Transparent Ultraviolet (UV)-Shielding Films Made from Waste Hemp Hurd and Polyvinyl Alcohol (PVA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Particles
2.3. Preparation of Hemp Hurd/PVA Film
2.4. Material Characterisation
2.4.1. Particle Size Analysis
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. X-ray Diffraction (XRD)
2.4.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.5. Mechanical Properties of the Films
2.4.6. Water Vapor Permeability
2.4.7. UV-Shielding
2.4.8. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Analysis
3.2. XRD Analysis
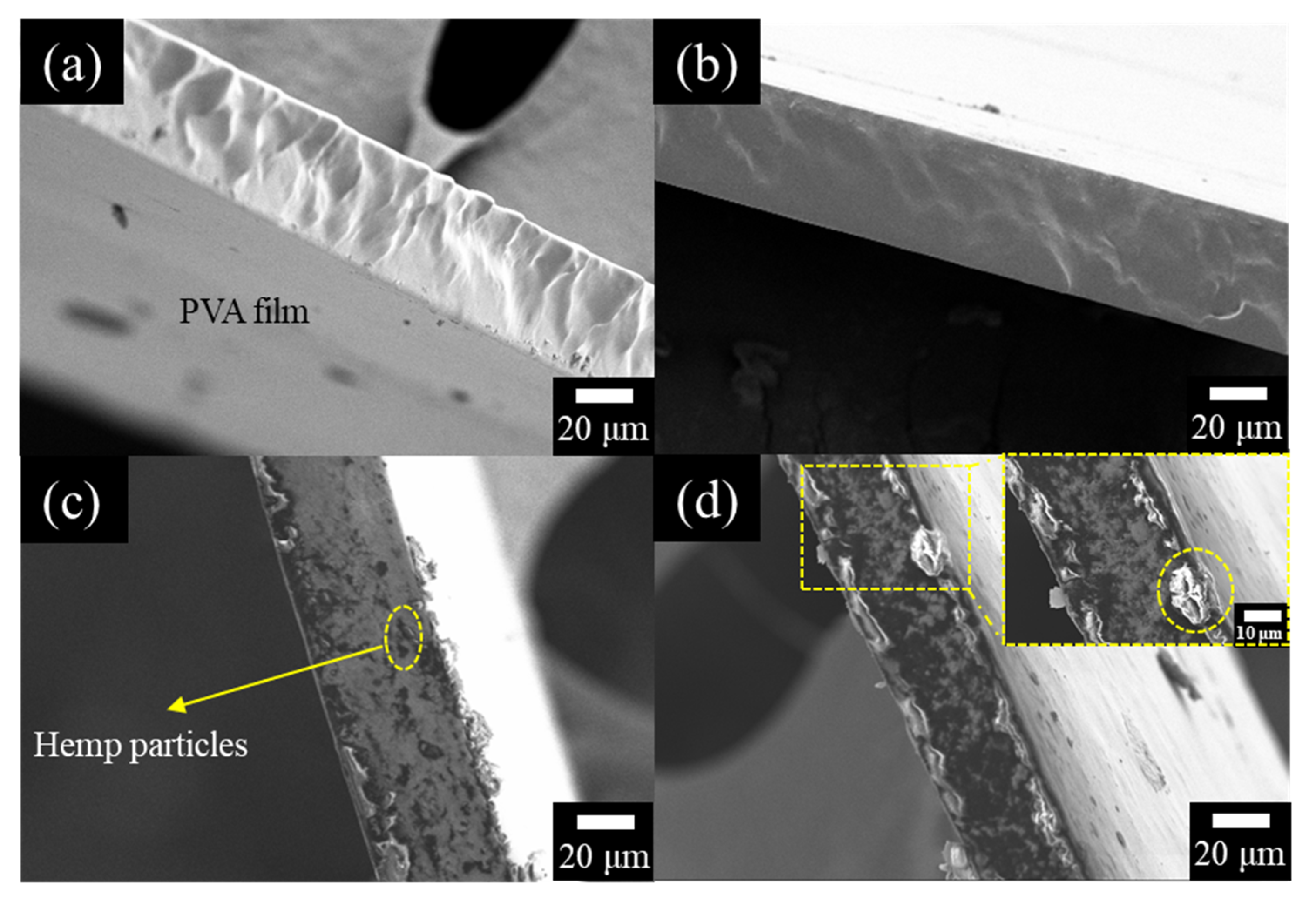
3.3. Morphology of the Films
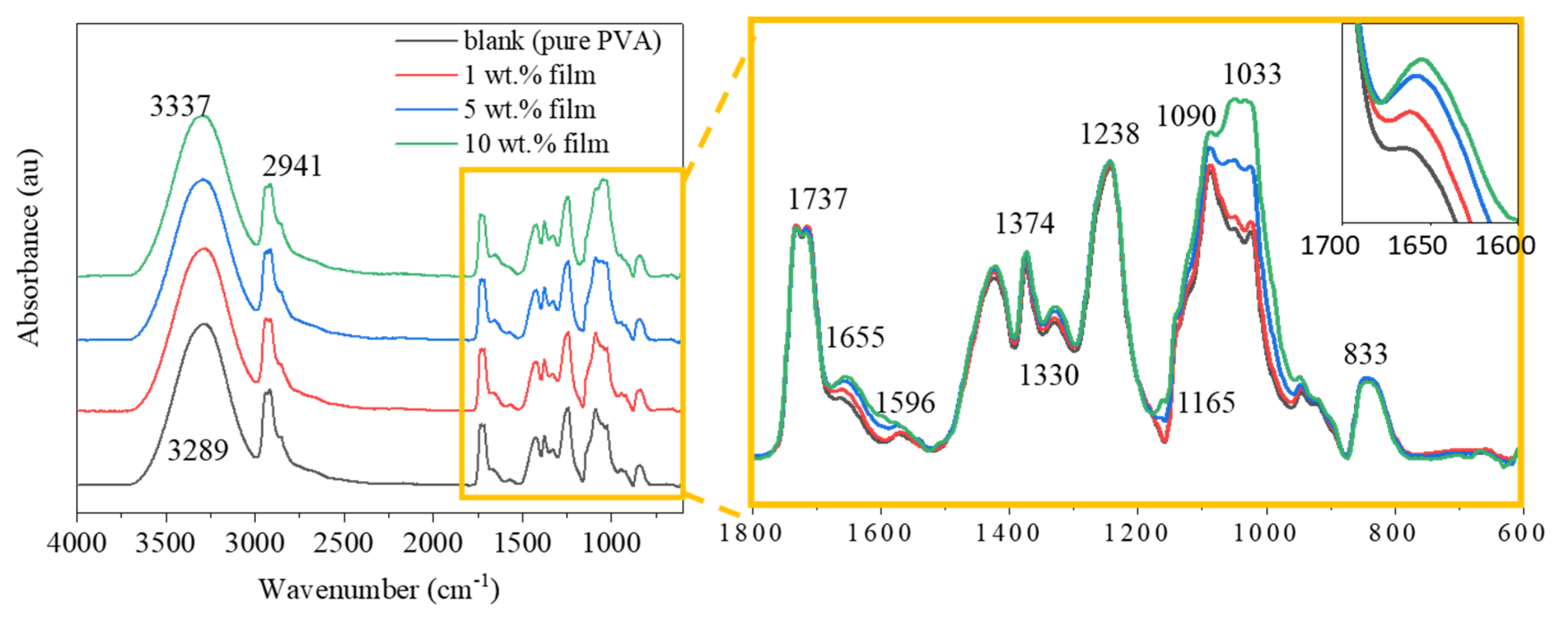
3.4. ATR-FTIR Analysis of the Films
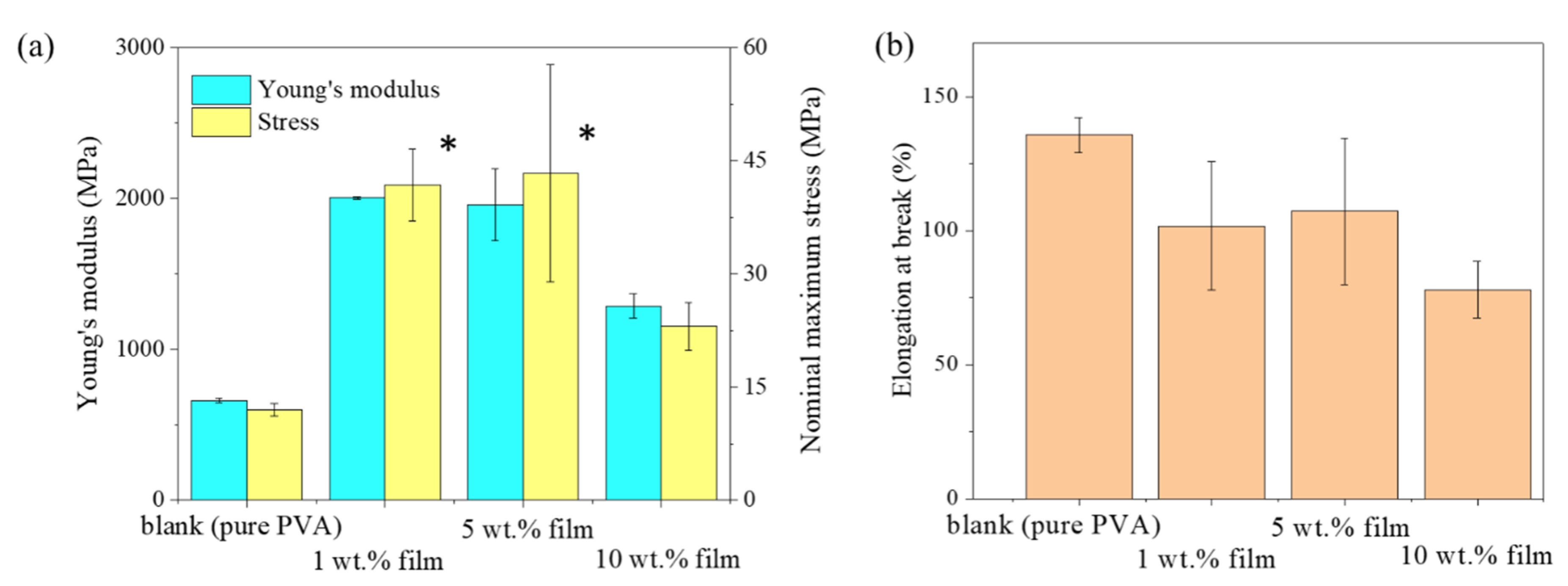
3.5. Mechanical Properties of the Film
3.6. Water Vapour Permeability (WVP) Properties of Hemp/PVA Composites
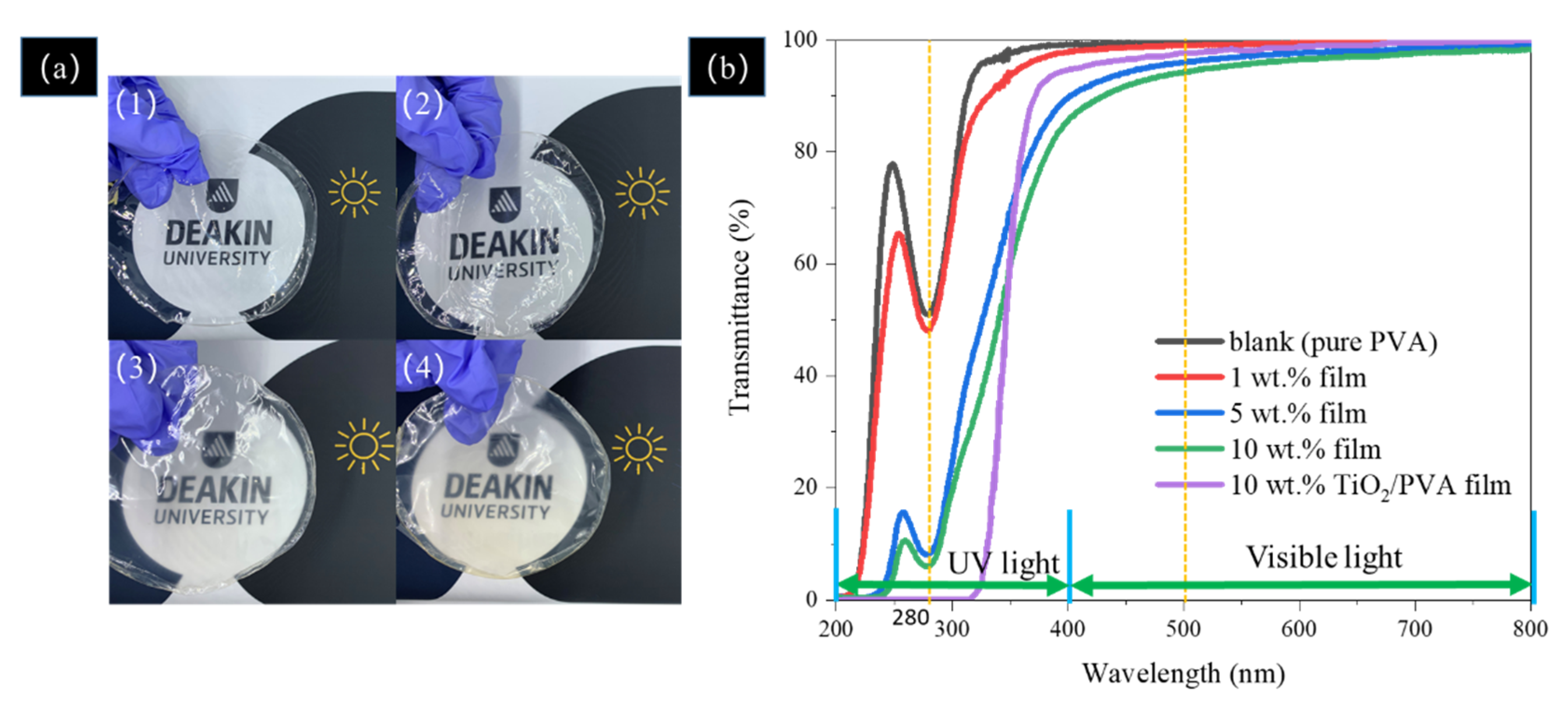
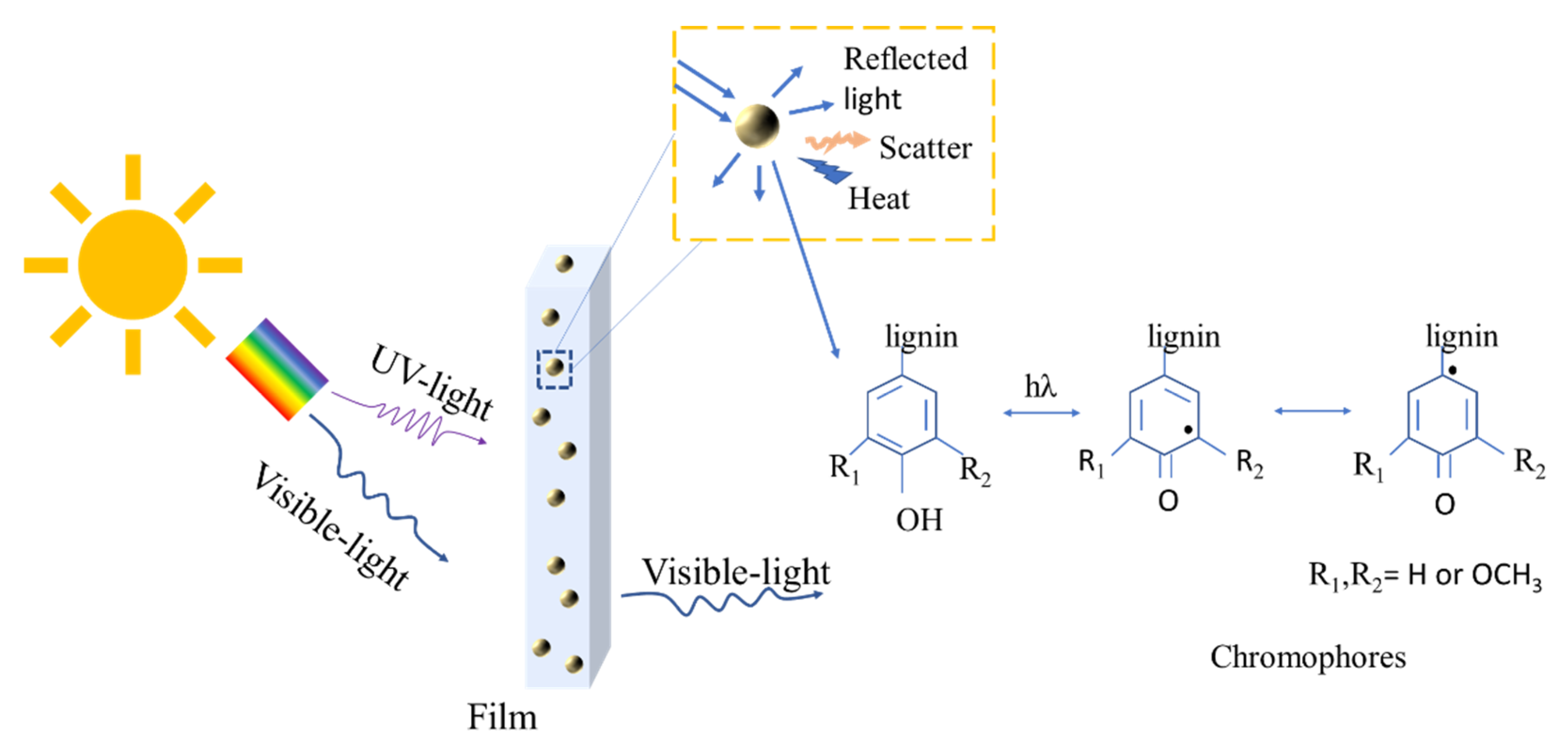
3.7. UV-Shielding Performance of the Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-Lignin Biodegradable and Flexible UV Protection Film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Tu, Y.; Zhou, L.; Jin, Y.Z.; Gao, C.; Ye, Z.Z.; Yang, Y.F.; Wang, Q.L. Transparent and flexible thin films of ZnO-polystyrene nanocomposite for UV-shielding applications. J. Mater. Chem. 2010, 20, 1594–1599. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Ma, P.; Bai, H.; Xie, Y.; Chen, M.; Dong, W. Simultaneous Enhancements of UV-Shielding Properties and Photostability of Poly(vinyl alcohol) via Incorporation of Sepia Eumelanin. ACS Sustain. Chem. Eng. 2016, 4, 2252–2258. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Heiskanen, J.P.; Liimatainen, H. UV-absorbing cellulose nanocrystals as functional reinforcing fillers in polymer nanocomposite films. J. Mater. Chem. A 2016, 4, 6368–6375. [Google Scholar] [CrossRef]
- Xie, W.; Pakdel, E.; Liu, D.; Sun, L.; Wang, X. Waste-Hair-Derived Natural Melanin/TiO 2 Hybrids as Highly Efficient and Stable UV-Shielding Fillers for Polyurethane Films. ACS Sustain. Chem. Eng. 2019, 8, 1343–1352. [Google Scholar] [CrossRef]
- Zhu, M.; Song, J.; Li, T.; Gong, A.; Wang, Y.; Dai, J.; Yao, Y.; Luo, W.; Henderson, D.; Hu, L. Highly Anisotropic, Highly Transparent Wood Composites. Adv. Mater. 2016, 28, 5181–5187. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and UV protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10. [Google Scholar] [CrossRef]
- Subramani, N.K.; Kasargod Nagaraj, S.; Shivanna, S.; Siddaramaiah, H. Highly Flexible and Visibly Transparent Poly(vinyl alcohol)/Calcium Zincate Nanocomposite Films for UVA Shielding Applications As Assessed by Novel Ultraviolet Photon Induced Fluorescence Quenching. Macromolecules 2016, 49, 2791–2801. [Google Scholar] [CrossRef]
- Lizundia, E.; Ruiz-Rubio, L.; Vilas, J.L.; León, L.M. Poly(l -lactide)/zno nanocomposites as efficient UV-shielding coatings for packaging applications. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Fang, Z.; Zhu, H.; Yuan, Y.; Ha, D.; Zhu, S.; Preston, C.; Chen, Q.; Li, Y.; Han, X.; Lee, S.; et al. Novel nanostructured paper with ultrahigh transparency and ultrahigh haze for solar cells. Nano Lett. 2014, 14, 765–773. [Google Scholar] [CrossRef]
- Xiong, F.; Wu, Y.; Li, G.; Han, Y.; Chu, F. Transparent Nanocomposite Films of Lignin Nanospheres and Poly(vinyl alcohol) for UV-Absorbing. Ind. Eng. Chem. Res. 2018, 57, 1207–1212. [Google Scholar] [CrossRef]
- Cai, Z.; Remadevi, R.; Al Faruque, M.A.; Setty, M.; Fan, L.; Haque, A.N.M.A.; Naebe, M. Fabrication of a cost-effective lemongrass (Cymbopogon citratus) membrane with antibacterial activity for dye removal. RSC Adv. 2019, 9, 34076–34085. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, S.H.; Won, K. Wood Powder as a New Natural Sunscreen Ingredient. Biotechnol. Bioprocess Eng. 2019, 24, 258–263. [Google Scholar] [CrossRef]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties Characterization of Chemically Modified Hemp Hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Phuong, M.; Ye, M.; Halasz, A.; Hawari, J. Isolation and characterization of herbaceous lignins for applications in biomaterials. Ind. Crops Prod. 2013, 41, 356–364. [Google Scholar] [CrossRef]
- Karus, M. European hemp industry: Cultivation, processing and product lines. Euphytica 2004, 140, 7–12. [Google Scholar] [CrossRef]
- Tan, K.B.; Ching, C.Y.; Poh, C.S.; Abdullah, C.L.; Gan, N.S. A Review of Natural Fiber Reinforced Poly(Vinyl Alcohol) Based Composites: Application and Opportunity. Polymers 2015, 7, 2205–2222. [Google Scholar] [CrossRef]
- Ng, T.S.; Ching, Y.C.; Awanis, N.; Ishenny, N.; Rahman, M.R. Effect of bleaching condition on thermal properties and UV transmittance of PVA/cellulose biocomposites. Mater. Res. Innov. 2014, 18, 400–404. [Google Scholar] [CrossRef]
- Ko, H.-U.; Zhai, L.; Park, J.H.; Lee, J.Y.; Kim, D.; Kim, J. Poly(vinyl alcohol)-lignin blended resin for cellulose-based composites. J. Appl. Polym. Sci. 2018, 135, 46655. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- ASTM International. D882-18 Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar] [CrossRef]
- Water Vapor Transmission Rate of Paper and Paperboard at High Temperature and Humidity, Test Method. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T464.aspx (accessed on 11 March 2020).
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Wang, X.; Naebe, M. Physicochemical properties of film fabricated from cotton gin trash. Mater. Chem. Phys. 2020, 239, 122009. [Google Scholar] [CrossRef]
- Al Faruque, M.A.; Remadevi, R.; Wang, X.; Naebe, M. Preparation and characterisation of mechanically milled particles from waste alpaca fibres. Powder Technol. 2019, 342, 848–855. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, Z.; Li, D.; Wu, M. Effects of ball milling processes on the microstructure and rheological properties of microcrystalline cellulose as a sustainable polymer additive. Materials 2018, 11, 1057. [Google Scholar] [CrossRef]
- Chandrakala, H.N.; Ramaraj, B. Optical properties and structural characteristics of zinc oxidecerium oxide doped polyvinyl alcohol films. J. Alloys Compd. 2014, 586, 333–342. [Google Scholar] [CrossRef]
- Hassan, M.; Zadeh, R.; Seifi, M.; Riva’i, I.; Wulandari, I.O.; Sulistyarti, H.; Sabarudin, A. Ex-Situ Synthesis of Polyvinyl alcohol(PVA)-coated Fe3O4 Nanoparticles by Coprecipitation-Ultrasonication Method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 1–8. [Google Scholar] [CrossRef]
- Corzo-González, Z.; Loria-Bastarrachea, M.I.; Hernández-Nuñez, E.; Aguilar-Vega, M.; González-Díaz, M.O. Preparation and characterization of crosslinked PVA/PAMPS blends catalytic membranes for biodiesel production. Polym. Bull. 2017, 74, 2741–2754. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. The formation of strong intermolecular interactions in immiscible blends of poly(vinyl alcohol) (PVA) and lignin. Biomacromolecules 2003, 4, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Anicuta, S.G.; Dobre, L.; Stroescu, M.; Jipa, I. Fourier Transform Infrared (FTIR) Spectroscopy for Characterization of Antimicrobial Films Containing Chitosan. 2010, pp. 1234–1240. Available online: https://pdfs.semanticscholar.org/0b63/6ec4355eb6aa82a0c8b5b1a7d0890febe79c.pdf?_ga=2.15658038.1246728340.1590139370-1225764345.1558607759 (accessed on 11 March 2020).
- Moosavinejad, S.M.; Madhoushi, M.; Vakili, M.; Rasouli, D. Evaluation of degradation in chemical compounds of wood in historical buildings using FT-IR and FT-Raman vibrational spectroscopy. Maderas. Cienc. y Tecnol. AHEAD 2019, 21, 381–392. [Google Scholar] [CrossRef]
- Gleadall, A. Mechanical properties of biodegradable polymers for medical applications. In Modelling Degradation of Bioresorbable Polymeric Medical Devices; Pen, J., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 163–199. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Imam, S.H.; Cinelli, P.; Gordon, S.H.; Chiellini, E. Characterization of Biodegradable Composite Films Prepared from Blends of Poly(Vinyl Alcohol), Cornstarch, and Lignocellulosic Fiber. J. Polym. Environ. 2005, 13, 47–55. [Google Scholar] [CrossRef]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water absorption and states of water in sernicrystalline poly(vinyl alcohol) films. Polymer (Guildf). 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Q.; Lv, L.; Feng, X.; Meng, X. Preparation of transparent PVA/TiO2 nanocomposite films with enhanced visible-light photocatalytic activity. Catal. Commun. 2015, 58, 30–33. [Google Scholar] [CrossRef]
- Baby, A.R.; Balogh, T.S.; Pedriali, C.A.; Kaneko, T.M.; Velasco, M.V.R. Uva I-protection effectiveness of bioactive compound and organic uv filters: An in vitro assessment. Quim. Nova 2009, 32, 1321–1323. [Google Scholar] [CrossRef]
- Dean, J.C.; Navotnaya, P.; Parobek, A.P.; Clayton, R.M.; Zwier, T.S. Ultraviolet spectroscopy of fundamental lignin subunits: Guaiacol, 4-methylguaiacol, syringol, and 4-methylsyringol. J. Chem. Phys. 2013, 139. [Google Scholar] [CrossRef]

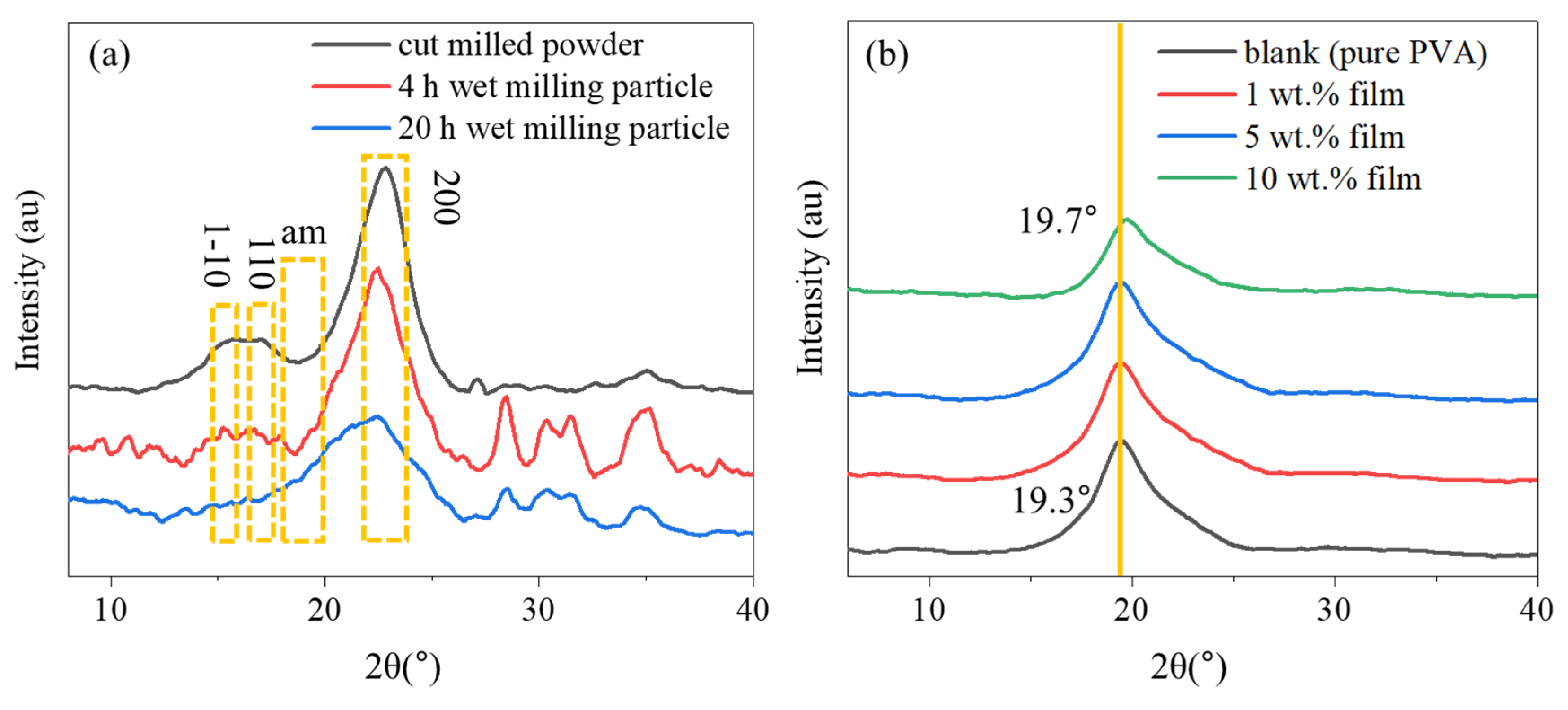


| CrI (%) | I200 | Iam | |
|---|---|---|---|
| cutter milled | 84.26 | 0.98759 | 0.1554 |
| 4 h wet ball-milling | 81.97 | 0.90891 | 0.16388 |
| 20 h wet ball-milling | 46.96 | 0.56037 | 0.29723 |
| Young’s Modulus | Nominal Maximum Stress | Elongation at Break (%) | ||
|---|---|---|---|---|
| p-Value | ||||
| pure PVA | 1 wt. % film | 0.033 | 0.027 | 0.318 |
| 5 wt. % film | 0.037 | 0.021 | 0.397 | |
| 10 wt. % film | 0.057 | 0.348 | 0.108 |
| UPF | Normalised UPF | Protection Grade | |
|---|---|---|---|
| PVA film | 2.03 | 6.15 | poor |
| 1 wt. % film | 2.76 | 8.28 | poor |
| 5 wt. % film | 15.07 | 43.39 | good |
| 10 wt. % film | 35.79 | 98.60 | very good |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Remadevi, R.; Hinestroza, J.P.; Wang, X.; Naebe, M. Transparent Ultraviolet (UV)-Shielding Films Made from Waste Hemp Hurd and Polyvinyl Alcohol (PVA). Polymers 2020, 12, 1190. https://doi.org/10.3390/polym12051190
Zhang Y, Remadevi R, Hinestroza JP, Wang X, Naebe M. Transparent Ultraviolet (UV)-Shielding Films Made from Waste Hemp Hurd and Polyvinyl Alcohol (PVA). Polymers. 2020; 12(5):1190. https://doi.org/10.3390/polym12051190
Chicago/Turabian StyleZhang, Yi, Rechana Remadevi, Juan P. Hinestroza, Xungai Wang, and Maryam Naebe. 2020. "Transparent Ultraviolet (UV)-Shielding Films Made from Waste Hemp Hurd and Polyvinyl Alcohol (PVA)" Polymers 12, no. 5: 1190. https://doi.org/10.3390/polym12051190
APA StyleZhang, Y., Remadevi, R., Hinestroza, J. P., Wang, X., & Naebe, M. (2020). Transparent Ultraviolet (UV)-Shielding Films Made from Waste Hemp Hurd and Polyvinyl Alcohol (PVA). Polymers, 12(5), 1190. https://doi.org/10.3390/polym12051190









