DFT Prediction of Factors Affecting the Structural Characteristics, the Transition Temperature and the Electronic Density of Some New Conjugated Polymers
Abstract
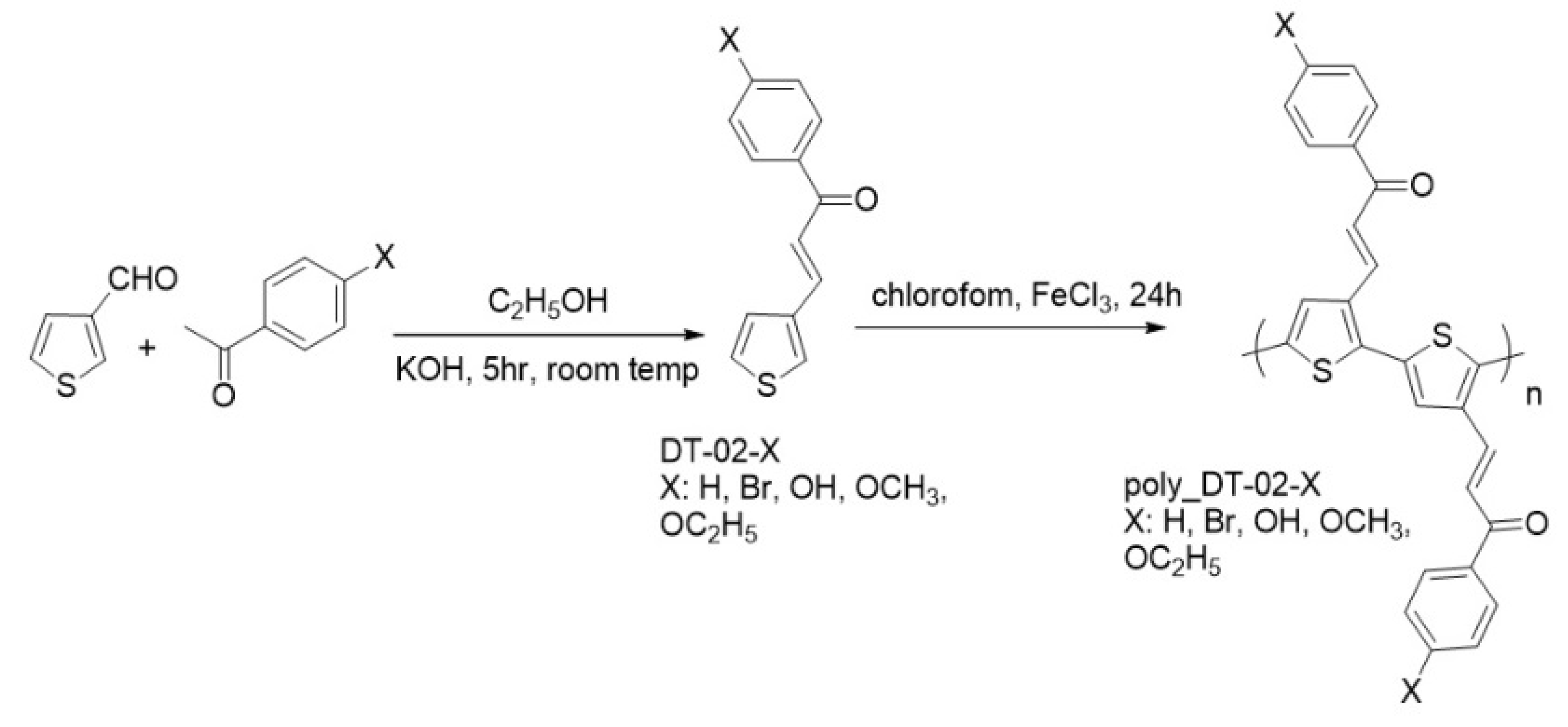
1. Introduction
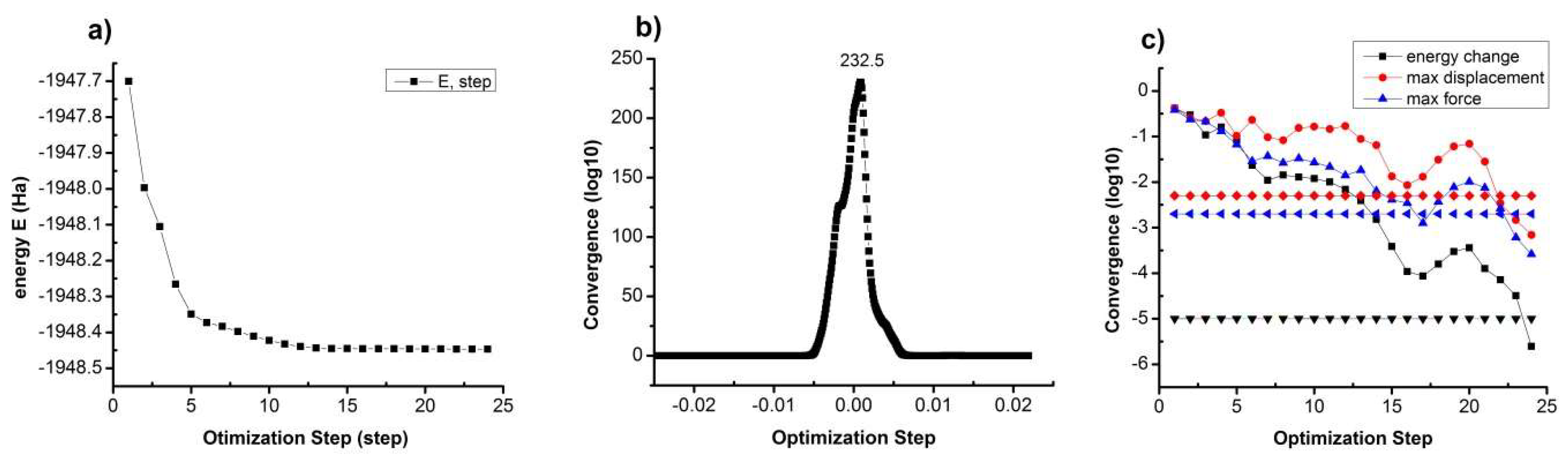
2. Method of Calculation
3. Results and Discussion
3.1. Effect of Distance between Layers
3.2. Effect of Impurities/Heterogeneity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Skotheim, T.A.E. Handbook of Conducting Polymers; Dekker: Michigan City, IN, USA, 1986. [Google Scholar]
- Chiang, C.K.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T.W. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Hajian, A.; Rafati, A.A.; Afraz, A.; Najafi, M. Electrosynthesis of polythiophene nanowires and their application for sensing of chlorpromazine. J. Electrochem. Soc. 2014, 161, B196–B200. [Google Scholar] [CrossRef]
- English, J.T.; Deore, B.A.; Freund, M.S. Biogenic amine vapor detection using poly (aniline boronic acid) films. Sens. Actuators B 2006, 115, 666–671. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Ahmad, R.; Shrestha, S.; Park, C.H.; Kim, C.S. In situ synthesis of cylindrical spongy polypyrrole doped protonated graphitic carbon nitride for cholesterol sensing application. Biosens Bioelectron. 2017, 94, 686–693. [Google Scholar] [CrossRef]
- Kumar, M.R.; Ryman, S.; Tareq, M.O.; Buchanan, D.; Freund, M.S. Chemical diversity in electrochemically deposited conducting polymer-based sensor arrays. Sens. Actuators B 2014, 202, 600–608. [Google Scholar] [CrossRef]
- Ayenimo, J.G.; Adeloju, S.B. Amperometric detection of glucose in fruit juices with polypyrrole-based biosensor with an integrated selective layer for the exclusion of interferences. Food Chem. 2017, 229, 127–135. [Google Scholar] [CrossRef]
- Chun, L.; Gaoquan, S. Polythiophene-based optical sensors for small molecules. ACS Appl. Mater. Interfaces 2013, 5, 4503–4510. [Google Scholar]
- Fang, Y.; Jiang, X.; Niu, L.; Wang, S. Constructing polypyrrole/aligned carbon nanotubes composite materials as electrodes for high-performance supercapacitors. Mater. Lett. 2017, 190, 232–235. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; He, T.; Bi, Q.; Sun, L.; Liu, Z. Fabrication of polypyrrole/vanadium oxide nanotube composite with enhanced electrochemical performance as a cathode in rechargeable batteries. Appl. Surf. Sci. 2017, 405, 146–151. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A.; Habib, A.; Awais, M.; Waje, S.B.; Atieh, M.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Sources 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Ebrahimi, I.; Gashti, M.P. Chemically reduced versus photo-reduced clay-Ag-polypyrrole ternary nanocomposites: Comparing thermal, optical, electrical and electromagnetic shielding properties. Mater. Res. Bull. 2016, 83, 96–107. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, L.; Lu, Y. Electromagnetic shielding effectiveness and serviceability of the multilayer structured cuprammonium fabric/polypyrrole/copper (CF/PPy/Cu) composite. ChemEng J. 2016, 297, 170–179. [Google Scholar] [CrossRef]
- Trung, V.Q.; van Hoan, P.; Phung, D.Q.; Duc, l.; Hang, l.T. Double corrosion protection mechanism of molybdate-doped polypyrrole/montmorillonite nanocomposites. J. Exp. Nanosci. 2014, 9, 282–292. [Google Scholar] [CrossRef]
- Rammelt, U.; Duc, L.M.; Plieth, W. Improvement of protection performance of polypyrrole by dopant anion. J. Appl. Electrochem. 2005, 35, 1225–1230. [Google Scholar] [CrossRef]
- Paliwoda-Porebska, G.; Rohwerder, M.; Stratmann, M.; Rammelt, U.; Duc, L.M.; Plieth, W. Release mechanism of electrodeposited polypyrrole doped with corrosion inhibitor anions. J. Solid State Electrochem. 2006, 10, 730–736. [Google Scholar] [CrossRef]
- Grari, O.; Taouil, A.E.; Dhouibi, L.; Buron, C.; Lallemand, F. Multilayered polymer role SiO2 composite coatings for functionalization of stainless steel: Characterization and corrosion protection behavior. Prog. Org. Coat. 2015, 88, 48–53. [Google Scholar] [CrossRef]
- Ilangovan, G.; Pillai, K.C. Preparation and characterization of monomeric molybdate (VI) anion-doped polypyrrole electrodes. J. Solid State Electrochem. 1999, 3, 474–477. [Google Scholar]
- Vera, R.; Schrebler, R.; Grez, P.; Romero, H. The corrosion-inhibiting effect of polypyrrole films doped with p -toluene-sulfonate, benzene-sulfonate or dodecyl-sulfate anions, as a coating on stainless steel in NaCl aqueous solutions. Prog. Org. Coat. 2014, 77, 853–858. [Google Scholar] [CrossRef]
- Frommer, J.E.; Chance, R.R. Encyclopedia of Polymer Science and Engineering; Wiley: New York, NY, USA, 1986; Volume 5, p. 462. [Google Scholar]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielec trics screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 99–805. [Google Scholar] [CrossRef]
- Dai, Y.; Chowdhury, S.; Blaisten-Barojas, E. Density functional theory study of the structure energetics of negatively charge doligopyrroles. Int. J. Quantum. Chem. 2011, 111, 2295–2305. [Google Scholar] [CrossRef]
- NIST. NIST Standard Reference Database 35; NIST/EPA Gas-Phase Infrared Database JCAMP Format; NIST: Gaithersburg, MD, USA, 2017.
- Ullah, H.; Shah, A.A.; Bilal, S.; Ayub, K. Doping and dedoping processes of polypyrrole: DFT study with hybrid functionals. J. Phys. Chem. C 2014, 118, 17819–17830. [Google Scholar] [CrossRef]
- Runge, E.; Gross, E.K.U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- van Hoang, H.; Nguyen, T.D.; Ha, N.N. Corrosion inhibition mechanism of pyridine ion iron and its alloys using DFT. Asian J. Chem. 2013, 25, 3117–3120. [Google Scholar] [CrossRef]
- Roth, S.; Bleier, H. Solitons in polyacetylene. Adv. Phys. 1987, 36, 385. [Google Scholar] [CrossRef]
- Heeger, A.J.; Kivelson, S.; Schrieffer, J.R.; Su, W.P. Solitons in conducting polymers. Rev. Mod. Phys. 1988, 60, 750–781. [Google Scholar] [CrossRef]
- Chiang, C.K.; Drury, M.A.; Gau, S.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Park, Y.D. Synthesis of highly conducting films of derivatives of polyacetylene. J. Am. Chem. Soc. 1978, 100, 1013–1015. [Google Scholar] [CrossRef]
- Bhavana, A.D.; Insun, Y.; Freund, M.S. A switchable self-doped polyaniline: Interconversion between self-doped and non-self-doped forms. J. Am. Chem. Soc. 2004, 126, 52–53. [Google Scholar]
- Winokur, W.; Moon, Y.B.; Heeger, A.J.; Barker, J.; Bott, J.C.; Shirakavm, H. X-ray scattering from sodium-doped polyacetylene: In commensurate-com mensurate and order-disorder transformations. Phys. Rev. Lett. 1987, 58, 2329. [Google Scholar] [CrossRef]
- Moon, Y.-B.; Winokur, M.; Heeger, A.J.; Barker, J.; Bott, D.C. X-ray scattering from oriented durham polyacetylene: Structural changes after electrochemical doping. Macromolecules 1987, 20, 2457. [Google Scholar] [CrossRef]
- Chiang, C.K.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G. Conducting polymers: Halogen doped polyacetylene. J. Chem. Phys. 1978, 69, 5098. [Google Scholar] [CrossRef]
- Chiang, C.K.; Gau, S.C.; Fincher, C.R.; Park, Y.W.; MacDiarmid, A.G.; Heeger, A.J. Polyacetylene, (CH) x: N-Type and p-Type doping and compensation. Appl. Phys. Lett. 1978, 33, 18. [Google Scholar] [CrossRef]
- Ma, C.-Q.; Mena-Osteritz, E.; Debaerdemaeker, T.; Wienk, M.M.; Janssen, R.A.J.; Bauerle, P. Functionalized 3D oligothiophene dendrons, and dendrimers-nove l macromolecules for organic electronics. Angew. Chem. Int. Ed. 2007, 46, 1679–1683. [Google Scholar] [CrossRef]
- Zamoshchik, N.; Salzner, U.; Bendikov, M. Nature of Charge Carriers in Long Doped Oligothiophenes: The Effect of Counterions. J. Phys. Chem. C 2008, 112, 8408–8418. [Google Scholar] [CrossRef]
- Zade, S.S.; Bendikov, M. Theoretical study of long oligothiophene polycations as a model for doped polythiophene. J. Phys. Chem. C 2007, 111, 10662–10672. [Google Scholar] [CrossRef]
- Salzner, U. Does the donor-acceptor concept work for designing synthetic metals? theoretical investigation of poly (3-cyano-3′-hydroxy bithiophene). J. Phys. Chem. B 2002, 106, 9214–9220. [Google Scholar] [CrossRef]
- Rittmeyer, S.P.; Gross, A. Structural and electronic properties of oligo- and polythiophenes modified by substituents. Beilstein J. Nanotechnol. 2012, 3, 909–919. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Parthasar, R.; Subra mania, V.; Somanathan, N. Vibrational analysis of heterocyclic polymers: A comparative study of polythiophene, polypyrrole, and polyisothia-naphthene. J. Chem. Phys. 2005, 123, 164905. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Ananthakrishnan, S.J.; Somanathan, N. Structure-property relationships of electroluminescent polythio-phenes: Role of nitrogen-based heterocycles as side chains. Bull. Mater. Sci. 2011, 34, 713–726. [Google Scholar] [CrossRef][Green Version]
- Patra, A.; Wijsboom, Y.H.; Leitus, G.; Bendikov, M. Tuning the band gap of low-band-gap polyselenophenes and polythiophenes: The effect of the heteroatom. Chem. Mater. 2011, 23, 896–906. [Google Scholar] [CrossRef]
- Guay, J.; Kasai, P.; Diaz, A.; Wu, R.; Tour, J.M.; Dao, L.H. Chain-length dependence of electrochemical and electronic proper-ties of neutral and oxidized soluble.alpha. Alpha Coupled Thiophene Oligomers Chem. Mater. 1992, 4, 1097–1110. [Google Scholar]
- Gao, J.; Niles, E.T.; Grey, J.K. J-aggregates promote efficient charge transfer doping of poly(3-hexylthiophene). J. Phys. Chem. Lett. 2013, 4, 2953–2957. [Google Scholar] [CrossRef]
- Mena-Osteritz, E.; Zhang, F.; Gotz, G.; Reineke, P.; Bauerle, P. Optical properties of fully conjugated cyclo [n] thiophenes-an experimental and theoretical approach. Beilstein J. Nanotechnol. 2011, 2, 720–726. [Google Scholar] [CrossRef]
- Quoc, T.V.; Thuy, D.T.T.; Thanh, T.D.; Ngoc, T.P.; Thien, V.N.; Thuy, C.N.; Meervelt, L.V. Some chalcones derived from thiophene-3-carbaldehyde: Synthesis and crystal structures. Acta Crystallograph. Sect. E 2019, 75, 957–963. [Google Scholar] [CrossRef]
- Vu, Q.-T.; Pavlik, M.; Hebestreit, N.; Rammelt, U.; Plieth, W.; Pfleger, J. Nanocomposites based on titanium dioxide and polythiophene: Structure and Properties. React. Funct. Polym. 2005, 65, 69–77. [Google Scholar] [CrossRef]
- Vu, Q.-T.; Pavlik, M.; Hebestreit, N.; Pfleger, J.; Rammelt, U.; Plieth, W. Electrophoretic deposition of nanocomposites formed from polythiophene and metal oxides. Electroch. Acta 2005, 51, 1117–1124. [Google Scholar] [CrossRef]
- Trung, V.Q.; Linh, N.N.; Linh, D.K.; Pfleger, J. Synthesis and characterization of polythiophenes from hydrazone derivatives side groups. Vietnam J. Chem. 2016, 54, 730–735. [Google Scholar]
- Trung, V.Q.; Linh, N.N.; Duong, T.T.T.; Chinh, N.T.; Linh, D.K.; Hung, H.M.; Oanh, D.T.Y. Synthesis and characterization of novel poly[4-phenyl-3-(thiophene-3-ylmethyl)-1H-1,2,4-triazole -5(4H)-thione]. Vietnam J. Chem. 2019, 57, 770–776. [Google Scholar] [CrossRef]
- Pettifor, D.G.; Cottrell, A.H. Electron Theory in Alloy Design; Maney Publishing: Leeds, UK, 1992. [Google Scholar]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Bernholc, J. Computational materials science: The era of applied quantummechanics. Phys. Today 1999, 52, 30–35. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalizedgradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S. Interaction energies of van der Waals and hydrogen-bonded systems calculated using density functional theory: Assessing the PW91 model. J. Chem. Phys. 2001, 114, 3949. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Hamann, D.R.; Schlüter, M.; Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 1979, 43, 1494–1497. [Google Scholar] [CrossRef]
- Akira, I.; Yoshiyuki, T.; Takeo, F.; Hirofumi, Y. Solid-state calculations of poly(vinylidene fluoride) using the hybrid DFT method: Spontaneous polarization of polymorphs. Polym. J. 2014, 46, 207–211. [Google Scholar]
- Yoshiaki, Y.; Yasuteru, M.; Masayoshi, T. Proposed Mechanism for the High-Yield Polymerization of Oxyethyl Propiolates with Rh Complex Catalyst Using the Density Functional Theory Method. Polymers 2019, 11, 93. [Google Scholar]
- Robert; Robert, G. Parr and Weitao Yang, Density-Functional Theory of Atoms and Molecules; Oxford University Press–Newyork Clarendon Press: New York, NY, USA, 1989. [Google Scholar]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory, 2nd ed.; Wiley–VCH: Hoboken, NJ, USA, 2001. [Google Scholar]
- Søndenå, R.; Stølen, S.; Ravindran, P. Corner- versus face-sharing octahedra in AMnO3 perovskites (A = Ca, Sr, and Ba). Phys. Rev. B 2007, 75, 184105. [Google Scholar] [CrossRef]
- Slater, J.C. A Simplification of the hartree-fock method. Phys. Rev. 1951, 81, 385–390. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Englisch, H.; Englisch, R. Hohenberg-Kohn theorem and non-vrepresentable densities. Phys. A 1983, 121, 253–268. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. A 1965, 140, 1133–1138. [Google Scholar] [CrossRef]
- Andersen, O.K.; Jepsen, O.; Glotzel, D. Highlights of Condensed Matter Theory; Elsevier: Amsterdam, The Netherlands, 1985; pp. 59–176. [Google Scholar]
- Ruban, A.V.; Skriver, H.L. Calculated surface segregation in transition metal alloys. Comput. Mater. Sci. 1999, 15, 119–143. [Google Scholar] [CrossRef]
- Wang, Y.; Perdew, J.P. Correlation hole of the spin-polarized electron gas, with exact small-wave-vector and high-density scaling. Phys. Rev. B 1991, 44, 298–307. [Google Scholar] [CrossRef]
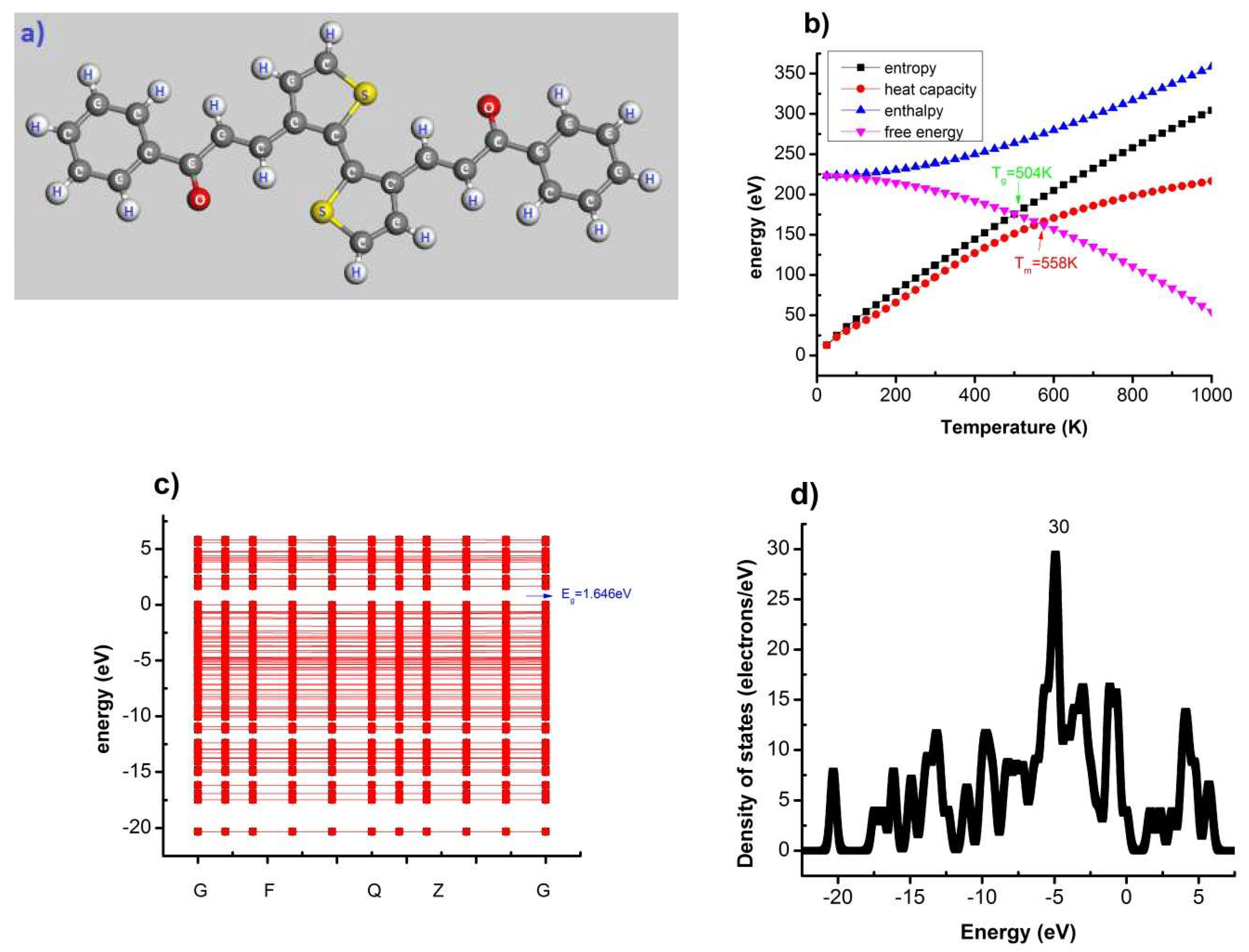

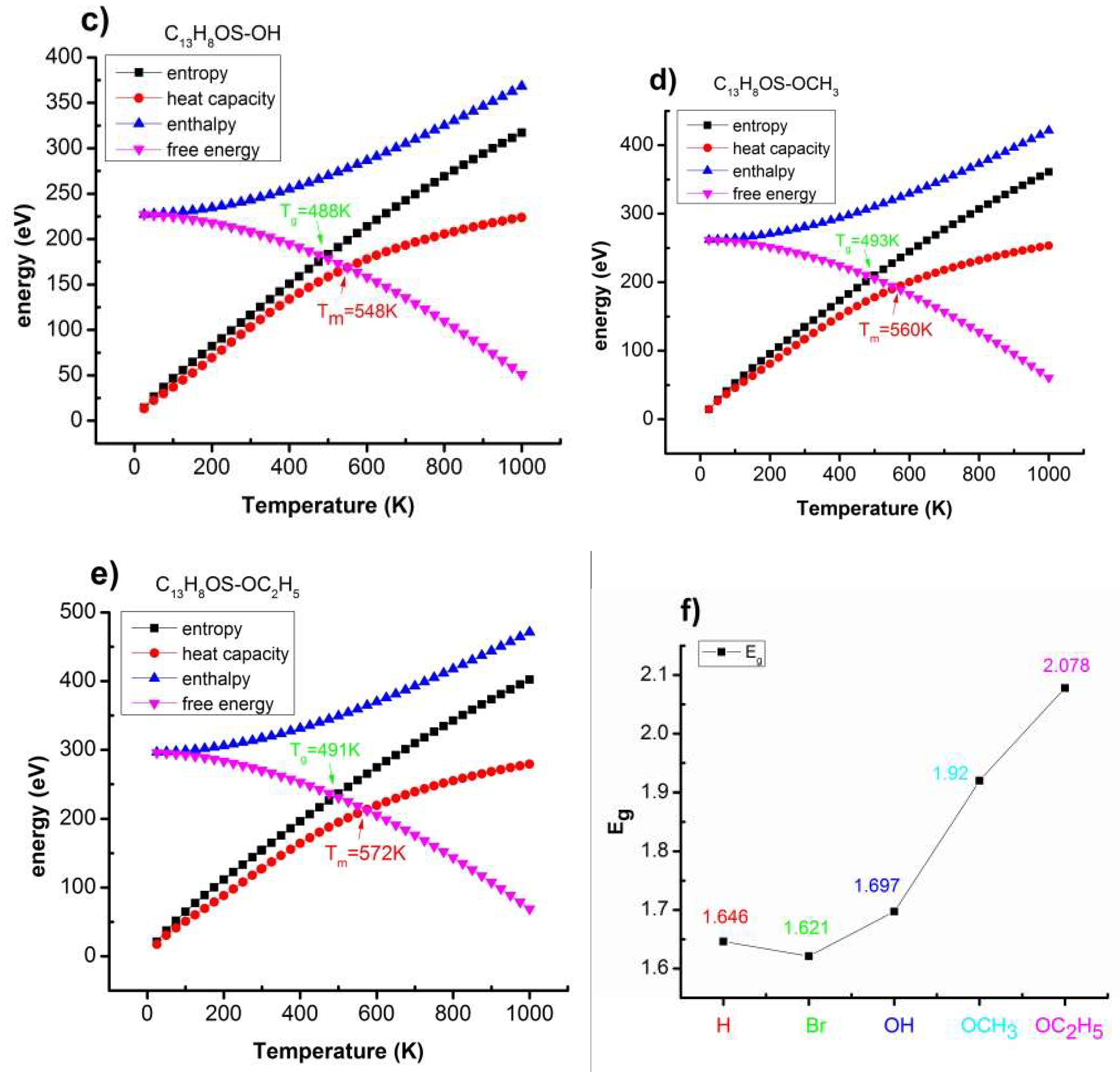
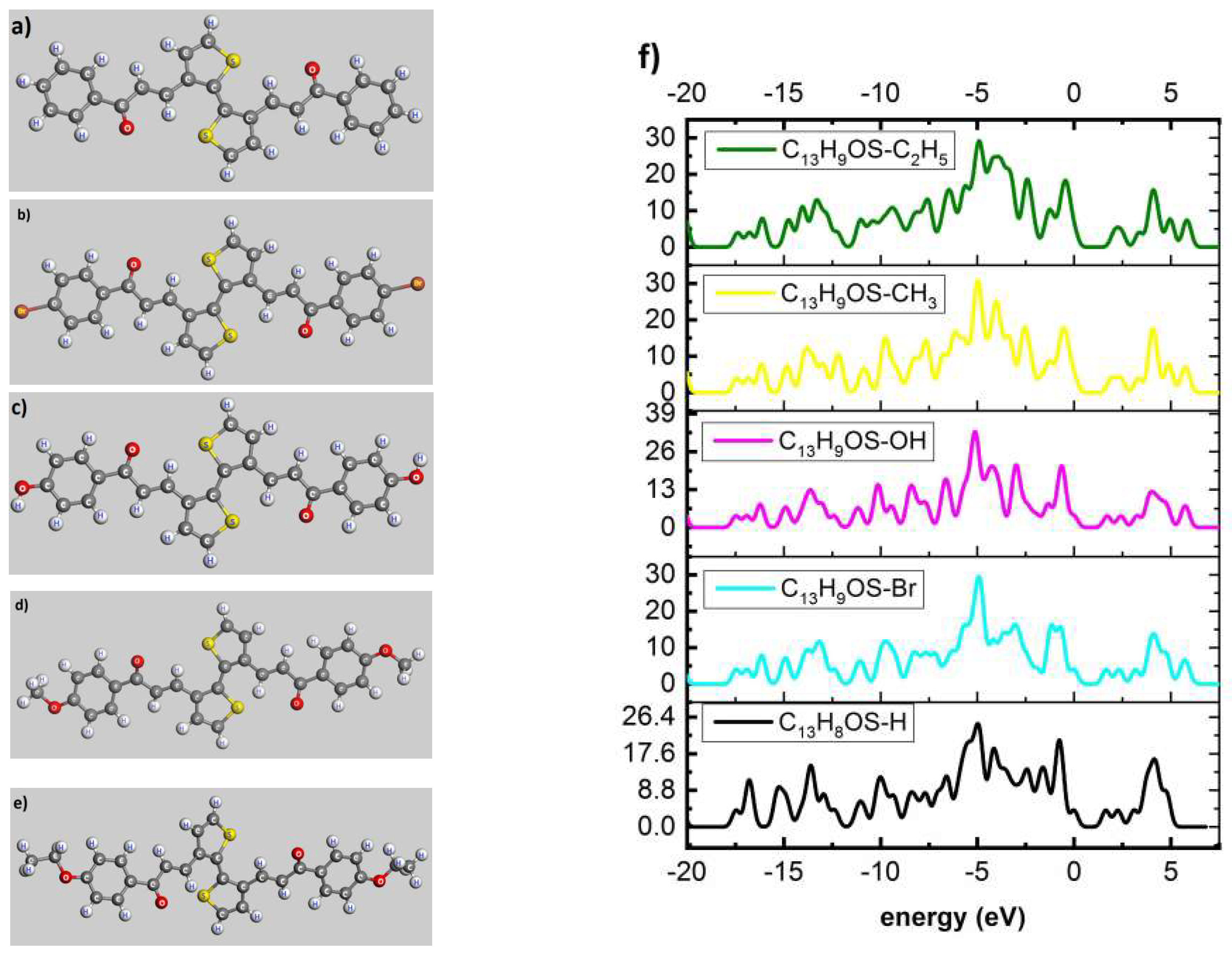
| Poly(C13H8OS–H) | −20 | −15 | −10 | −5 | −2.5 | 0 | 2.5 | 5 |
|---|---|---|---|---|---|---|---|---|
| Electron density | 2.334 | 6.625 | 9.423 | 29.462 | 8.925 | 4.011 | 2.629 | 4.151 |
| d(Å) | 6 | 9 | 12 | 15 |
|---|---|---|---|---|
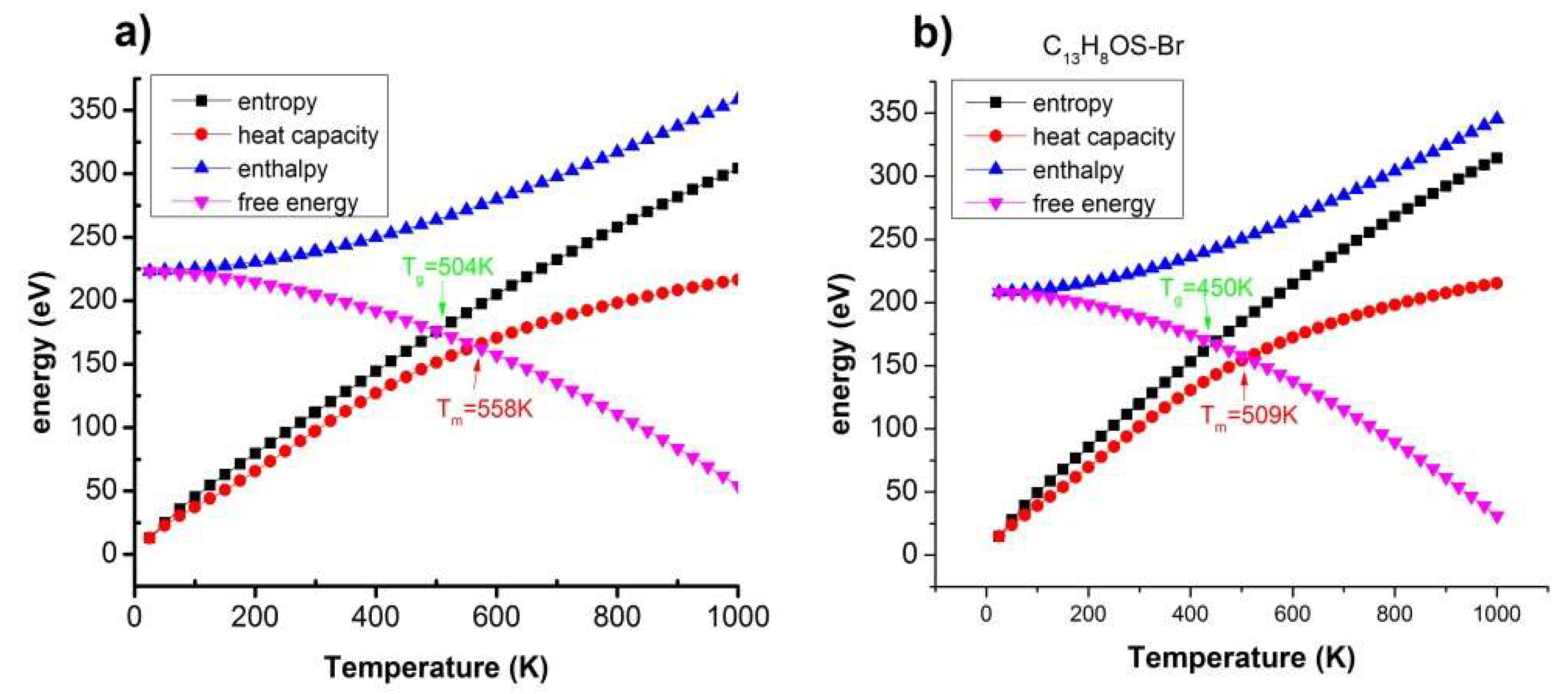
| T(K) | 504 < T < 558 | 504 < T < 564 | 504 < T < 564 | 504 < T < 564 |
| Eg(eV) | 1.646 | 1.675 | 1.675 | 1.675 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, Q.-T.; Tran, T.-T.-D.; Nguyen, T.-C.; Nguyen, T.V.; Nguyen, H.; Vinh, P.V.; Nguyen-Trong, D.; Dinh Duc, N.; Nguyen-Tri, P. DFT Prediction of Factors Affecting the Structural Characteristics, the Transition Temperature and the Electronic Density of Some New Conjugated Polymers. Polymers 2020, 12, 1207. https://doi.org/10.3390/polym12061207
Vu Q-T, Tran T-T-D, Nguyen T-C, Nguyen TV, Nguyen H, Vinh PV, Nguyen-Trong D, Dinh Duc N, Nguyen-Tri P. DFT Prediction of Factors Affecting the Structural Characteristics, the Transition Temperature and the Electronic Density of Some New Conjugated Polymers. Polymers. 2020; 12(6):1207. https://doi.org/10.3390/polym12061207
Chicago/Turabian StyleVu, Quoc-Trung, Thi-Thuy-Duong Tran, Thuy-Chinh Nguyen, Thien Vuong Nguyen, Hien Nguyen, Pham Van Vinh, Dung Nguyen-Trong, Nguyen Dinh Duc, and Phuong Nguyen-Tri. 2020. "DFT Prediction of Factors Affecting the Structural Characteristics, the Transition Temperature and the Electronic Density of Some New Conjugated Polymers" Polymers 12, no. 6: 1207. https://doi.org/10.3390/polym12061207
APA StyleVu, Q.-T., Tran, T.-T.-D., Nguyen, T.-C., Nguyen, T. V., Nguyen, H., Vinh, P. V., Nguyen-Trong, D., Dinh Duc, N., & Nguyen-Tri, P. (2020). DFT Prediction of Factors Affecting the Structural Characteristics, the Transition Temperature and the Electronic Density of Some New Conjugated Polymers. Polymers, 12(6), 1207. https://doi.org/10.3390/polym12061207









