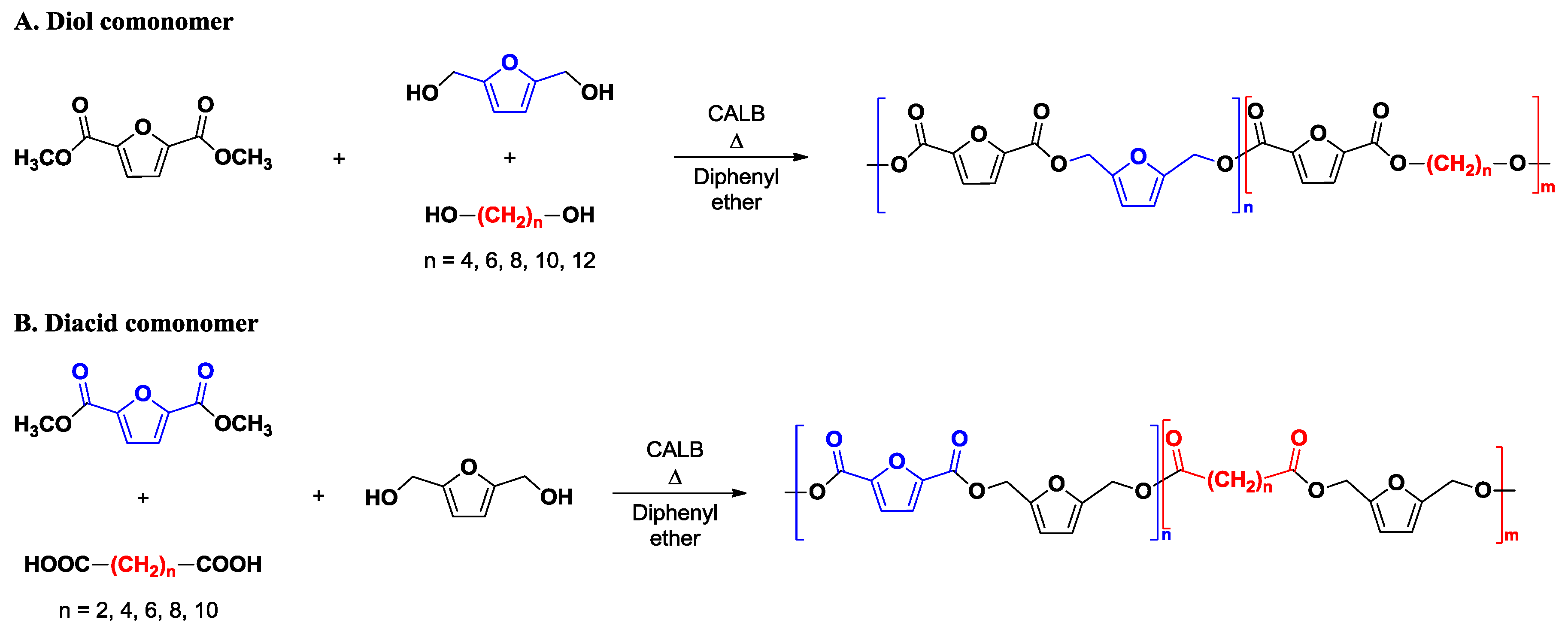
2.3. Enzymatic Synthesis
2.4. Reactive Blending
3. Thermal Properties
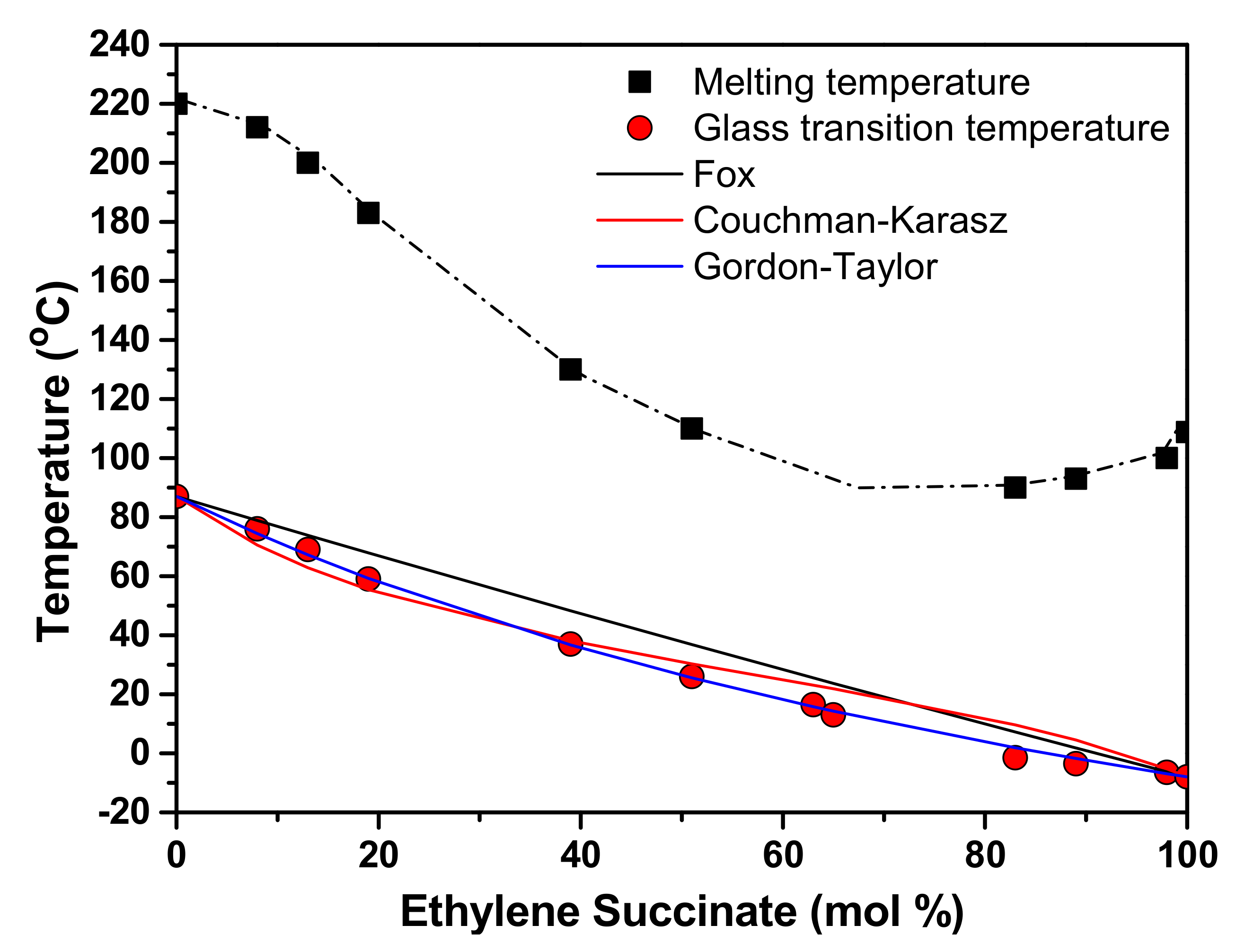
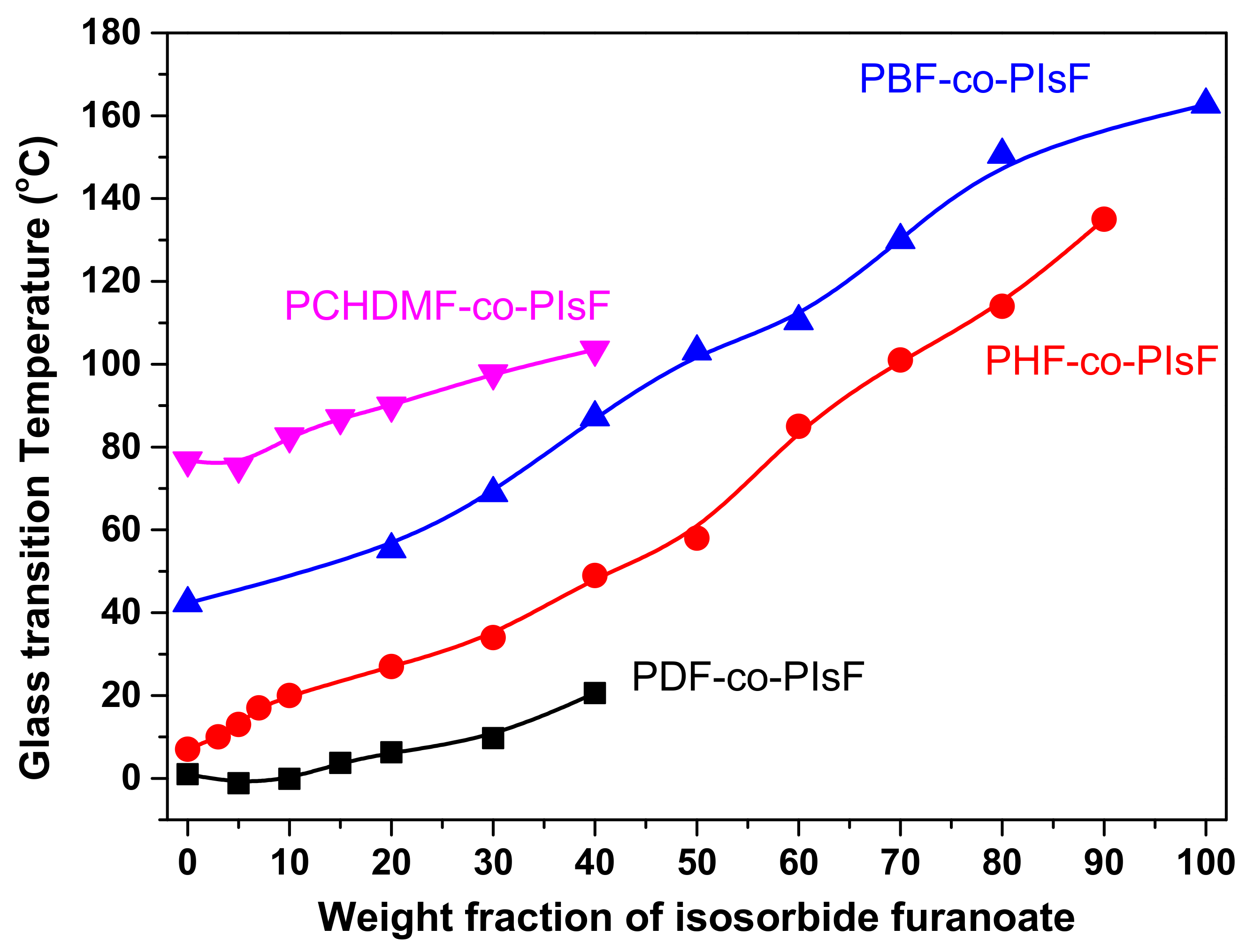
3.1. Glass Transition (Tg)
3.2. Melting and Crystallization
3.2.1. Block Copolymers
3.2.2. Random Copolymers
3.3. Thermal Stability
3.3.1. Copolymers with Cyclic Diols
3.3.2. Copolymers with Cyclic Diacids
3.3.3. Copolymers with Acyclic Comonomers
4. Mechanical and Thermomechanical Properties
4.1. Tensile Properties
4.1.1. Copolysters of FDCA with Comonomers Containing Cyclic Units
4.1.2. Copolyesters of FDCA with Acyclic Comonomers
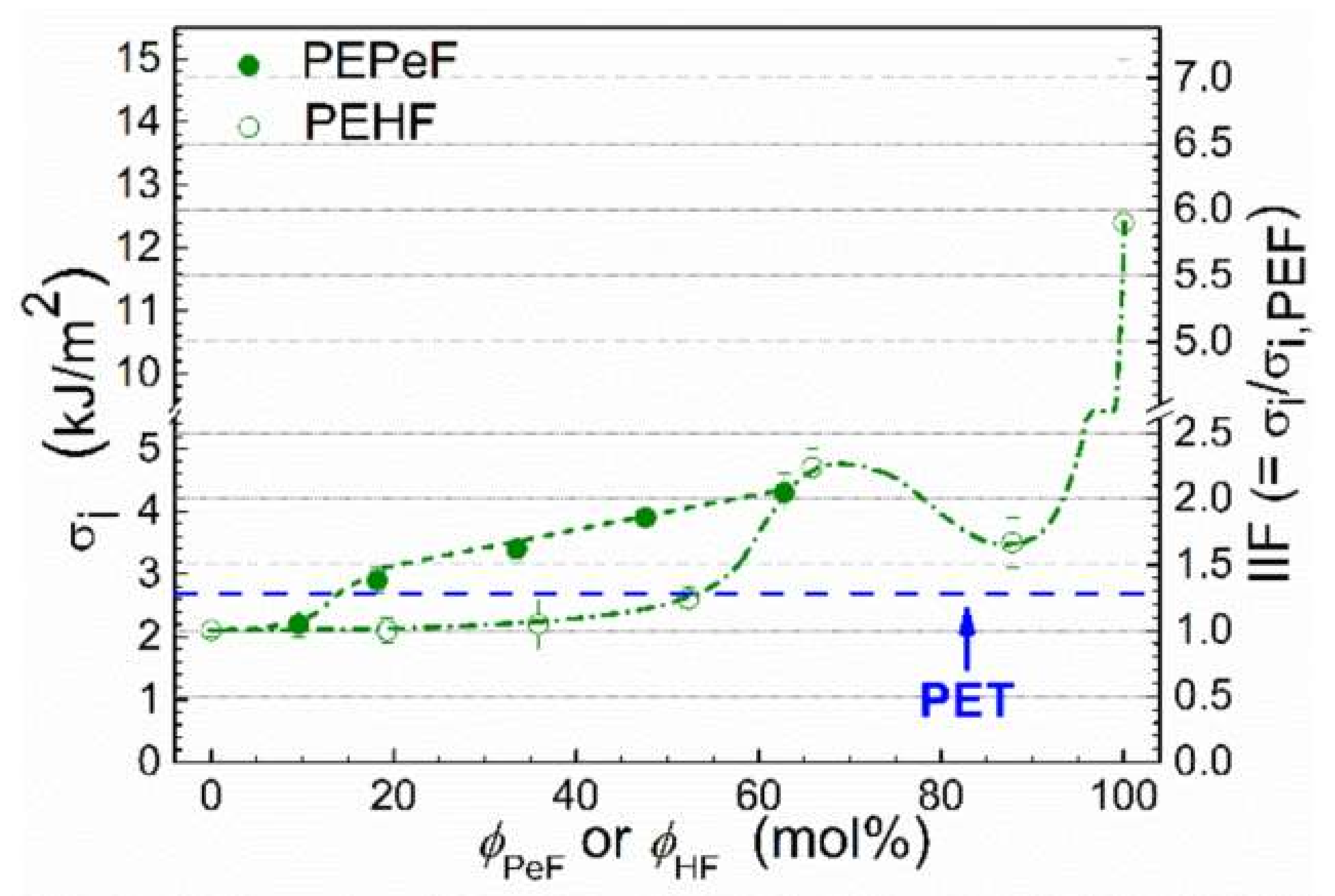
4.2. Impact Properties
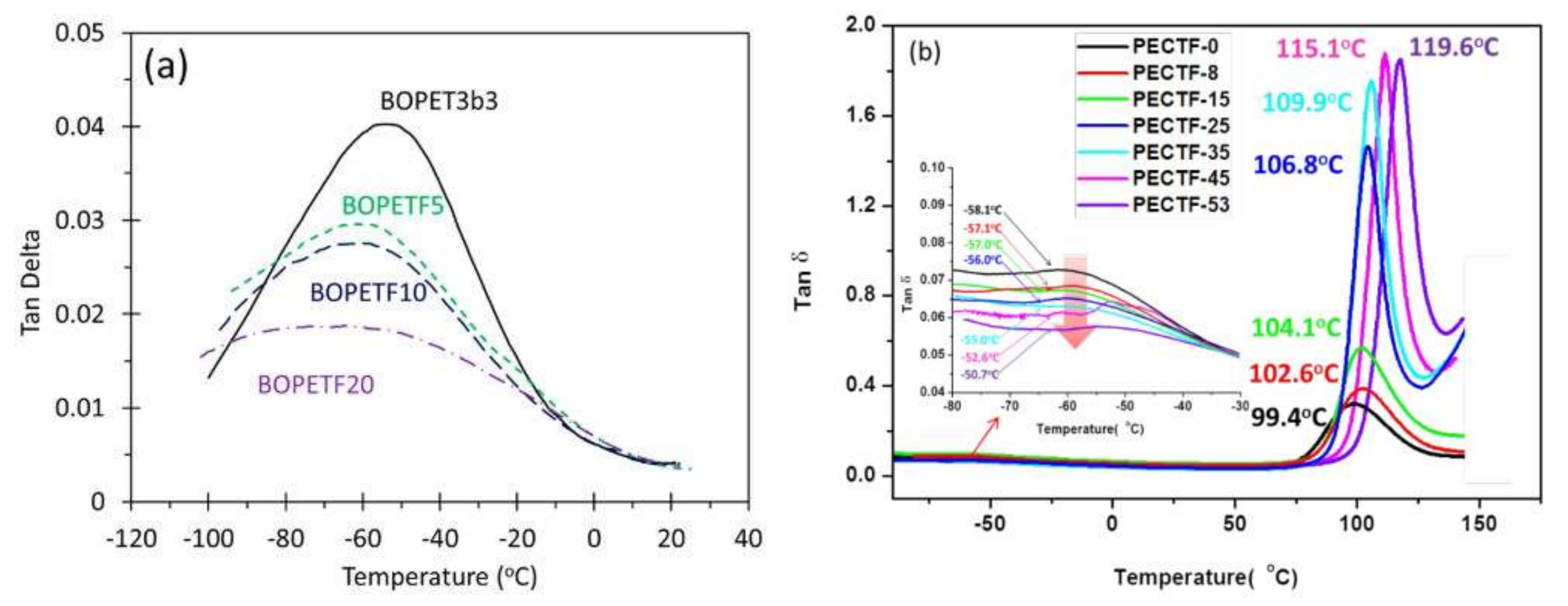
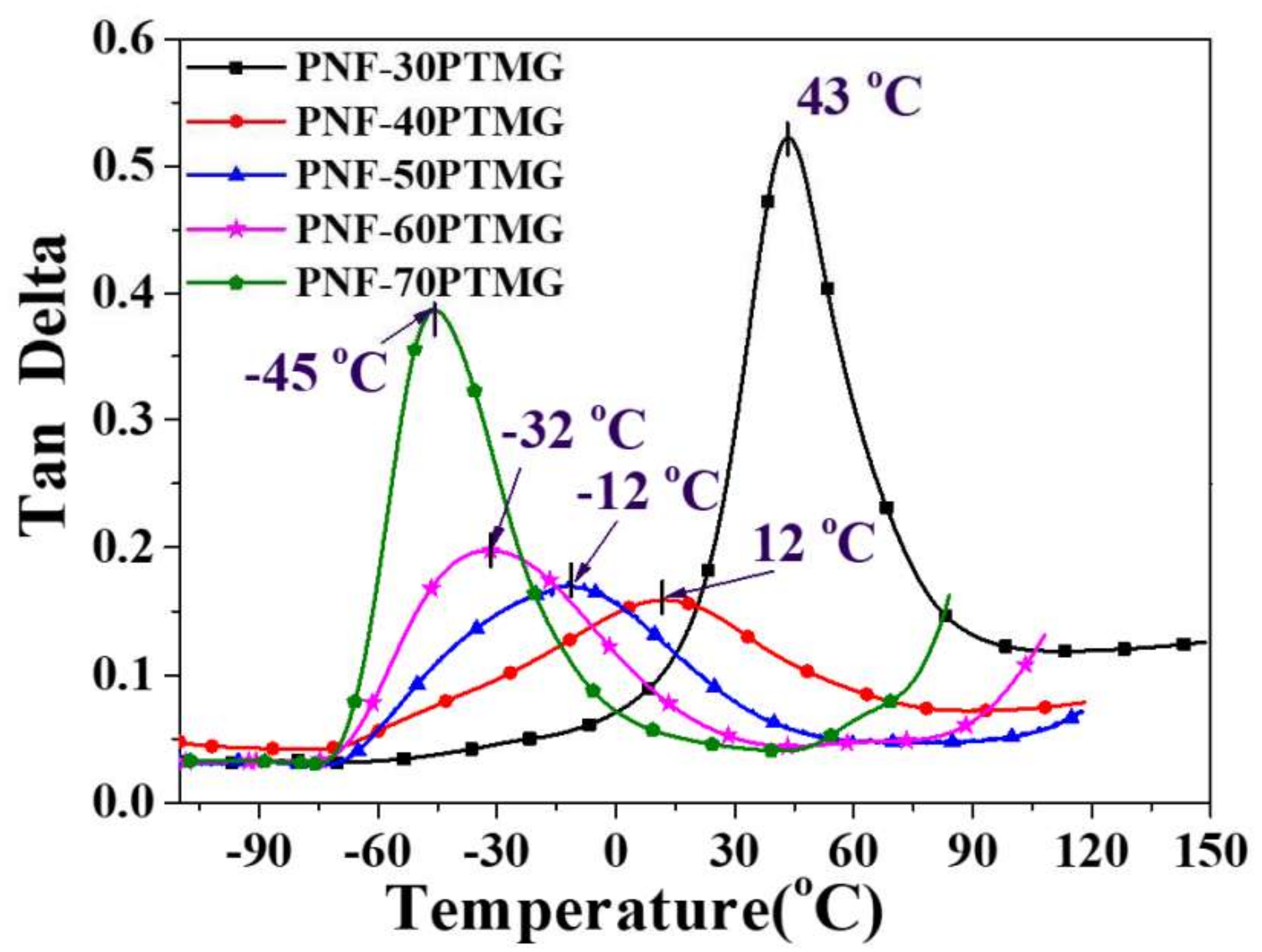
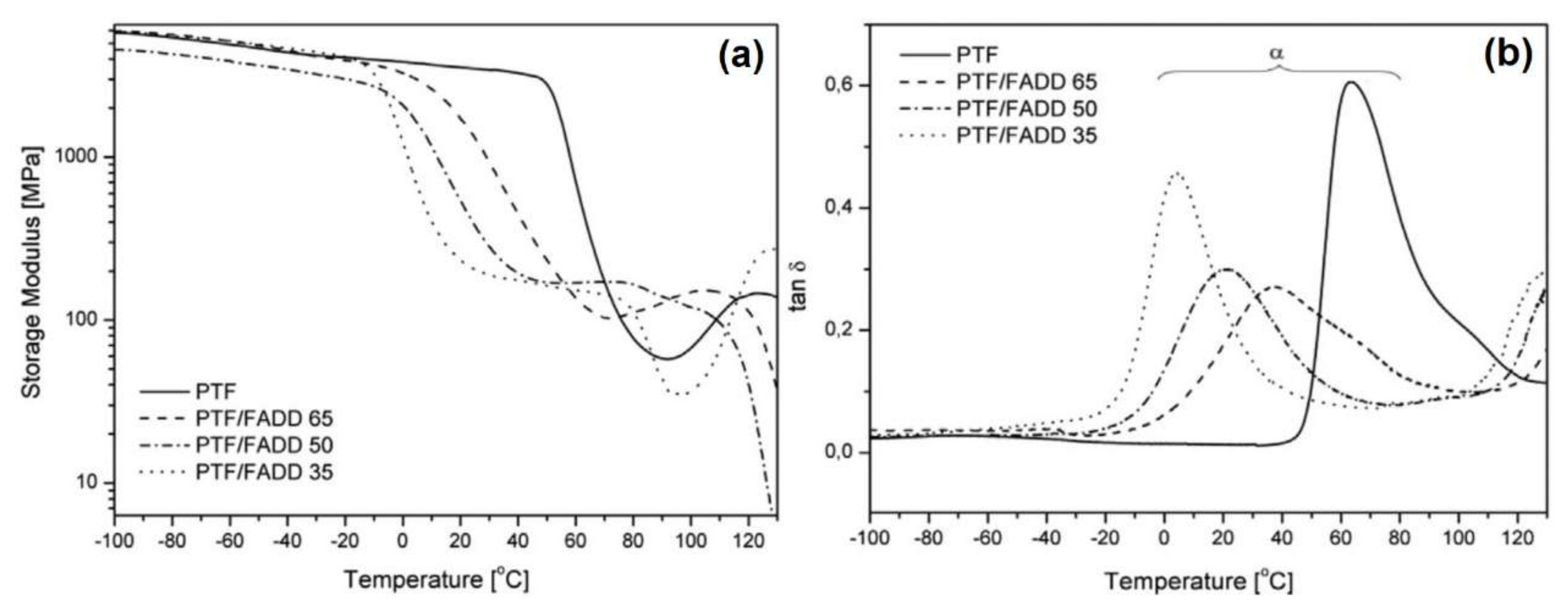
4.3. Dynamic Mechanical Analysis
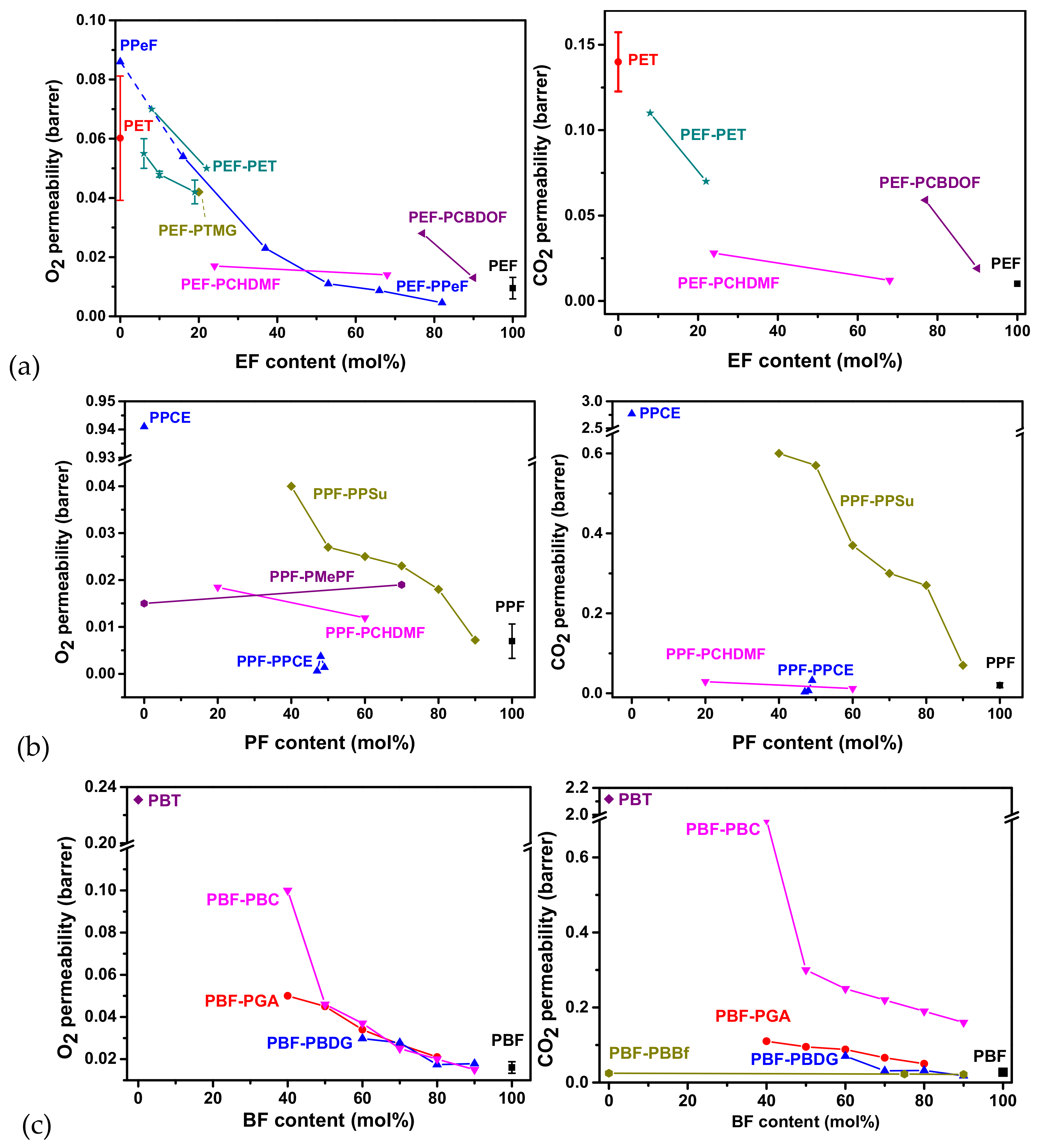
5. Gas Barrier Properties
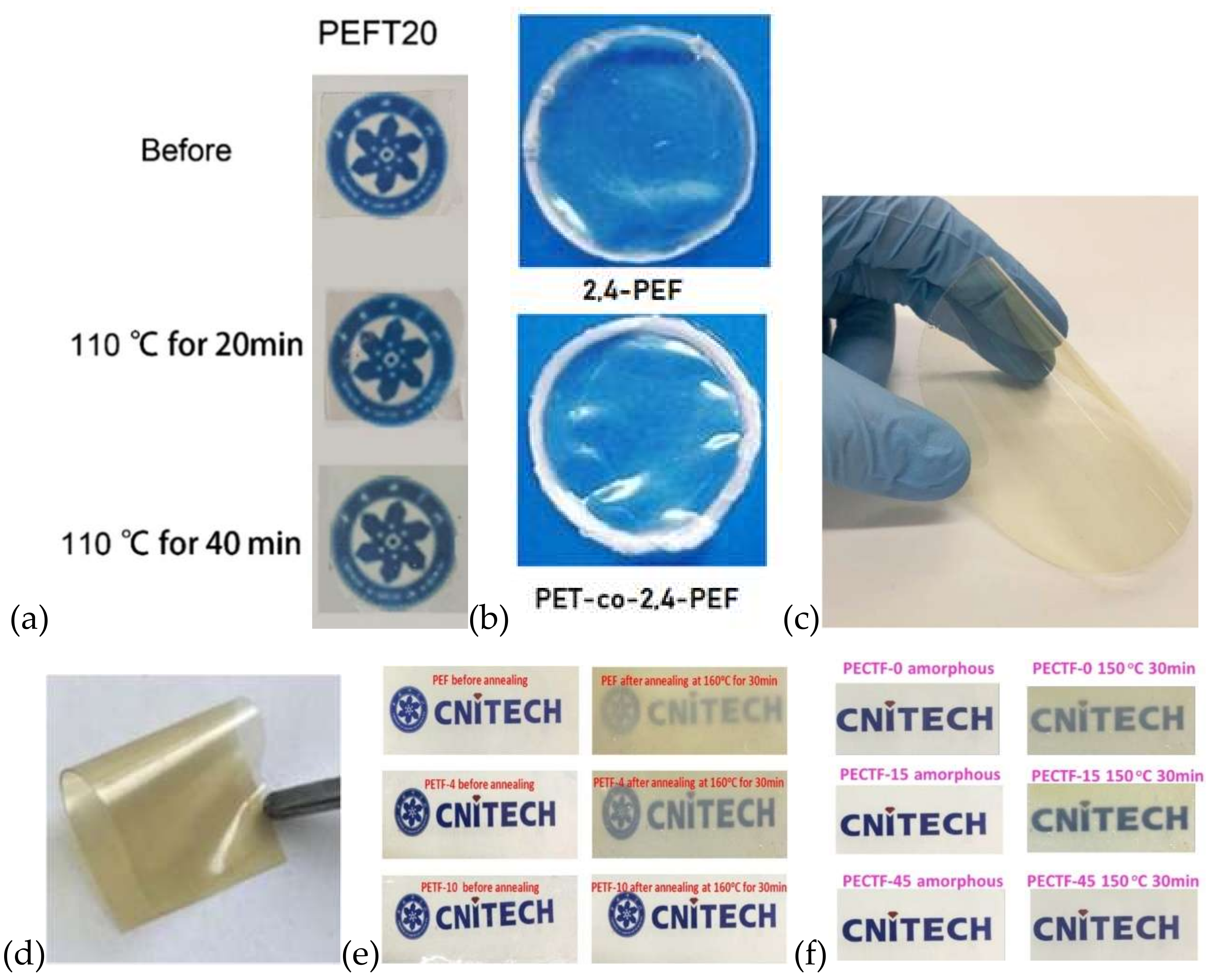
6. Optical Properties
7. Biodegradation
8. Potential Applications
9. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BDO | 1,4-butanediol |
| CALB | Candida arctica lipase B |
| CBDO | 2,2,4,4-tetramethyl-1,3-cyclobutanediol |
| CH | 1,4-cyclohexane dicarboxylic acid |
| CHDM | 1,4-Cyclohexanedimethanol |
| CL | ε-caprolactone |
| DMA | Dynamic Mechanical Analysis |
| DMFD | dimethyl furan dicarboxylate |
| DSC | Diffential Scanning Calorimetry |
| E | Young’s modulus |
| E’ | Storage Modulus |
| EC | European Commision |
| EF | ethylene furanoate |
| EG | ethylene glycol |
| FADD | dimerized fatty acid diol |
| FDCA | furan dicarboxylic acid |
| FSC | Fast Scanning Calorimetry |
| HDO | 1,6-hexanediol |
| Mw | molecular weight |
| PAA | poly(p-acetobenzoic acid) |
| PBAd | poly(butylene adipate) |
| PBbF | poly(butylene bis-2,5-furan dicarboxylate) |
| PBC | poly(butylene carbonate) |
| PBdGA | poly(Butylene Diglycolate) |
| PBF | poly(butylene 2,5-furan dicarboxylate) |
| PBI | poly(butylene isophthalate) |
| PBS | poly(butylene succinate) |
| PBSeb | poly(butylene sebacate) |
| PBT | poly(butylene terepthalate) |
| PC | polycarbonate |
| PCBDOF | poly(2,2,4,4-tetramethyl-1,3-cyclobutanediol 2,5-furan dicarboxylate) |
| PCHDMF | poly(1,4-cyclohexanedimethylene 2,5-furandicarboxylate) |
| PCL | poly(ε-caprolactone) |
| PDABPHF | poly(4,4′-diacetoxybiphenyl 2,5-furan dicarboxylate) |
| poly(decylene 2,5-furan dicarboxylate) | |
| PDO | 1,3-propanediol |
| PDoF | poly(dodecylene 2,5-furan dicarboxylate) |
| PEAd | poly(ethylene adipate) |
| PECH | poly(ethylene 1,4-cyclohexanedicarboxylate) |
| PeDO | 1,5-pentanediol |
| PEF | poly(ethylene 2,5-furan dicarboxylate) |
| PEG | poly(ethylene glycol) |
| PEI | poly(ethylene isophthalate) |
| PES | poly(ethylene succinate) |
| PESeb | poly(ethylene sebacate) |
| PET | poly(ethylene terepthlalate) |
| PFDMS | poly(2,5-furandimethylene succinate) |
| PGA | poly(glycolic acid) |
| PhBS | phosphate buffer solution |
| PHCEPPA | poly(hexamethylene 2-carboxyethyl (phenyl) phosphinic acid) |
| PHF | poly(hexylene 2,5-furan dicarboxylate) |
| PHT | poly(hexylene terepthalate) |
| PIC | poly(isosorbide carbonate) |
| PImF | poly(isomannide furandicarboxylate) |
| PIsF | poly(isosorbide 2,5-furandicarboxylate) |
| PLA | poly(lactic acid) |
| PMePF | poly(2-methyl-1,3-propanediol 2,5-furandicarboxylate) |
| PNF | poly(neopentyl glycol 2,5-furandicarboxylate) |
| PNoF | poly(nonylene 2,5-furan dicarboxylate) |
| POF | poly(octylene 2,5-furan dicarboxylate) |
| POT | poly(octylene terepthlalate) |
| PPCH | poly(propylene cyclohexane dicarboxylate) |
| PPeF | poly(pentylene 2,5-furan dicarboxylate) |
| PPEGF | poly((poly(ethylene glycol)) 2,5-furandicarboxylate)- |
| PPF | poly(propylene 2,5-furan dicarboxylate) |
| PPO | poly(propylene oxide) |
| PPPOF | poly(poly(propylene oxide) 2,5-furan dicarboxylate)) |
| PPS | poly(propylene succinate) |
| PPT | poly(propylene terepthalate) |
| PPTMGF | poly(tetramethylene glycol) 2,5-furan dicarboxylate) |
| PRF | poly(di-O-2-(hydroxyethyl) resorcinol 2,5-furandicarboxylate) |
| PTMG | poly(tetramethylene glycol) |
| ROP | ring opening polymerization |
| Sb2O3 | antimony(III) oxide |
| SBF | simulated body fluid |
| Sn(oct)2 | Stannous octoate |
| TBT | titanium(IV) butoxide |
| Td, 5% | Temperature that corresponds to 5% mass loss |
| Td.max | temperature at which degradation occurs with the fastest rate |
| Tg | glass transition temperature |
| TGA | thermogravimetric analysis |
| Tm | melting temperature |
| TPA | terepthalic acid |
| Xc | % crystallinity |
| εb | elongation at break |
| σb | tensile stress at break |
| σγ | tensile stress at yield |
Appendix A
| Polymer | Tensile Strength (σb) | Yield Point (σγ) | Young’s Modulus (E) | Elongation (εb) | ΔHm | Mw | [η] | Reference |
|---|---|---|---|---|---|---|---|---|
| MPa | MPa | MPa | % | J/g | g/mol | dL/g | ||
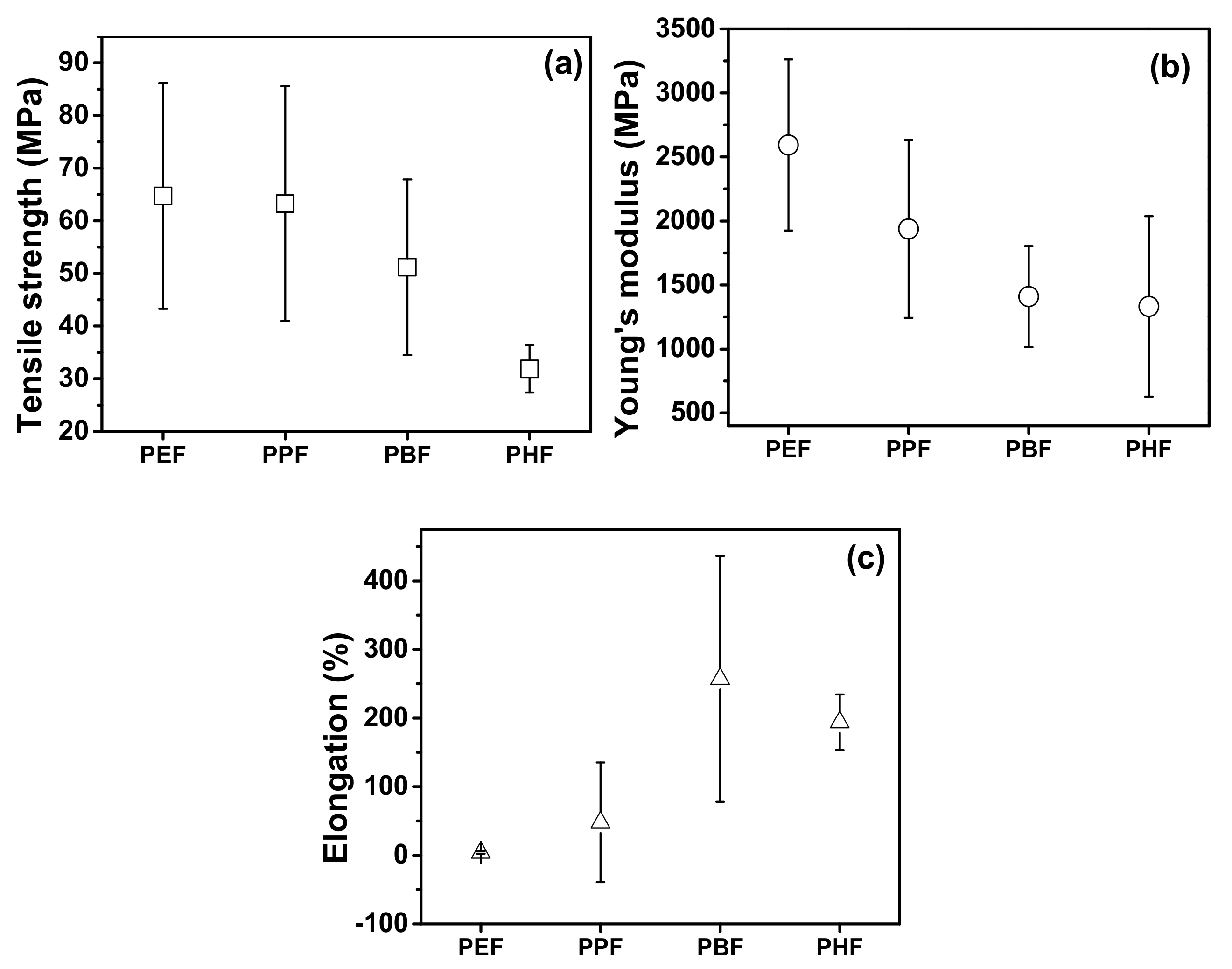
| PEF | 85 ± 9 | - | 2800 ± 120 | 5 ± 1 | 29.6 | 46,900 | 0.82 | [30,34,35] |
| 35 ± 8 | - | 2450 ± 220 | 2.81 ± 0.69 | - | 1520 | ND | [131] | |
| 56 ± 9 | - | 2511 ± 148 | 7 ± 1 | 24.4 | ND | 0.43 | [33] | |
| 25.6 | - | 1555 | 1.5 | - | 43,140 | ND | [42] | |
| 82 ± 5 | - | 3340 ± 490 | 4 ± 1 | 5.9 | ND | 0.82 | [37,45] | |
| 72 ± 5 | - | ND | 3 ± 1 | - | 90,300 | ND | [47] | |
| 84 ± 2 | - | 3430 ± 160 | 3 ± 1 | 1.2 | ND | ND | [56] | |
| 39 ± 3 | - | 2067 ± 212 | 6 ± 2 | ND | ND | 0.3 | [49] | |
| PPF | 53 ± 2 | - | 2700 ± 30 | 50 ± 7 | - | 6500 | 0.88 | [34] |
| 31 ± 3 | - | 1363 ± 158 | 3 ± 1 | 7 | ND | ND | [61] | |
| 90 ± 6 | - | 2460 ± 280 | 222 ± 20 | 3.21 | 41,300 | 0.74 | [59] | |
| 42 | - | 1055 | 4.2 | - | 56,080 | 0.81 | [42] | |
| 72 ± 5 | - | 2080 ± 100 | 3 ± 1 | - | 252,000 | ND | [51] | |
| 70.3 ± 2 | - | 1085.2 ± 14.6 | 6.3 ± 0.3 | - | 24,846 | 0.65 | [60] | |
| 98.5 ± 0.4 | - | 2600 ± 53 | 5 ± 1 | - | 7128 | 0.95 | [62] | |
| PBF | 65.6 ± 2.2 | 61.0 ± 1.8 | 1360 ± 32 | 310 ± 15 | ND | 62,000 | 1.06 | [92] |
| 62 ± 3 | ND | 2000 ± 30 | 290 ± 6 | ND | 76,000 | 0.98 | [34] | |
| 56.8 | ND | 1483 | 5.2 | ND | 44,040 | ND | [42] | |
| 58.9 ± 2.2 | ND | 2000 ± 100 | 4 ± 0.3 | ND | ND | 0.77 | [63] | |
| 53 ± 2 | 39 ± 2 | 1502 ± 101 | 685 ± 32 | 30.6 | ND | 1.23 | [68] | |
| 20.2 ± 1.7 | 20.1 ± 1.5 | 907.7 ± 42.1 | 184.3 ± 17.8 | 51.7 | ND | 0.81 | [71] | |
| 73.9 ± 0.9 | ND | 1351 ± 64 | 289 ± 12 | 27.1 | ND | 1.02 | [89] | |
| 35 ± 2.6 | ND | 875 ± 18 | 55 ± 10 | 37 | ND | ND | [72,94] | |
| 38 ± 3.1 | ND | 926 ± 11 | 90 ± 8 | ND | ND | ND | [94] | |
| 29 ± 4 | 34 ± 5 | 1283 ± 126 | 102 ± 51 | 32 | ND | 0.621 | [77] | |
| 66 ± 2 | ND | 1360 ± 32 | 310 ± 15 | ND | 62,000 | ND | [70] | |
| 55.6 ± 1.6 | ND | 1860 ± 160 | 256 ± 19 | 33.7 | ND | ND | [73] | |
| PPeF | 14 ± 2 | ND | 6 ± 1 | 320 ± 11 | - | ND | 0.82 | [37] |
| PHF | 30 ± 2 | ND | 1830 ± 170 | 237 ± 33 | 52.7 | ND | 0.72 | [45] |
| 37.4 | ND | 833 | 156.7 | ND | 27,260 | ND | [42] | |
| 28.6 ± 0.7 | ND | ND | 188 ± 22 | 41.2 | 97,400 | 0.90 | [96] | |
| POF | 26.5 ± 1.5 | 23.9 ± 1.7 | 310.5 ± 21 | 160 ± 15 | 63.9 | 62,085 | 0.43 | [132] |
| PNoF | 21.0 ± 1.6 | 19.0 ± 1.4 | 251.7 ± 19 | 149 ± 11 | 4.3 | 67,284 | 0.50 | [132] |
| 11.4 ± 1.2 | 10.6 ± 1.4 | 201.9 ± 15 | 135 ± 17 | 64.2 | 57,025 | 0.47 | [132] | |
| PDoF | 10.8 ± 0.9 | 9.5 ± 1.1 | 180.7 ± 16 | 130 ± 10 | 69.6 | 68,965 | 0.49 | [132] |
| PCHDMF | 62 ± 4 | ND | 2100 ± 200 | 18 ± 4 | 49.2 | 30,400 | 0.72 | [30,59] |
| PNoF | 68.1 ± 1.5 | ND | 1976.9 ± 30 | 6.0 ± 0.6 | 29.5 | ND | 0.72 | [102] |
| 74.5 ± 2.3 | ND | 2315.1 ± 13 | 4.9 ± 0.3 | 39.5 | ND | 0.72 | [102] |
| Copolymer | Degradation Medium | Temperature (°C) | pH | Specimen | Time (days) | Maximum Mass Loss (%) | Reference |
|---|---|---|---|---|---|---|---|
| PEF-PLA | SBF | 37 | 6.9 | Square 12–50 mg | 84 | 60 | [54] |
| SBF | 35 | 7.4 | 20 × 20 × 2 mm | 55 | 25 | [55] | |
| Garden soil | ND | ND | 20 × 20 × 2 mm | 55 | 65 | ||
| PEF-PEG | PBS | 37 | 7.2 | 1 cm × 3 cm ×(0.1–0.3) mm | 100 | 15 | [48] |
| PBF-PEG | PBS | 37 | 7.4 | ND | 35 | 44 | [89] |
| NaOH 0.01 M | 37 | 12 | ND | 35 | 100 | ||
| H2O | 37 | 7 | ND | 49 | 27 | [90] | |
| PBS | 37 | 7.2-7.4 | ND | 49 | 24 | ||
| NaOH 0.0001 M | 37 | 10 | ND | 49 | 51 | ||
| NaOH 0.01 M | 37 | 12 | ND | 3 | 100 | ||
| PBF-PPOF | PBS | 37 | 7 | Square 69–113 mg | 84 | 1.5 | [93] |
| PBS/porcine pancreas lipase | 37 | 7 | Square 69–113 mg | 84 | 2.3 | ||
| PBF-PGA | PBS | 37 | 7.4 | films | 35 | 8 | [92] |
| PBS/porcine pancreas lipase | 37 | 7.4 | films | 35 | 60 | ||
| PBF-PBDG | Mautre compost | 60 | ND | 20 × 40 × 0.2 mm | 62 | 43 | [77] |
| PEF-PESu | PBS/R delemar, P cepacia | 50 | 7.2 | 5 × 5 × 2 mm | 30 | 12.5 | [52] |
| PPF-PPSu | PBS/porcine pancreas lipase | 37 | 7.4 | 2 × 2 cm × 0.3 mm | 28 | 35 | [62] |
| PBF-PBSu | Compost (ISO 14855-1:2005) | 58 | ND | ND | 100 | 120 | [82] |
| PBS | 25 | 7 | ND | 2 | 154 | [75] | |
| Sodium acetate/sodium hydrogen phosphate buffer | 25 | 4 | ND | 1.4 | 154 | [74] | |
| Sodium acetate/sodium hydrogen phosphate buffer | 25 | 12 | ND | 14 | 154 | ||
| Compost (ISO 14855-1:2005) | 58 | Film 20 × 20 mm | 91 | 100 | |||
| Citric acid buffer | 37 | 2 | Discs diameter 10mm, thickness 200 um, mass 20–30 mg | 30 | 10 | [83] | |
| Sodium phosphate buffer | 37 | 7.4 | Discs diameter 10mm, thickness 200 um, mass 20–30 mg | 30 | 5 | ||
| Sodium phosphate buffer/porcine pancreas lipase | 37 | 7.4 | Discs diameter 10mm, thickness 200 um, mass 20–30 mg | 30 | 17.5 | ||
| PNF-PNGS | PBS | 37 | 7.4 | Film 0.5 mm | 70 | 7 | [102] |
| PBS/Candida antarctica lipase B | 37 | 7.4 | Film 0.5 mm | 70 | 12 | ||
| PEF-PEAd | PBS/R oryzae, P cepacia | 37 | 7.2 | 5 × 5 × 0.4 mm | 30 | 100 | [50] |
| PBF-PBAd | PBS/lipase from porcine pancreas | 37 | 7.2 | 10 × 10 × 0.3 mm | 28 | 95 | [72] |
| PBS | 25 | 7 | 100 um thickness | 5 | 154 | [75] | |
| Sodium acetate/sodium hydrogen phosphate buffer | 25 | 4 | ND | 1.8 | 154 | [74] | |
| Sodium acetate/sodium hydrogen phosphate buffer | 25 | 12 | ND | 52 | 154 | ||
| Compost (ISO 14855-1:2005) | 58 | Film 20 × 20 mm | 98 | 100 | |||
| PBF-PCL | Sodium phosphate buffer | 37 | 7.4 | 10 mm diameter, 20–30 mg | 1.5 | 40 | [86] |
| Sodium phosphate buffer/porcine pancreas lipase | 37 | 7.4 | 10 mm diameter, 20–30 mg | 40 | 40 | ||
| PBS | 37 | 7.4 | 2 × 2 × 0.03 cm | 10 | 56 | [85] | |
| PBS/porcine pancreas lipase | 37 | 7.4 | 2 × 2 × 0.03 cm | 20 | 56 | ||
| PBS/Candida Antarctica lipase B | 37 | 7.4 | 2 × 2 × 0.03 cm | 32 | 56 | ||
| PPeF-PCL | PBS/P cepacia, R oryzae | 37 | 7.2 | 5 × 5 cm × 0.4 mm | 25 | 15 | [95] |
| PHeF-PCL | PBS/P cepacia, R oryzae | 37 | 7.2 | 5 × 5 cm × 0.4 mm | 25 | 32 | |
| PBF-PC | PBS/porcine pancreas lipase | 37 | 7.4 | 2 × 2 × 0.5 mm | 14.5 | 28 | [84] |
| PBF-PIsF/PIC/PBSu | PBS | 37 | 7.4 | 20 × 10mm, 20–30 mg | 2.5 | 28 | [70] |
| PBS/porcine pancreas lipase | 37 | 7.4 | 20 × 10mm, 20–30 mg | 12.5 | 28 | ||
| PBF-PImF | Sodium phosphate buffer | 37 | 7.4 | 10 mm diameter,20−30 mg weight disks | 20 | 30 | [66] |
| Sodium phosphate buffer/porcine pancreas lipase | 37 | 7.4 | 10 mm diameter,20−30 mg weight disks | 50 | 30 | ||
| PDF-PIsF | Garden soil | ND | 6.5 | 20 × 10 × 0.1 mm | 15 | 161 | [100] |
References
- Ligtvoet, A.; Maier, F.; Sijtsma, L.; van den Broek, L.A.M.; Safi, C.; Doranova, A.; Eaton, D.; Guznajeva, T.; Kals, J.; Le Gallou, M. Blue Bioeconomy Forum: Highlights: Summary of the Roadmap and A Selection of Viable and Innovative Projects; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Comission A sustainable Bioeconomy for Europe: Strengthening the Connection Between Economy, Society and the Environment. Updat. Bioecon. Strateg. Available online: https//ec.europa.eu/research/bioeconomy/2018 (accessed on 28 February 2020).
- ABOUT BBI, JU. Available online: https://www.bbi-europe.eu/about/about-bbi (accessed on 14 February 2020).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Holladay, J.E.; White, J.F.; Bozell, J.J.; Johnson, D. Top Value-Added Chemicals from Biomass-Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 2007. [Google Scholar]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Gandini, A. Furans as offsprings of sugars and polysaccharides and progenitors of an emblematic family of polymer siblings. In Green Polymerisation Methods: Renewable Starting Materials, Catalysis and Waste Reduction, Wiley-VCH, Weinheim; Wiley Online Library: Weinheim, Germany, 2011; Volume 29. [Google Scholar]
- New Market Data 2019: Bioplastics Industry Continues Dynamic Grow over The Next Five Years. Available online: https://www.european-bioplastics.org/new-market-data-2019-bioplastics-industry-continues-dynamic-grow-over-the-next-five-years/ (accessed on 14 February 2020).
- Global Polyethylene Furanoate Market 2018-2025 - Ongoing Quest to Decrease the Global Carbon Footprint is a Major Driver. Available online: https://www.prnewswire.com/news-releases/global-polyethylene-furanoate-market-2018-2025---ongoing-quest-to-decrease-the-global-carbon-footprint-is-a-major-driver-300689405.html (accessed on 28 February 2020).
- Tullo, A. DuPont, ADM Unveil Route To Biobased Polyester. Available online: https://cen.acs.org/articles/94/i4/DuPont-ADM-Unveil-Route-Biobased.html (accessed on 24 January 2020).
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2, 5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Vouvoudi, E.; Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. Thermal degradation kinetics and decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and low molecular weight aliphatic diols. J. Anal. Appl. Pyrolysis 2015, 112, 369–378. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Bikiaris, D.N.; Papageorgiou, G.Z. Crystallization and Polymorphism of Poly(ethylene furanoate). Cryst. Growth Des. 2015, 15, 5505–5512. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Fully biobased poly (propylene 2, 5-furandicarboxylate) for packaging applications: Excellent barrier properties as a function of crystallinity. Green Chem. 2015, 17, 4162–4166. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Poly (propylene 2, 5-thiophenedicarboxylate) vs. Poly (propylene 2, 5-furandicarboxylate): Two examples of high gas barrier bio-based polyesters. Polymers 2018, 10, 785. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly (ethylene furanoate) compared to poly (ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly (ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly (ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Daniel, B.Y.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly (ethylene furanoate) compared to poly (ethylene terephthalate). Part 2: Kinetic sorption. Polymer 2014, 55, 6870–6882. [Google Scholar] [CrossRef]
- Yamamoto, M.; Witt, U.; Skupin, G.; Beimborn, D.; Müller, R. Biodegradable aliphatic-aromatic polyesters:“Ecoflex®. ” Biopolym. Online Biol. Chem. Biotechnol. Appl. 2005, 4. [Google Scholar] [CrossRef]
- Kriegel, R.M.; Shi, Y.; Moffitt, R.D. Poly(ethylenefuranoate) copolymers and methods 2015. U.S. Patent Application No. 14/475,488, 5 March 2015. [Google Scholar]
- Eritate, S. Polyester resin, method of producing the same, composition for molded article and molded article 2015. U.S. Patent 8,420,769, 16 April 2013. [Google Scholar]
- Bhattacharjee, D.; Sehanobish, K. FDCA-based polyesters made with isosorbide 2017. U.S. Patent 9,580,542, 28 February 2017. [Google Scholar]
- Sipos, L. Process for preparing a polymer having a 2, 5-furandicarboxylate moiety within the polymer backbone and such (co) polymers 2017. U.S. Patent No. 9,567,431, 14 February 2017. [Google Scholar]
- Gandini, A. Furans as offspring of sugars and polysaccharides and progenitors of a family of remarkable polymers: A review of recent progress. Polym. Chem. 2010, 1, 245–251. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2, 5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Qi, Z.; He, L.; Peng, L. Progress in the Synthesis and Properties of 2, 5-Furan Dicarboxylate based Polyesters. BioResources 2020, 15, 4502–4527. [Google Scholar]
- Thiyagarajan, S.; Meijlink, M.A.; Bourdet, A.; Vogelzang, W.; Knoop, R.; Esposito, A.; Dargent, E.; Van Es, D.S.; Van Haveren, J. Synthesis and thermal properties of bio-based copolyesters from the mixtures of 2, 5-and 2, 4-furandicarboxylic acid with different diols. ACS Sustain. Chem. Eng. 2019, 7, 18505–18516. [Google Scholar] [CrossRef]
- Ma, J.; Pang, Y.; Wang, M.; Xu, J.; Ma, H.; Nie, X. The copolymerization reactivity of diols with 2,5-furandicarboxylic acid for furan-based copolyester materials. J. Mater. Chem. 2012, 22, 3457–3461. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, Y.; Liu, F.; Zhu, J. Modification of poly(ethylene 2,5-furandicarboxylate) with 1,4-cyclohexanedimethylene: Influence of composition on mechanical and barrier properties. Polymer 2016, 103, 1–8. [Google Scholar] [CrossRef]
- Xu, W.; Zhong, L.; Xu, F.; Song, W.; Wang, J.; Zhu, J.; Chou, S. Ultraflexible Transparent Bio-Based Polymer Conductive Films Based on Ag Nanowires. Small 2019, 15, 1805094. [Google Scholar] [CrossRef]
- Hong, S.; Min, K.-D.; Nam, B.-U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 5142–5150. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Liu, S.; Wang, G. Biobased copolyesters: Synthesis, structure, thermal and mechanical properties of poly(ethylene 2,5-furandicarboxylate-co-ethylene 1,4-cyclohexanedicarboxylate). Polym. Degrad. Stab. 2018, 154, 96–102. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhu, J.; Jiang, Y. Copolyesters based on 2,5-furandicarboxylic acid (FDCA): Effect of 2,2,4,4-tetramethyl-1,3-cyclobutanediol units on their properties. Polymers 2017, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Jia, Z.; Liu, Y.; Sun, L.; Zhu, J. Synthesis of bio-based poly(ethylene 2,5-furandicarboxylate) copolyesters: Higher glass transition temperature, better transparency, and good barrier properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3298–3307. [Google Scholar] [CrossRef]
- Wang, J.; Mahmud, S.; Zhang, X.; Zhu, J.; Shen, Z.; Liu, X. Biobased Amorphous Polyesters with High Tg: Trade-Off between Rigid and Flexible Cyclic Diols. ACS Sustain. Chem. Eng. 2019, 7, 6401–6411. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.-G.; Dubois, P. Modification of poly (ethylene 2, 5-furandicarboxylate) with biobased 1, 5-pentanediol: Significantly toughened copolyesters retaining high tensile strength and O2 barrier property. Biomacromolecules 2018, 20, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.F.; Matos, M.; Freire, C.S.R.; Silvestre, A.J.D.; Coelho, J.F.J. New copolyesters derived from terephthalic and 2, 5-furandicarboxylic acids: A step forward in the development of biobased polyesters. Polymer 2013, 54, 513–519. [Google Scholar] [CrossRef]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly (ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Wang, J.; Liu, F.; Jia, Z.; Liu, X.; Zhu, J. 2,5-Furandicarboxylic acid as a sustainable alternative to isophthalic acid for synthesis of amorphous poly(ethylene terephthalate) copolyester with enhanced performance. J. Appl. Polym. Sci. 2019, 136, 47186. [Google Scholar] [CrossRef]
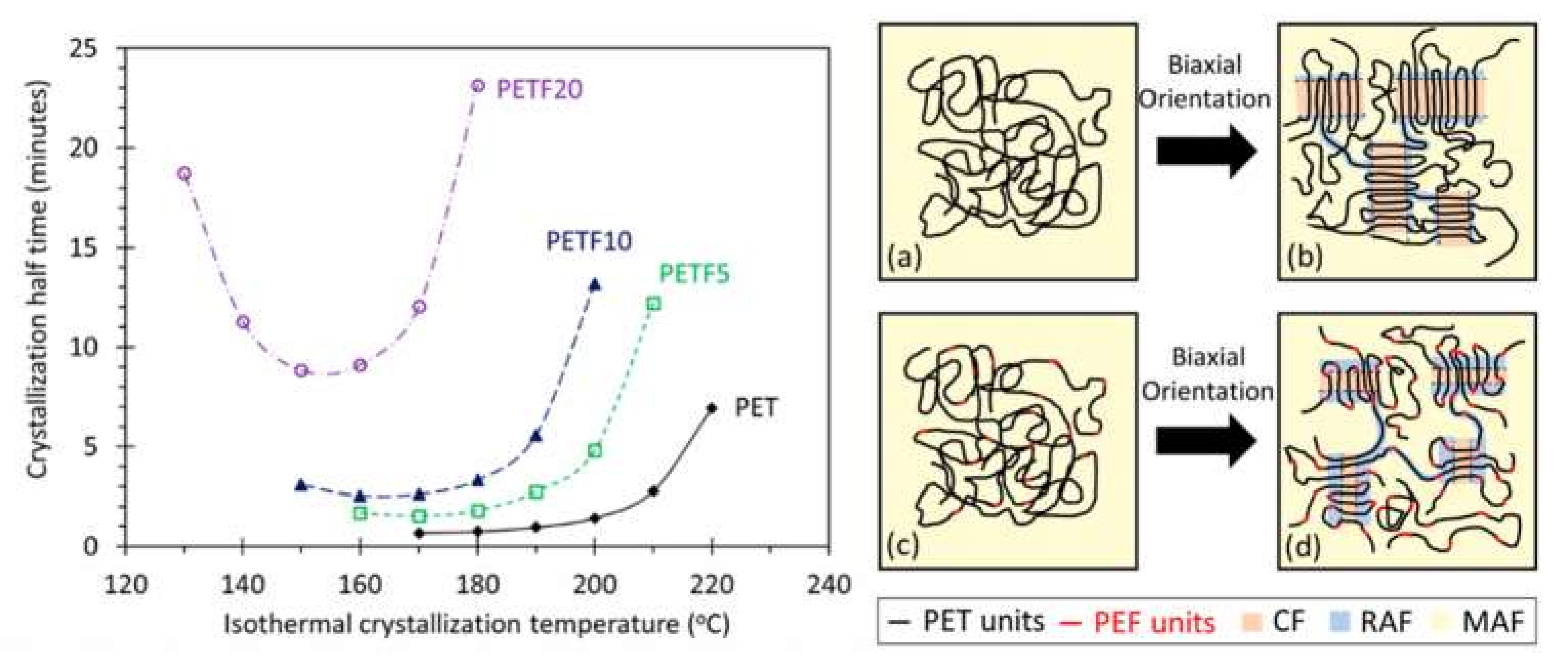
- Joshi, A.S.; Lawrence, J.G.; Coleman, M.R. Effect of Biaxial Orientation on Microstructure and Properties of Renewable Copolyesters of Poly(ethylene terephthalate) with 2,5-Furandicarboxylic Acid for Packaging Application. ACS Appl. Polym. Mater. 2019, 1, 1798–1810. [Google Scholar] [CrossRef]
- Min, J.; Tingting, L.; Qiang, Z.; Ying, C.; Guangyuan, Z. From fossil resources to renewable resources: Synthesis, structure, properties and comparison of terephthalic acid-2, 5-furandicarboxylic acid-diol copolyesters. J. Renew. Mater. 2015, 3, 120–141. [Google Scholar] [CrossRef]
- Wang, P.; Huang, W.; Zhang, Y.; Lin, J.; Chen, P. An evoluted bio-based 2,5-furandicarboxylate copolyester fiber from poly(ethylene terephthalate). J. Polym. Sci. 2020, 58, 320–329. [Google Scholar] [CrossRef]
- Zaidi, S.; Thiyagarajan, S.; Bougarech, A.; Sebti, F.; Abid, S.; Majdi, A.; Silvestre, A.J.D.; Sousa, A.F. Highly transparent films of new copolyesters derived from terephthalic and 2, 4-furandicarboxylic acids. Polym. Chem. 2019, 10, 5324–5332. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.G.; Dubois, P. Biobased Poly(ethylene-co-hexamethylene 2,5-furandicarboxylate) (PEHF) Copolyesters with Superior Tensile Properties. Ind. Eng. Chem. Res. 2018, 57, 13094–13102. [Google Scholar] [CrossRef]
- Sousa, A.F.; Coelho, J.F.J.; Silvestre, A.J.D. Renewable-based poly ((ether) ester) s from 2, 5-furandicarboxylic acid. Polymer 2016, 98, 129–135. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Zhou, G. Biobased multiblock copolymers: Synthesis, properties and shape memory performance of poly(ethylene 2,5-furandicarboxylate)-b-poly(ethylene glycol). Polym. Degrad. Stab. 2017, 144, 121–127. [Google Scholar] [CrossRef]
- Ji, P.; Lu, D.; Zhang, S.; Zhang, W.; Wang, C.; Wang, H. Modification of Poly (Ethylene 2, 5-Furandicarboxylate) with Poly (Ethylene glycol) for Biodegradable Copolyesters with Good Mechanical Properties and Spinnability. Polymers 2019, 11, 2105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Wang, Q.; Li, J.; Wang, G. Synthesis and characterization of poly(ethylene 2,5-furandicarboxylate-co-ε-caprolactone) copolyesters. Eur. Polym. J. 2018, 109, 191–197. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Magaziotis, A.; Nerantzaki, M.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and characterization of novel poly(ethylene furanoate-co-adipate) random copolyesters with enhanced biodegradability. Polym. Degrad. Stab. 2018, 156, 32–42. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Zhou, G. Biobased copolyesters: Synthesis, crystallization behavior, thermal and mechanical properties of poly(ethylene glycol sebacate-co-ethylene glycol 2,5-furan dicarboxylate). RSC Adv. 2017, 7, 13798–13807. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Biobased poly(ethylene furanoate-co-ethylene succinate) copolyesters: Solid state structure, melting point depression and biodegradability. RSC Adv. 2016, 6, 84003–84015. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Cao, F.; Wen, B.; Zhu, X.; Wei, P. Chemosynthesis and characterization of fully biomass-based copolymers of ethylene glycol, 2,5-furandicarboxylic acid, and succinic acid. J. Appl. Polym. Sci. 2013, 130, 1415–1420. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Fonseca, A.C.; Freire, C.S.; Coelho, J.F.; Silvestre, A.J. A New Generation of Furanic Copolyesters with Enhanced Degradability: Poly(ethylene 2,5-furandicarboxylate)-co-poly(lactic acid) copolyesters). Macromol. Chem. Phys. 2014, 215, 2175–2184. [Google Scholar] [CrossRef]
- Wu, H.; Wen, B.; Zhou, H.; Zhou, J.; Yu, Z.; Cui, L.; Huang, T.; Cao, F. Synthesis and degradability of copolyesters of 2, 5-furandicarboxylic acid, lactic acid, and ethylene glycol. Polym. Degrad. Stab. 2015, 121, 100–104. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.-G.; Dubois, P. Poly(ethylene 2,5-furandicarboxylate-mb-poly(tetramethylene glycol)) multiblock copolymers: From high tough thermoplastics to elastomers. Polymer 2018, 155, 89–98. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Kowalczyk, I.; Szymczyk, A.; Gorący, K. Effect of thermal aging on the crystalline structure and mechanical performance of fully bio-based, furan-ester, multiblock copolymers. Polimery 2018, 63, 594–602. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Kowalczyk, I.; Kwiatkowski, K.; Szymczyk, A.; Rosłaniec, Z. Fully biobased multiblock copolymers of furan-aromatic polyester and dimerized fatty acid: Synthesis and characterization. Polymer 2016, 99, 503–512. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, J.; Sun, L.; Liu, F.; Zhu, J.; Liu, X. Copolyesters developed from bio-based 2,5-furandicarboxylic acid: Synthesis, sequence distribution, mechanical, and barrier properties of poly(propylene-co-1,4-cyclohexanedimethylene 2,5-furandicarboxylate)s. J. Appl. Polym. Sci. 2019, 136, 47291. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Chen, C.-W.; Rwei, S.-P. Influence of asymmetric substituent group 2-methyl-1, 3-propanediol on bio-based poly (propylene furandicarboxylate) copolyesters. Soft Matter 2020, 16, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Genovese, L.; Soccio, M.; Gigli, M.; Munari, A.; Siracusa, V.; Lotti, N. Block Copolyesters Containing 2,5-Furan and trans-1,4-Cyclohexane Subunits with Outstanding Gas Barrier Properties. Int. J. Mol. Sci. 2019, 20, 2187. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Wang, J.; Ying, W.B.; Zhu, J. Fully bio-based poly(propylene succinate-co-propylene furandicarboxylate) copolyesters with proper mechanical, degradation and barrier properties for green packaging applications. Eur. Polym. J. 2018, 102, 101–110. [Google Scholar] [CrossRef]
- Kainulainen, T.P.; Hukka, T.I.; Özeren, H.D.; Sirviö, J.A.; Hedenqvist, M.S.; Heiskanen, J.P. Utilizing Furfural-based Bifuran Diester as Monomer and Comonomer for High-Performance Bioplastics: Properties of Poly (butylene furanoate), Poly (butylene bifuranoate), and their Copolyesters. Biomacromolecules 2019, 21, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Morales-Huerta, J.C.; Martínez De Ilarduya, A.; Muñoz-Guerra, S. Sustainable Aromatic Copolyesters via Ring Opening Polymerization: Poly(butylene 2,5-furandicarboxylate-co-terephthalate)s. ACS Sustain. Chem. Eng. 2016, 4, 4965–4973. [Google Scholar] [CrossRef]
- Diao, L.; Su, K.; Li, Z.; Ding, C. Furan-based co-polyesters with enhanced thermal properties: Poly(1,4-butylene-co-1,4-cyclohexanedimethylene-2,5-furandicarboxylic acid). RSC Adv. 2016, 6, 27632–27639. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martínez De Ilarduya, A.; León, S.; Muñoz-Guerra, S. Isomannide-Containing Poly(butylene 2,5-furandicarboxylate) Copolyesters via Ring Opening Polymerization. Macromolecules 2018, 51, 3340–3350. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Liu, S.; Sun, T.; Wang, G. Synthesis and properties of poly (isosorbide 2, 5-furandicarboxylate-co-ε-caprolactone) copolyesters. Polym. Test. 2020, 81, 106284. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Liu, S.; Wang, G. Synthesis and characterization of poly (isosorbide-co-butylene 2, 5-furandicarboxylate) copolyesters. Eur. Polym. J. 2019, 115, 70–75. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; de Ilarduya, A.M.; Muñoz-Guerra, S. Partially renewable poly(butylene 2,5-furandicarboxylate-co-isophthalate) copolyesters obtained by ROP. Polymers 2018, 10, 483. [Google Scholar] [CrossRef]
- Ouyang, Q.; Liu, J.; Li, C.; Zheng, L.; Xiao, Y.; Wu, S.; Zhang, B. A facile method to synthesize bio-based and biodegradable copolymers from furandicarboxylic acid and isosorbide with high molecular weights and excellent thermal and mechanical properties. Polym. Chem. 2019, 10, 5594–5601. [Google Scholar] [CrossRef]
- Bi, T.; Qiu, Z. Synthesis, Thermal and Mechanical Properties of Fully Biobased Poly (butylene-co-propylene 2, 5-furandicarboxylate) Copolyesters with Low Contents of Propylene 2, 5-furandicarboxylate Units. Polymer 2019, 186, 122053. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Yang, B.; Xu, Y.; Zhang, W.; Zhang, Y.; Ji, J. Synthesis, physical properties and enzymatic degradation of bio-based poly(butylene adipate-co-butylene furandicarboxylate) copolyesters. Polym. Degrad. Stab. 2013, 98, 2177–2183. [Google Scholar] [CrossRef]
- Wu, B.; Xu, Y.; Bu, Z.; Wu, L.; Li, B.G.; Dubois, P. Biobased poly(butylene 2,5-furandicarboxylate) and poly(butylene adipate-co-butylene 2,5-furandicarboxylate)s: From synthesis using highly purified 2,5-furandicarboxylic acid to thermo-mechanical properties. Polymer 2014, 55, 3648–3655. [Google Scholar] [CrossRef]
- Peng, S.; Wu, L.; Li, B.G.; Dubois, P. Hydrolytic and compost degradation of biobased PBSF and PBAF copolyesters with 40–60 mol% BF unit. Polym. Degrad. Stab. 2017, 146, 223–228. [Google Scholar] [CrossRef]
- Peng, S.; Wu, B.; Wu, L.; Li, B.-G.; Dubois, P. Hydrolytic degradation of biobased poly(butylene succinate-co-furandicarboxylate) and poly(butylene adipate-co-furandicarboxylate) copolyesters under mild conditions. J. Appl. Polym. Sci. 2017, 134, 44674. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Silva, N.H.C.S.; Freire, C.S.R.; Andrade, M.; Mendes, A.; Silvestre, A.J.D. Furanoate-based nanocomposites: A case study using poly(butylene 2,5-furanoate) and poly(butylene 2,5-furanoate)-co-(butylene diglycolate) and bacterial cellulose. Polymers 2018, 10, 810. [Google Scholar] [CrossRef]
- Soccio, M.; Costa, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Salatelli, E.; Manaresi, P.; Munari, A. Novel fully biobased poly(butylene 2,5-furanoate/diglycolate) copolymers containing ether linkages: Structure-property relationships. Eur. Polym. J. 2016, 81, 397–412. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Tong, X.; Xue, S.; Zhou, G. Biobased copolyesters: Synthesis, sequence distribution, crystal structure, thermal and mechanical properties of poly(butylene sebacate-co-butylene furandicarboxylate). Polym. Degrad. Stab. 2017, 143, 1–8. [Google Scholar] [CrossRef]
- Wu, L.; Mincheva, R.; Xu, Y.; Raquez, J.M.; Dubois, P. High molecular weight poly(butylene succinate-co-butylene furandicarboxylate) copolyesters: From catalyzed polycondensation reaction to thermomechanical properties. Biomacromolecules 2012, 13, 2973–2981. [Google Scholar] [CrossRef]
- Maniar, D.; Jiang, Y.; Woortman, A.J.J.; van Dijken, J.; Loos, K. Furan-Based Copolyesters from Renewable Resources: Enzymatic Synthesis and Properties. ChemSusChem 2019, 12, 990–999. [Google Scholar] [CrossRef]
- Oishi, A.; Iida, H.; Taguchi, Y. Synthesis of poly (butylene succinate) copolymer including 2, 5-furandicarboxylate. Kobunshi Ronbunshu 2010, 67, 541–543. [Google Scholar] [CrossRef][Green Version]
- Jacquel, N.; Saint-Loup, R.; Pascault, J.P.; Rousseau, A.; Fenouillot, F. Bio-based alternatives in the synthesis of aliphatic-aromatic polyesters dedicated to biodegradable film applications. Polymer 2015, 59, 234–242. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Ciulik, C.B.; de Ilarduya, A.M.; Muñoz-Guerra, S. Fully bio-based aromatic–aliphatic copolyesters: Poly (butylene furandicarboxylate-co-succinate) s obtained by ring opening polymerization. Polym. Chem. 2017, 8, 748–760. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Wang, J.; Ying, W.B.; Zhu, J. Synthesis and Structure-Property Relationship of Biobased Biodegradable Poly(butylene carbonate- co-furandicarboxylate). ACS Sustain. Chem. Eng. 2018, 6, 7488–7498. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Ying, W.B.; Kong, Z.; Wang, K.; Wang, J.; Zhu, J. Biodegradable Elastomer from 2,5-Furandicarboxylic Acid and ϵ-Caprolactone: Effect of Crystallization on Elasticity. ACS Sustain. Chem. Eng. 2019, 7, 17778–17788. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Blocky poly(ɛ-caprolactone-co-butylene 2,5-furandicarboxylate) copolyesters via enzymatic ring opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 290–299. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Zang, X.L.; Wang, G.X.; Wang, P.L.; Lu, B.; Ji, J.H. Poly(butylene 2,5-furandicarboxylate-ε-caprolactone): A new bio-based elastomer with high strength and biodegradability. Express Polym. Lett. 2017, 11, 611–621. [Google Scholar] [CrossRef]
- Sousa, A.F.; Guigo, N.; Pożycka, M.; Delgado, M.; Soares, J.; Mendonça, P.V.; Coelho, J.F.J.; Sbirrazzuoli, N.; Silvestre, A.J.D. Tailored design of renewable copolymers based on poly (1, 4-butylene 2, 5-furandicarboxylate) and poly (ethylene glycol) with refined thermal properties. Polym. Chem. 2018, 9, 722–731. [Google Scholar] [CrossRef]
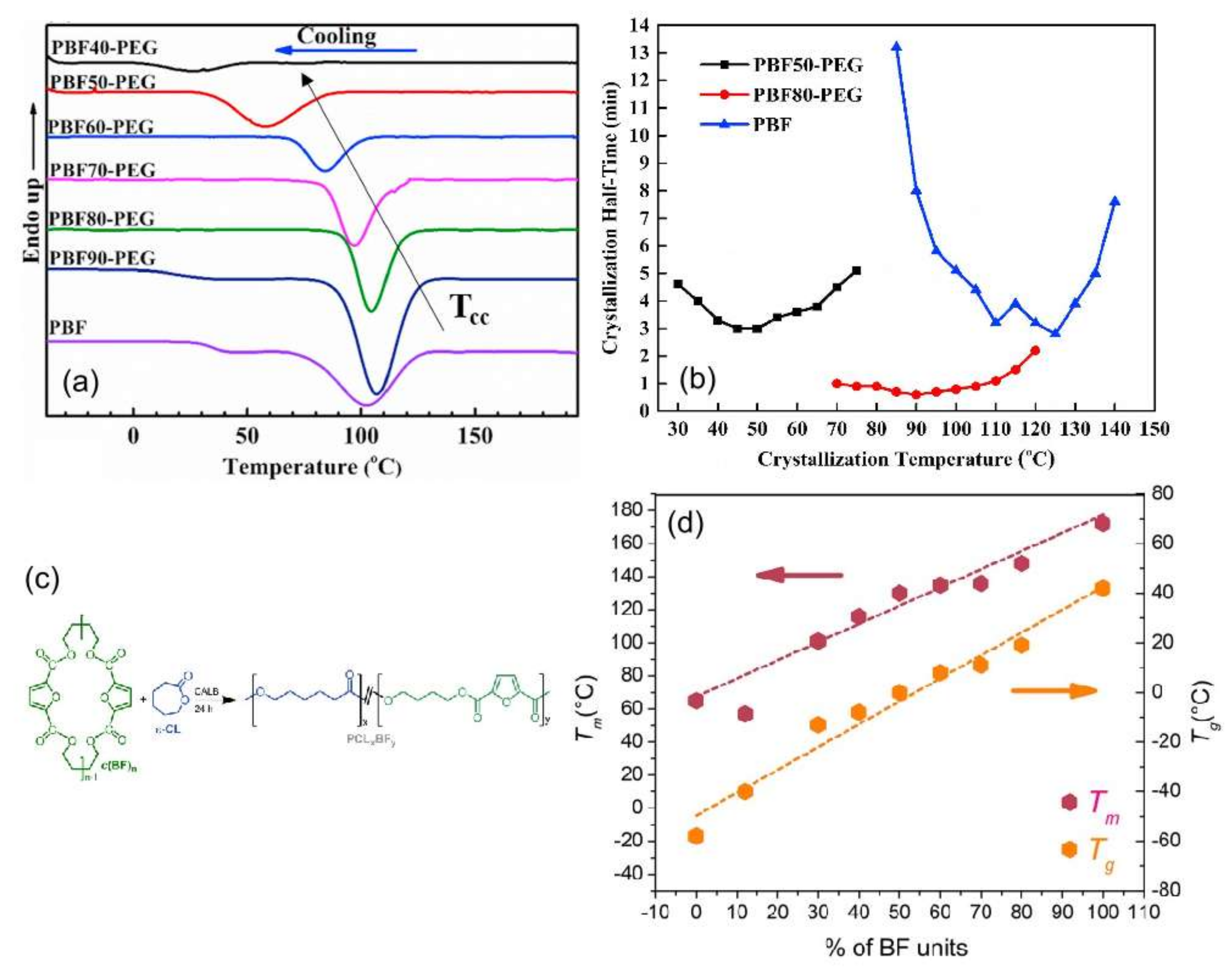
- Hu, H.; Zhang, R.; Sousa, A.; Long, Y.; Ying, W.B.; Wang, J.; Zhu, J. Bio-based poly(butylene 2,5-furandicarboxylate)-b-poly(ethylene glycol) copolymers with adjustable degradation rate and mechanical properties: Synthesis and characterization. Eur. Polym. J. 2018, 106, 42–52. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Kong, Z.; Wang, K.; Ying, W.B.; Wang, J.; Zhu, J. Bio-based poly (butylene furandicarboxylate)-b-poly (ethylene glycol) copolymers: The effect of poly (ethylene glycol) molecular weight on thermal properties and hydrolysis degradation behavior. Adv. Ind. Eng. Polym. Res. 2019, 2, 167–177. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Kowalczyk, I.; Kwiatkowski, K.; Zubkiewicz, A. Microstructure and Mechanical/Elastic Performance of Biobased Poly (Butylene Furanoate)–Block–Poly (Ethylene Oxide) Copolymers: Effect of the Flexible Segment Length. Polymers 2020, 12, 271. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Wang, J.; Ying, W.B.; Shi, L.; Yao, C.; Kong, Z.; Wang, K.; Zhu, J. A mild method to prepare high molecular weight poly(butylene furandicarboxylate- co -glycolate) copolyesters: Effects of the glycolate content on thermal, mechanical, and barrier properties and biodegradability. Green Chem. 2019, 21, 3013–3022. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Mendonça, P.V.; Silvestre, A.J.D. Co-polymers based on Poly(1,4-butylene 2,5-furandicarboxylate) and Poly(propylene oxide) with tuneable thermal properties: Synthesis and characterization. Materials 2019, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, Y.; Xu, Y.; Wang, P.; Gao, L.; Zhang, W.; Ji, J. Synthesis and characterization of bio-based poly(butylene furandicarboxylate)-b-poly(tetramethylene glycol) copolymers. Polym. Degrad. Stab. 2014, 109, 21–26. [Google Scholar] [CrossRef]
- Kasmi, N.; Wahbi, M.; Papadopoulos, L.; Terzopoulou, Z.; Guigo, N.; Sbirrazzuoli, N.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and characterization of two new biobased poly (pentylene 2, 5-furandicarboxylate-co-caprolactone) and poly (hexamethylene 2, 5-furandicarboxylate-co-caprolactone) copolyesters with enhanced enzymatic hydrolysis properties. Polym. Degrad. Stab. 2019, 160, 242–263. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Qu, X.; Zhou, G. Poly(hexamethylene 2,5-furandicarboxylate) copolyesters containing phosphorus: Synthesis, crystallization behavior, thermal, mechanical and flame retardant properties. Polym. Degrad. Stab. 2018, 153, 272–280. [Google Scholar] [CrossRef]
- Kasmi, N.; Ainali, N.M.; Agapiou, E.; Papadopoulos, L.; Papageorgiou, G.Z.; Bikiaris, D.N. Novel high Tg fully biobased poly(hexamethylene-co-isosorbide-2,5-furan dicarboxylate) copolyesters: Synergistic effect of isosorbide insertion on thermal performance enhancement. Polym. Degrad. Stab. 2019, 169, 108983. [Google Scholar] [CrossRef]
- Flores, I.; Martínez de Ilarduya, A.; Sardon, H.; Müller, A.J.; Muñoz-Guerra, S. ROP and crystallization behaviour of partially renewable triblock aromatic-aliphatic copolymers derived from L-Lactide. Eur. Polym. J. 2019, 122, 109321. [Google Scholar] [CrossRef]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and crystallization of new fully renewable resources-based copolyesters: Poly(1,4-cyclohexanedimethanol-co-isosorbide 2,5-furandicarboxylate). Polym. Degrad. Stab. 2018, 152, 177–190. [Google Scholar] [CrossRef]
- Chebbi, Y.; Kasmi, N.; Majdoub, M.; Cerruti, P.; Scarinzi, G.; Malinconico, M.; Dal Poggetto, G.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis, Characterization, and Biodegradability of Novel Fully Biobased Poly(decamethylene- co-isosorbide 2,5-furandicarboxylate) Copolyesters with Enhanced Mechanical Properties. ACS Sustain. Chem. Eng. 2019, 7, 5501–5514. [Google Scholar] [CrossRef]
- Chi, D.; Liu, F.; Na, H.; Chen, J.; Hao, C.; Zhu, J. Poly(neopentyl glycol 2,5-furandicarboxylate): A Promising Hard Segment for the Development of Bio-based Thermoplastic Poly(ether-ester) Elastomer with High Performance. ACS Sustain. Chem. Eng. 2018, 6, 9893–9902. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Jiang, Y.; Shi, L.; Wang, J.; Ying, W.B.; Zhu, J. Toward Biobased, Biodegradable, and Smart Barrier Packaging Material: Modification of Poly(Neopentyl Glycol 2,5-Furandicarboxylate) with Succinic Acid. ACS Sustain. Chem. Eng. 2019, 7, 4255–4265. [Google Scholar] [CrossRef]
- Yoshie, N.; Yoshida, S.; Matsuoka, K. Self-healing of biobased furan polymers: Recovery of high mechanical strength by mild heating. Polym. Degrad. Stab. 2019, 161, 13–18. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Noordover, B.A.J.; Rastogi, S. Aromatic thermotropic polyesters based on 2,5-furandicarboxylic acid and vanillic acid. Polymer 2014, 55, 2432–2439. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Modulating the Tg of poly (alkylene succinate) s by inserting bio-based aromatic units via ring-opening copolymerization. Polymers 2017, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Todea, A.; Bîtcan, I.; Aparaschivei, D.; Păușescu, I.; Badea, V.; Péter, F.; Gherman, V.D.; Rusu, G.; Nagy, L.; Kéki, S. Biodegradable oligoesters of ε-caprolactone and 5-hydroxymethyl-2-furancarboxylic acid synthesized by immobilized lipases. Polymers 2019, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulou, N.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Sustainable Polymers from Renewable Resources: Polymer Blends of Furan-Based Polyesters. Macromol. Mater. Eng. 2018, 303, 1800153. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Pipertzis, A.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Green polymeric materials: On the dynamic homogeneity and miscibility of furan-based polyester blends. Polymer 2019, 174, 187–199. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phs. Soc. 1952, 1, 123. [Google Scholar]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers. I. Non-crystalline copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Couchman, P.R.; Karasz, F.E. A classical thermodynamic discussion of the effect of composition on glass-transition temperatures. Macromolecules 1978, 11, 117–119. [Google Scholar] [CrossRef]
- Kwei, T.K. The effect of hydrogen bonding on the glass transition temperatures of polymer mixtures. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 307–313. [Google Scholar] [CrossRef]
- Lavilla, C.; Muñoz-Guerra, S. Sugar-based aromatic copolyesters: A comparative study regarding isosorbide and diacetalized alditols as sustainable comonomers. Green Chem. 2013, 15, 144–151. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and Properties of Novel Biodegradable/Biocompatible Poly [propylene-co-(ethylene succinate)] Random Copolyesters. Macromol. Chem. Phys. 2009, 210, 1408–1421. [Google Scholar] [CrossRef]
- Ceccorulli, G.; Scandola, M.; Kumar, A.; Kalra, B.; Gross, R.A. Cocrystallization of random copolymers of ω-pentadecalactone and ε-caprolactone synthesized by lipase catalysis. Biomacromolecules 2005, 6, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Bourdet, A.; Esposito, A.; Thiyagarajan, S.; Delbreilh, L.; Affouard, F.; Knoop, R.J.I.; Dargent, E. Molecular Mobility in Amorphous Biobased Poly (ethylene 2, 5-furandicarboxylate) and Poly (ethylene 2, 4-furandicarboxylate). Macromolecules 2018, 51, 1937–1945. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Guigo, N.; Tsanaktsis, V.; Papageorgiou, D.G.; Exarhopoulos, S.; Sbirrazzuoli, N.; Bikiaris, D.N. On the bio-based furanic polyesters: Synthesis and thermal behavior study of poly(octylene furanoate) using fast and temperature modulated scanning calorimetry. Eur. Polym. J. 2015, 68, 115–127. [Google Scholar] [CrossRef]
- Menard, K.P.; Menard, N.R. Dynamic mechanical analysis in the analysis of polymers and rubbers. Encycl. Polym. Sci. Technol. 2002. [Google Scholar] [CrossRef]
- Gubbels, E.; Jasinska-Walc, L.; Noordover, B.A.J.; Koning, C.E. Linear and branched polyester resins based on dimethyl-2,5-furandicarboxylate for coating applications. Eur. Polym. J. 2013, 49, 3188–3198. [Google Scholar] [CrossRef]
- Saywell, C.; Mai, S.; Romeu, C.C.; Neves, J.C.L. Process for the production of poly (ethylene 2, 5-furandicarboxylate) from 2, 5-furandicarboxylic acid and use thereof, polyester compound and blends thereof 2015. US Patent 14/369,887, 21 May.
- Zhang, J.; Liang, Q.; Xie, W.; Peng, L.; He, L.; He, Z.; Chowdhury, S.P.; Christensen, R.; Ni, Y. An Eco-Friendly Method to Get a Bio-Based Dicarboxylic Acid Monomer 2, 5-Furandicarboxylic Acid and Its Application in the Synthesis of Poly (hexylene 2, 5-furandicarboxylate)(PHF). Polymers 2019, 11, 197. [Google Scholar] [CrossRef]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mormann, W. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Prieto, A. To be, or not to be biodegradable… that is the question for the bio-based plastics. Microb. Biotechnol. 2016, 9, 652–657. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Nanocomposites of aliphatic polyesters: An overview of the effect of different nanofillers on enzymatic hydrolysis and biodegradation of polyesters. Polym. Degrad. Stab. 2013, 98, 1908–1928. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Mueller, R.-J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Bollinger, A.; Thies, S.; Knieps-Grünhagen, E.; Gertzen, C.; Kobus, S.; Höppner, A.; Ferrer, M.; Gohlke, H.; Smits, S.H.J.; Jaeger, K.-E. A Novel Polyester Hydrolase From the Marine Bacterium Pseudomonas aestusnigri–Structural and Functional Insights. Front. Microbiol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Haernvall, K.; Pichler, C.M.; Ghazaryan, G.; Breinbauer, R.; Guebitz, G.M. Enzymatic hydrolysis of poly (ethylene furanoate). J. Biotechnol. 2016, 235, 47–53. [Google Scholar] [CrossRef]
- García González, M.N.; Börjesson, P.; Levi, M.; Turri, S. Development and Life Cycle Assessment of Polyester Binders Containing 2,5-Furandicarboxylic Acid and Their Polyurethane Coatings. J. Polym. Environ. 2018, 26, 3626–3637. [Google Scholar] [CrossRef]
- Visser, R. One:20 in Detail A Combination Fit for Future Sustainable Barrier Packaging Solutions Pet and PEF. Available online: https://www.petnology.com/competence-magazine/news-details/pet-and-pef.html (accessed on 14 February 2020).
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Papageorgiou, G.Z.; Bikiaris, D.N. A facile method to synthesize high-molecular-weight biobased polyesters from 2, 5-furandicarboxylic acid and long-chain diols. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2617–2632. [Google Scholar] [CrossRef]















| Copolymer | Repeating Units (Abbrev.) | Structure | Reference | |
|---|---|---|---|---|
| PEF with comonomers containing cyclic units | ||||
| poly(ethylene 2,5-furandicarboxylate-co-ethylene 2,4-furan dicarboxylate) (PEF-co-2,4 PEF) | 2,5-PEF | 2,4-PEF |  | [28] |
| poly(ethylene 2,5-furandicarboxylate)-co-butylene 2,5-furandicarboxylate) (PEF-co-PBF) | PEF | PBF |  | [29] |
| poly(ethylene 2,5-furandicarboxylate-co-1,4-cyclohexanedimethylene 2,5-furandicarboxylate) (PEF-co-PCHDMF) | PEF | PCHDMF |  | [30,31,32] |
| poly(ethylene 2,5-furandicarboxylate-co-ethylene 1,4-cyclohexanedicarboxylate) (PEF-co-PECH) | PEF | PECH |  | [33] |
| poly(ethylene 2,5-furandicarboxylate-co-2,2,4,4-tetramethyl-1,3-cyclobutanediol 2,5-furan dicarboxylate) (PEF-co-PCBDOF) | PEF | PCBDOF | 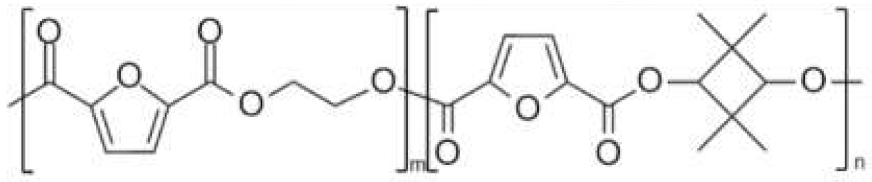 | [34,35] |
| poly(ethylene 2,5-furandicarboxylate-co-1,4-cyclohexyldimethylene 2,5-furandicarboxylate-co-2,2,4,4-tetramethyl-1,3-cyclobutanediol 2,5-furandicarboxylate) (PEF-co-PCHDMF-co-PCBDOF) | PEF | PCHDMF PCBDOF | 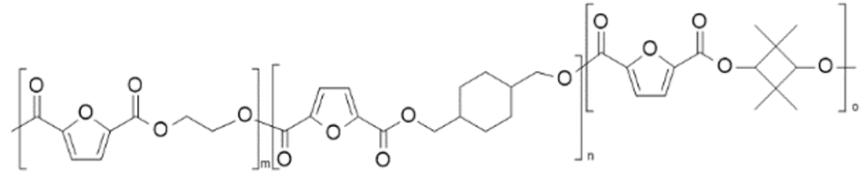 | [36] |
| poly(ethylene 2,5-furandicarboxylate-co-pentylene 2,5-furandicarboxylate) (PEF-co-PPeF) | PEF | PPeF | 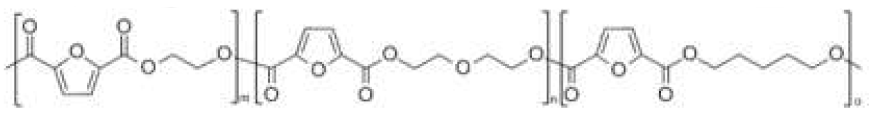 | [37] |
| poly(ethylene 2,5-furan dicarboxylate-co-ethylene terephthalate) (PEF-co-PET) | PEF | PET | 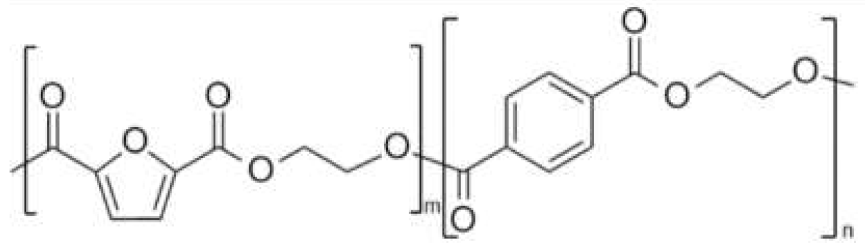 | [38,39,40,41,42,43,44] |
| poly(ethylene 2,5-furan dicarboxylate-co-hexamethylene 2,5-furandicarboxylate) (PEF-co-PHF) | PEF | PHF | 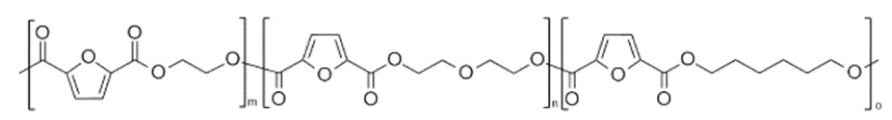 | [45] |
| poly((poly(ethylene glycol)) 2,5-furandicarboxylate)-co-poly(isosorbide 2,5-furandicarboxylate) (PPEGF-co-PIsF) | PEGF | PIsF |  | [46] |
| poly(ethylene 2,5-furan dicarboxylate)-co-poly((poly(ethylene glycol) 2,5-furandicarboxylate) (PEF-co-PPEGF) | PEF | PPEGF | 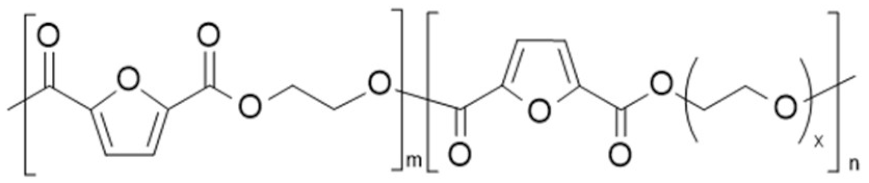 | [47,48] |
| poly(ethylene 2,5-furandicarboxylate-co-ε-caprolactone) (PEF-co-PCL) | PEF | PCL |  | [49] |
| poly(ethylene 2,5-furandicarboxylate-co-ethylene adipate) (PEF-co-PEAd) | PEF | PEAd | 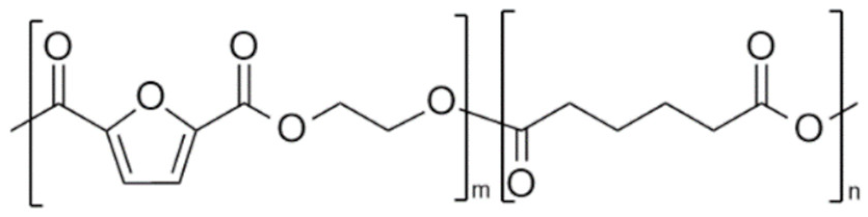 | [50] |
| poly(ethylene 2,5-furan dicarboxylate-co-ethylene sebacate) (PEF-co-PESeb) | PEF | PESeb |  | [51] |
| poly(ethylene 2,5-furan dicarboxylate-co-ethylene succinate) (PEF-co-PES) | PEF | PES |  | [52,53] |
| Poly(ethylene 2,5-furandicarboxylate-co-lactic acid) (PEF-co-PLA) | PEF | PLA |  | [54,55] |
| Poly(ethylene 2,5-furandicarboxylate-mb-poly(tetramethylene glycol)) (PEF-co-PPTMGF) | PEF | PPTMGF | 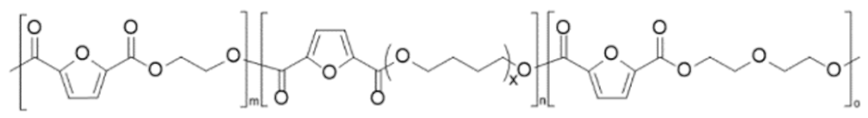 | [56] |
| PPF with comonomers containing cyclic units | ||||
| Poly(propylene 2,5-furandicarboxylate-co-propylene 2,4-furan dicarboxylate) (PPF-co-2,4 PPF) | 2,5-PPF | 2,4-PPF | 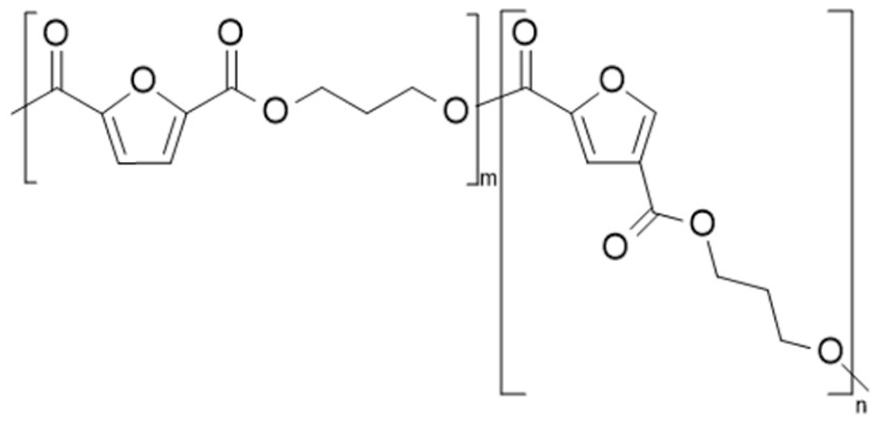 | [28] |
| Poly(propylene 2,5-furandicarboxylate-b-dimerized fatty acid diol) (PPF-b-PFADDF) | PPF | FADD | 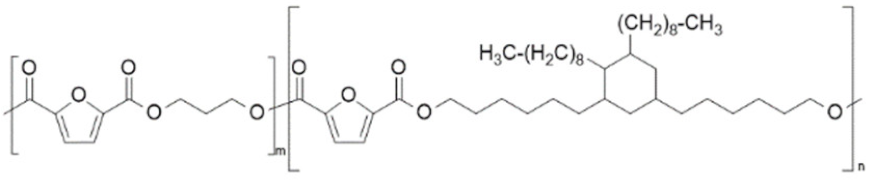 | [57,58] |
| poly(propylene 2,5-furandicarboxylate-co-1,4-cyclohexanedimethylene 2,5-furandicarboxylate) (PPF-co-PCHDMF) | PPF | PCHDMF |  | [59] |
| poly(propylene 2,5-furandicarboxylate-co-2,2,4,4-tetramethyl-1,3-cyclobutanediol 2,5-furandicarboxylate) (PPF-co-PCBDOF) | PPF | PCBDOF | 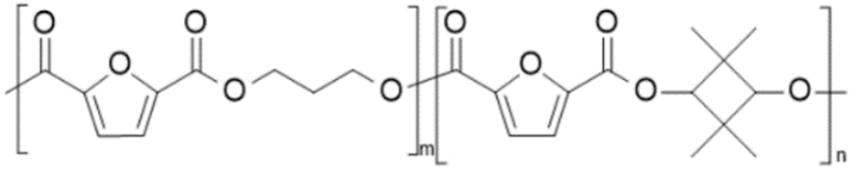 | [34] |
| poly(propylene 2,5-furandicarboxylate-co-2-methyl-1,3-propanediol 2,5-furandicarboxylate) (PPF-co-PMePF) | PPF | PMePF |  | [60] |
| poly(propylene 2,5-furandicarboxylate-co-propylene cyclohexane dicarboxylate) (PPF-co-PPCH) | PPF | PPCH |  | [61] |
| poly(propylene 2,5-furan dicarboxylate-co-propylene terephthalate) (PPF-co-PPT) | PPF | PPT | 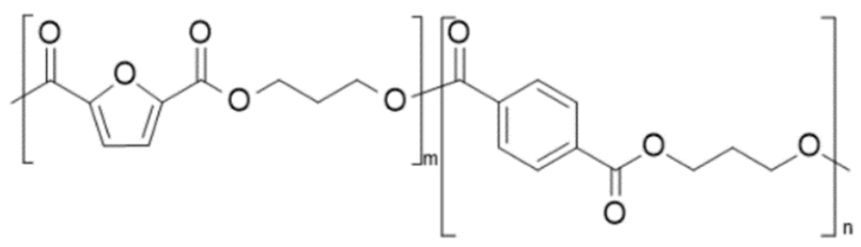 | [42] |
| PPF with comonomers containing linear units | ||||
| poly(propylene 2,5-furandicarboxylate-co-succinate) (PPF-co-PPS) | PPF | PPS | 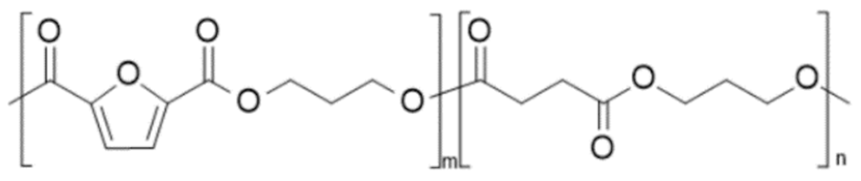 | [62] |
| PBF with comonomers containing cyclic units | ||||
| Poly(butylene 2,5-furandicarboxylate-co-butylene 2,4-furan dicarboxylate) (PBF-co-2,4 PBF) | 2,5-PBF | 2,4-PBF | 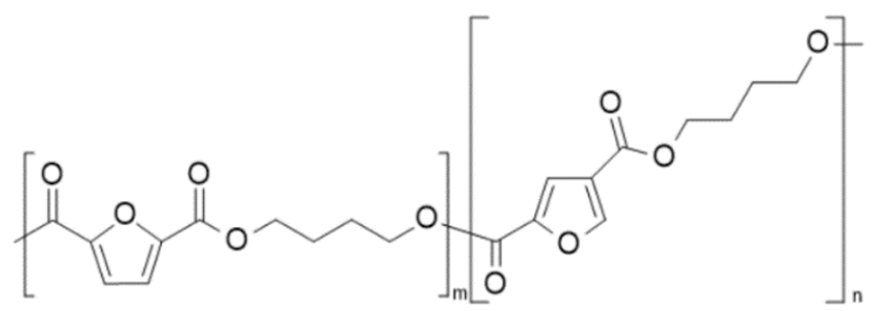 | [28] |
| Poly(butylene 2,5-furan dicarboxylate-co-butylene bis-2,5-furan dicarboxylate) (PBF-co-PBbF) | PBF | PBbF | 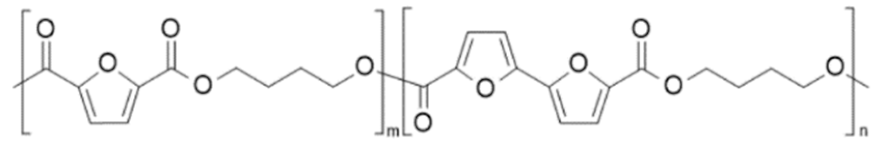 | [63] |
| poly(butylene 2,5-furandicarboxylate-co-butylene terephthalate) (PBF-co-PBT) | PBF | PBT |  | [42,64] |
| poly(butylene 2,5-furandicarboxylate-co-2,2,4,4-tetramethyl-1,3-cyclobutanediol 2,5-furandicarboxylate) (PBF-co-PCBDOF) | PBF | PCBDOF |  | [34] |
| poly(butylene 2,5-furandicarboxylate-co-1,4-cyclohexanedimethylene 2,5-furandicarboxylate) (PBF-co-PCHDMF) | PBF | PCDHDMF |  | [65] |
| poly(butylene 2,5-furandicarboxylate-co-isomannide 2,5-furandicarboxylate) (PBF-co-PImF) | PBF | PImF | 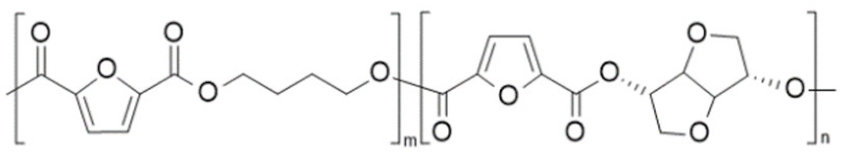 | [66] |
| poly(butylene 2,5-furandicarboxylate-co-isosorbide 2,5-furandicarboxylate) (PBF-co-PIsF) | PBF | PIsF | 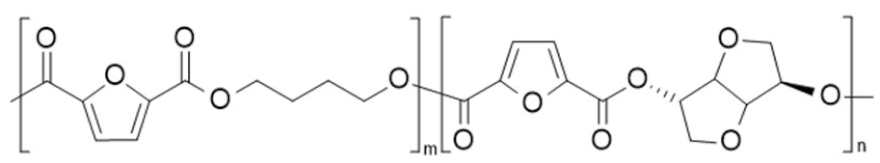 | [67,68] |
| poly(butylene 2,5-furandicarboxylate-co-butylene isophthalate) (PBF-co-PBI) | PBF | PBI |  | [69] |
| poly(butylene 2,5-furandicarboxylate-co-butylene succinate-co-isosorbide carbonate) (PBF-co-PIsC-co-PBS) | PBF | PBSu/PIsC | 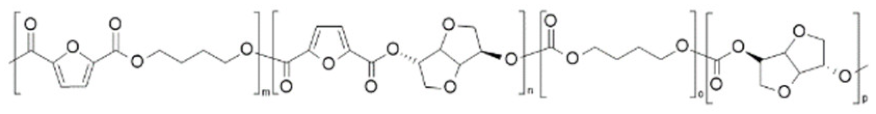 | [70] |
| poly(butylene 2,5-furandicarboxylate-co-propylene 2,5-furandicarboxylate) (PBF-co-PPF) | PBF | PPF | 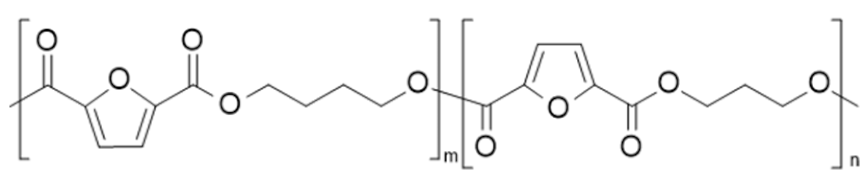 | [71] |
| PBF with comonomers containing linear units | ||||
| poly(butylene 2,5-furan dicarboxylate-co-butylene adipate) (PBF-co-PBAd) | PBF | PBAd | 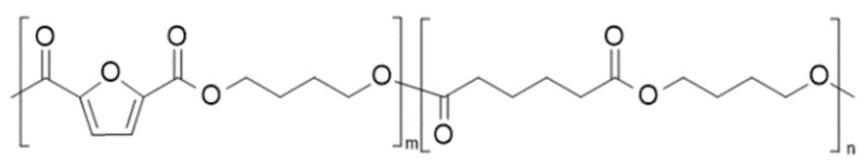 | [72,73,74,75] |
| poly(butylene 2,5-furan dicarboxylate-co-butylene diglycolate) (PBF-co-PBdGA) | PBF | PBdGA | 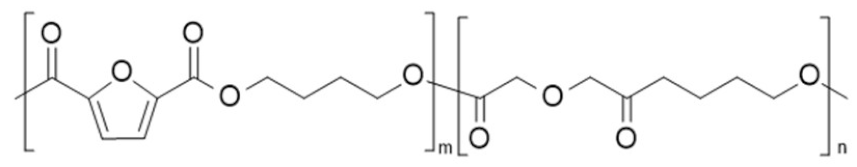 | [76,77] |
| poly(butylene 2,5-furan dicarboxylate-co-butylene sebacate) (PBF-co-PBSeb) | PBF | PBSeb |  | [78] |
| poly(butylene 2,5-furan dicarboxylate-co-butylene succinate) (PBF-co-PBS) | PBF | PBS | 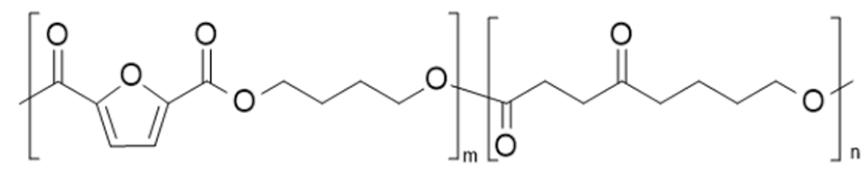 | [74,75,79,80,81,82,83] |
| poly(butylene 2,5-furan dicarboxylate-co-butylene carbonate) (PBF-co-PBC) | PBF | PBC | 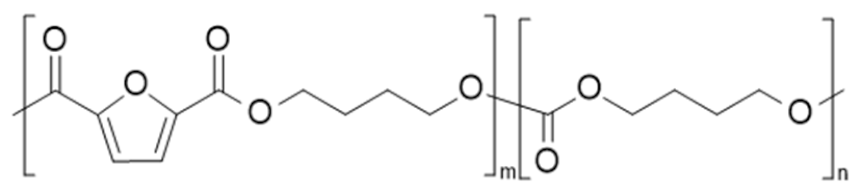 | [84] |
| poly(butylene 2,5-furan dicarboxylate-co-ε-caprolactone) (PBF-co-PCL) | PBF | PCL |  | [85,86,87] |
| poly(butylene 2,5-furandicarboxylate)-b-poly(ethylene glycol) 2,5-furan dicarboxylate) (PBF-b-PEG) | PBF | PPEGF | 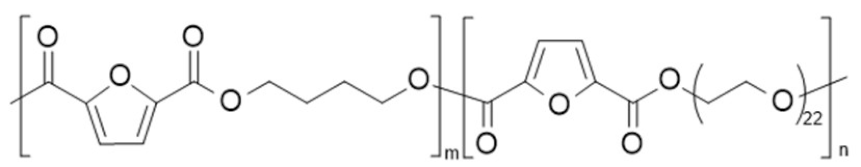 | [88,89,90,91] |
| poly(butylene 2,5-furan dicarboxylate-co-glycolate) (PBF-co-PGA) | PBF | PGA |  | [92] |
| poly(butylene 2,5-furan dicarboxylate-co-poly(propylene oxide) 2,5-furan dicarboxylate) (PBF-co-PPPOF) | PBF | PPPOF | 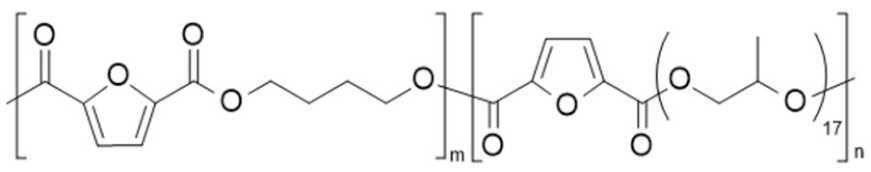 | [93] |
| poly(butylene 2,5-furan dicarboxylate)-b-poly(tetramethylene glycol) 2,5-furan dicarboxylate) (PBF-co-PPTMGF) | PBF | PPTMGF | 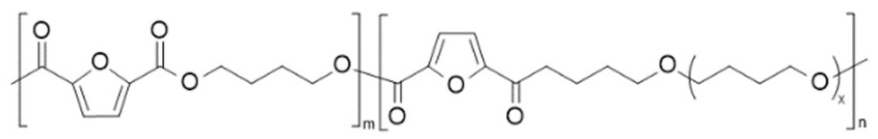 | [94] |
| PPeF copolymers | ||||
| poly(pentylene 2,5-furandicarboxylate-co-ε-caprolactone) (PPeF-co-PCL) | PPeF | PCL |  | [95] |
| PHF copolymers | ||||
| poly(hexamethylene 2,5-furandicarboxylate-co-hexamethylene 2-carboxyethyl (phenyl) phosphinic acid) (PHF-co-PHPCEPPA) | PHF | PCEPPA |  | [96] |
| poly(hexamethylene 2,5-furandicarboxylate-co-caprolactone) (PHF-co-PCL) | PHF | PCL | 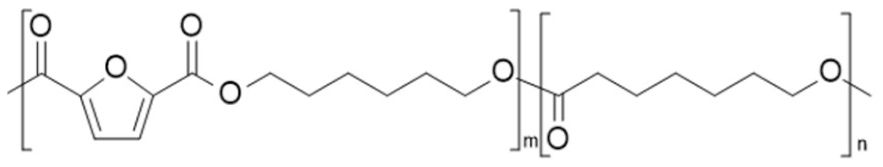 | [95] |
| poly(hexylene 2,5-furandicarboxylate-co-hexylene terephthalate) (PHF-co-PHT) | PHF | PHT | 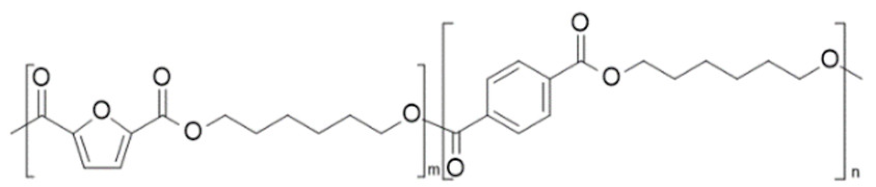 | [42] |
| poly(hexylene 2,5-furan dicarboxylate-co-isosorbide-2,5-furan dicarboxylate) (PHF-co-PIsF) | PHF | PIsF |  | [97] |
| poly(lactic acid-b-hexylene 2,5-furan dicarboxylate-b-lactic acid) (PHF-b-PLA) | PHF | PLA |  | [98] |
| Other furan-based copolymers | ||||
| poly(1,4-cyclohexanedimethanol-co-isosorbide 2,5-furandicarboxylate) (PCHDMF-co-PIsF) | PCHDMF | PIsF | 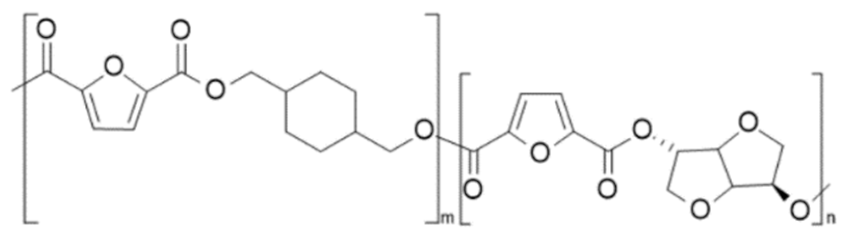 | [99] |
| poly(decamethylene 2,5-furan dicarboxylate-co-isosorbide-2,5-furan dicarboxylate) (PDF-co-PIsF) | PIsF |  | [100] | |
| poly(neopentyl glycol 2,5-furandicarboxylate-co-poly(tetramethylene glycol) 2,5-furan dicarboxylate) (PNF-co-PPTMGF) | PNF | PPTMGF | 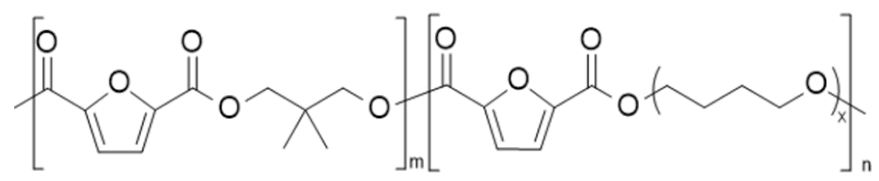 | [101] |
| poly(neopentyl glycol 2,5-furandicarboxylate-co-neopentyl glycol succinate) (PNF-co-PNS) | PNF | PNGS |  | [102] |
| poly(isosorbide 2,5-furandicarboxylate-co-ε-caprolactone) (PIsF-co-PCL) | PIsF | PCL | 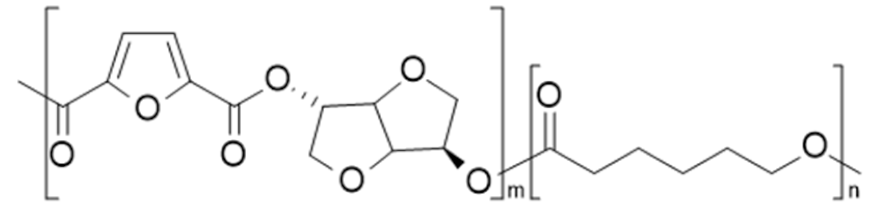 | [67] |
| poly(2,5-furan dimethylene succinate-co-propylene succinate) (PFDMS-co-PPS) | PFDMS | PPS |  | [103] |
| poly(octylene 2,5-furandicarboxylate-co-octylene terephthalate) (POF-co-POT) | POF | POT | 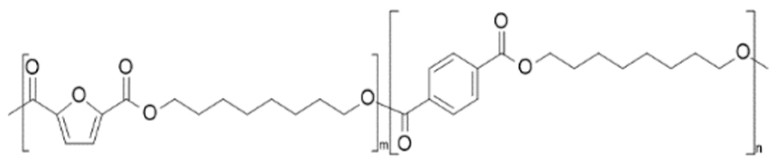 | [42] |
| poly(p-acetobenzoic acid-co-4,4′-diacetoxybiphenyl 2,5-furan dicarboxylate) (PAA-co-PDABPHF) | PAA | PDABPHF |  | [104] |
| poly(di- O -2-(hydroxyethyl) resorcinol 2,5-furandicarboxylate-co-ethylene succinate)€(PRF-co-PES) | PRF | PES | 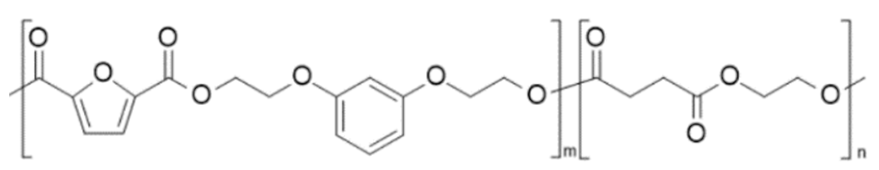 | [105] |
| poly(di- O -2-(hydroxyethyl) resorcinol 2,5-furandicarboxylate-co-butylene succinate)€(PRF-co-PBS) | PRF | PBS | 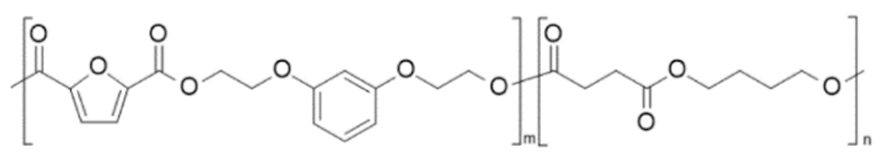 | [105] |
| Sample | Tensile Strength (σb) | Young’s Modulus (E) | Elongation (εb) | [η] |
|---|---|---|---|---|
| MPa | MPa | % | dL/g | |
| PIs80B20F | 77 ± 3 | 1900 ± 60 | 15 ± 3 | 0.45 |
| PIs80CBF20 | 29.7 ± 2.5 | 1117 ± 28 | 3 ± 1 | 0.50 |
| PIs80CBF10S10 | 69 ± 8.1 | 1330 ± 38 | 7 ± 1 | 0.77 |
| PIs60B40F | 134 ± 3 | 1590 ± 42 | 32 ± 2 | 0.58 |
| PIs60CBF40 | 40.3 ± 1.5 | 1186 ± 30 | 5 ± 1 | 0.58 |
| PIs60CBF30S10 | 71.5 ± 2.2 | 1242 ± 31 | 23 ± 7 | 0.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzopoulou, Z.; Papadopoulos, L.; Zamboulis, A.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Tuning the Properties of Furandicarboxylic Acid-Based Polyesters with Copolymerization: A Review. Polymers 2020, 12, 1209. https://doi.org/10.3390/polym12061209
Terzopoulou Z, Papadopoulos L, Zamboulis A, Papageorgiou DG, Papageorgiou GZ, Bikiaris DN. Tuning the Properties of Furandicarboxylic Acid-Based Polyesters with Copolymerization: A Review. Polymers. 2020; 12(6):1209. https://doi.org/10.3390/polym12061209
Chicago/Turabian StyleTerzopoulou, Zoi, Lazaros Papadopoulos, Alexandra Zamboulis, Dimitrios G. Papageorgiou, George Z. Papageorgiou, and Dimitrios N. Bikiaris. 2020. "Tuning the Properties of Furandicarboxylic Acid-Based Polyesters with Copolymerization: A Review" Polymers 12, no. 6: 1209. https://doi.org/10.3390/polym12061209
APA StyleTerzopoulou, Z., Papadopoulos, L., Zamboulis, A., Papageorgiou, D. G., Papageorgiou, G. Z., & Bikiaris, D. N. (2020). Tuning the Properties of Furandicarboxylic Acid-Based Polyesters with Copolymerization: A Review. Polymers, 12(6), 1209. https://doi.org/10.3390/polym12061209