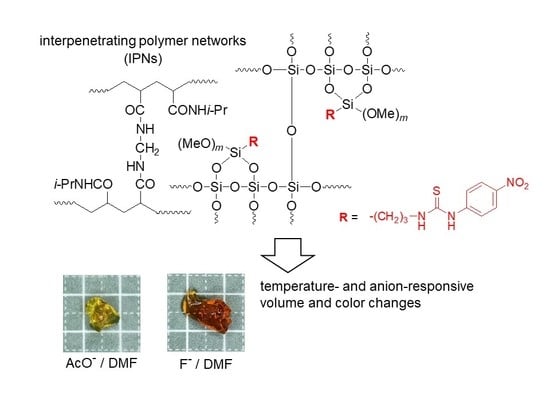
Multi-Responsive Polysiloxane/Poly(N-isopropylacrylamide) Interpenetrating Networks Containing Urea and Thiourea Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Materials
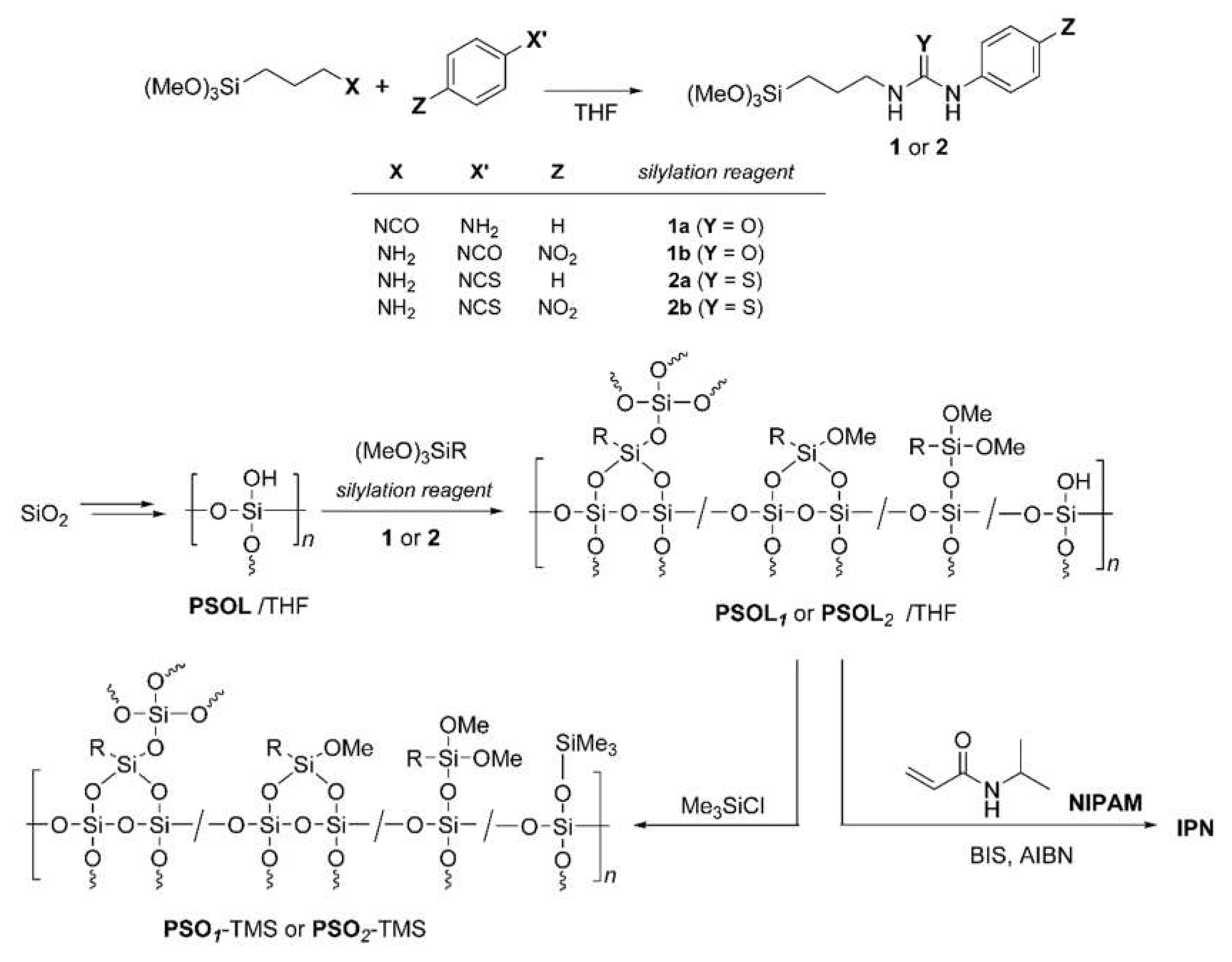
2.3. IPN Synthesis
3. Results and Discussion
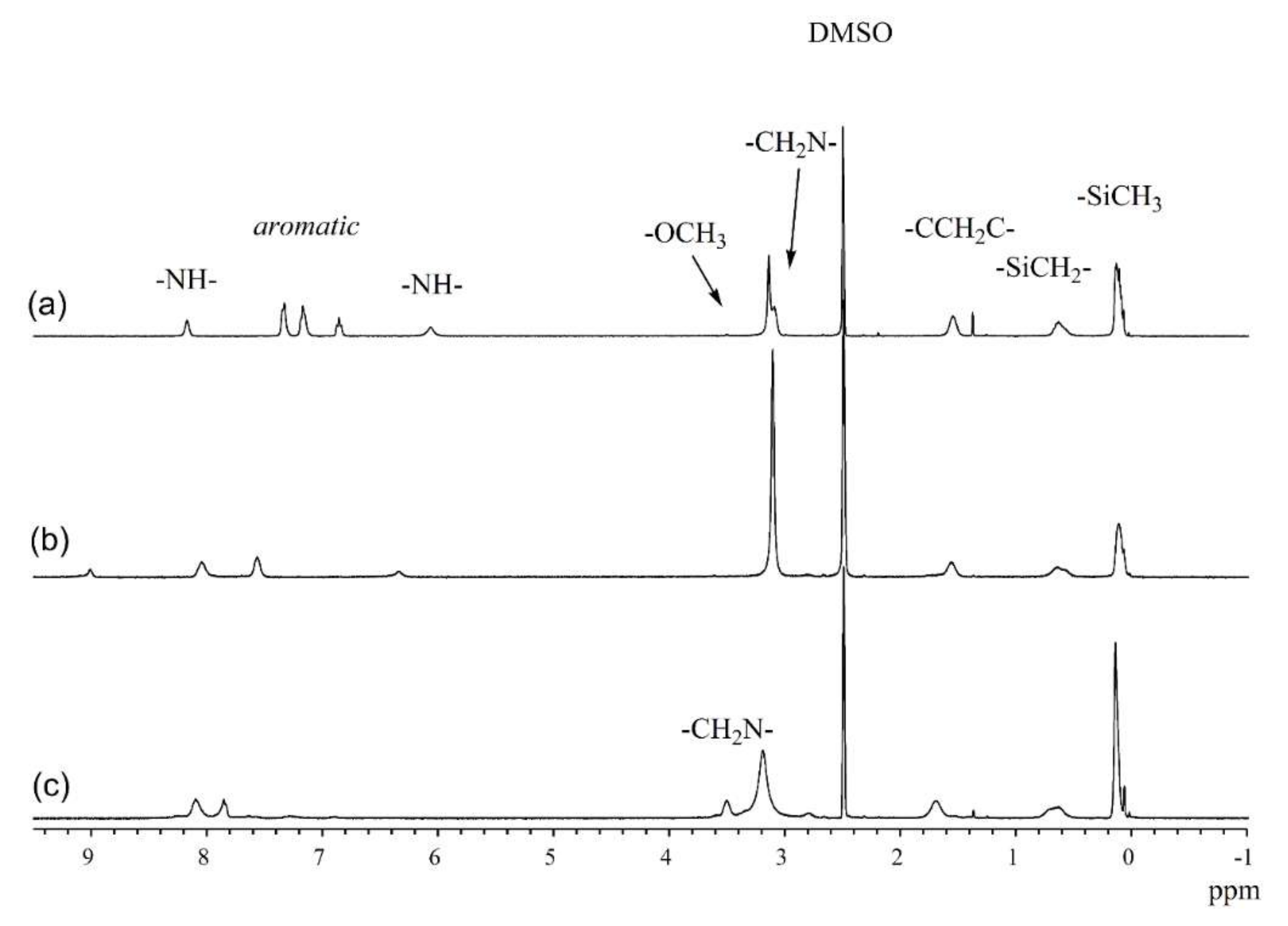
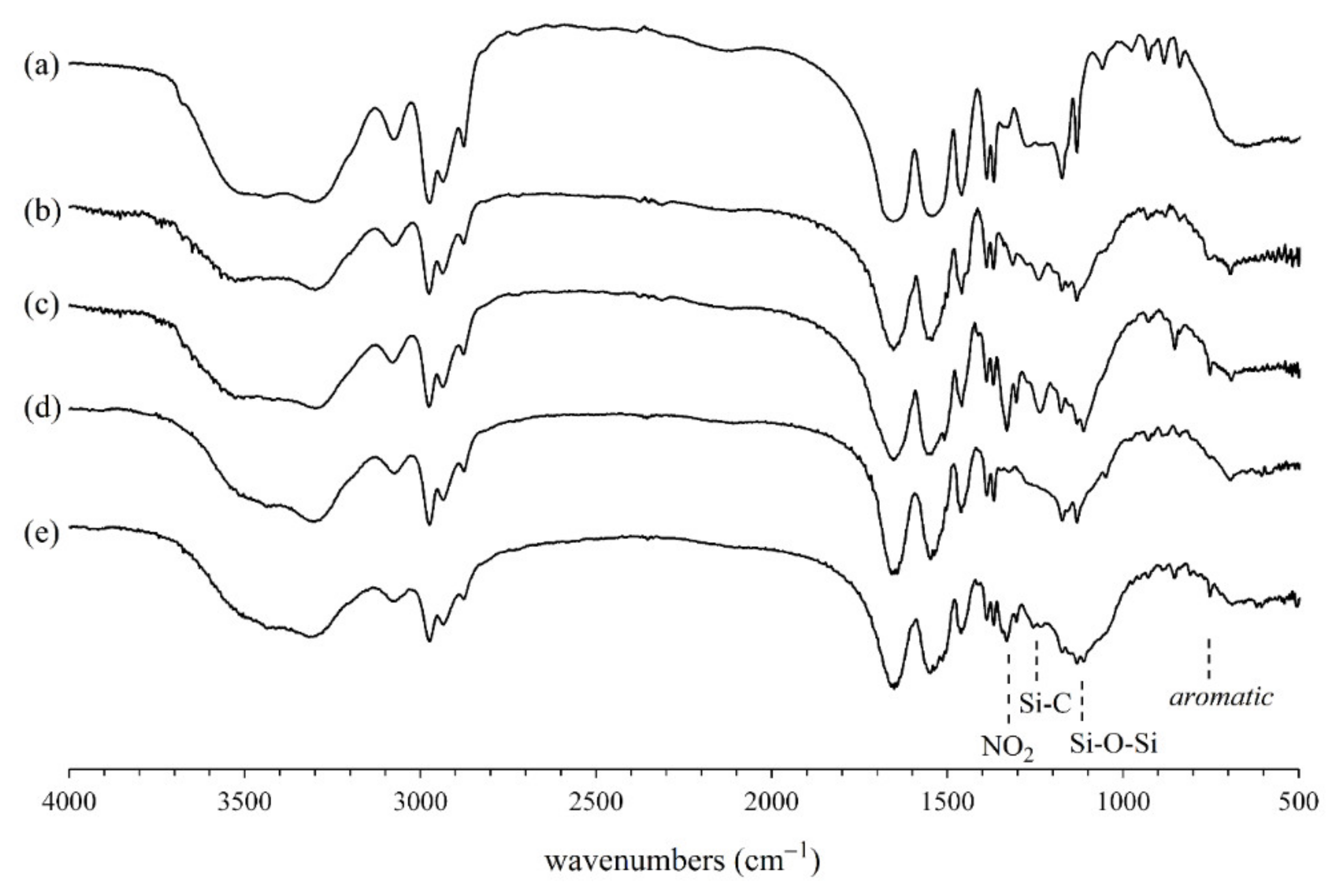
3.1. Structure of Silicone Polymers
3.2. Synthesis of IPNs
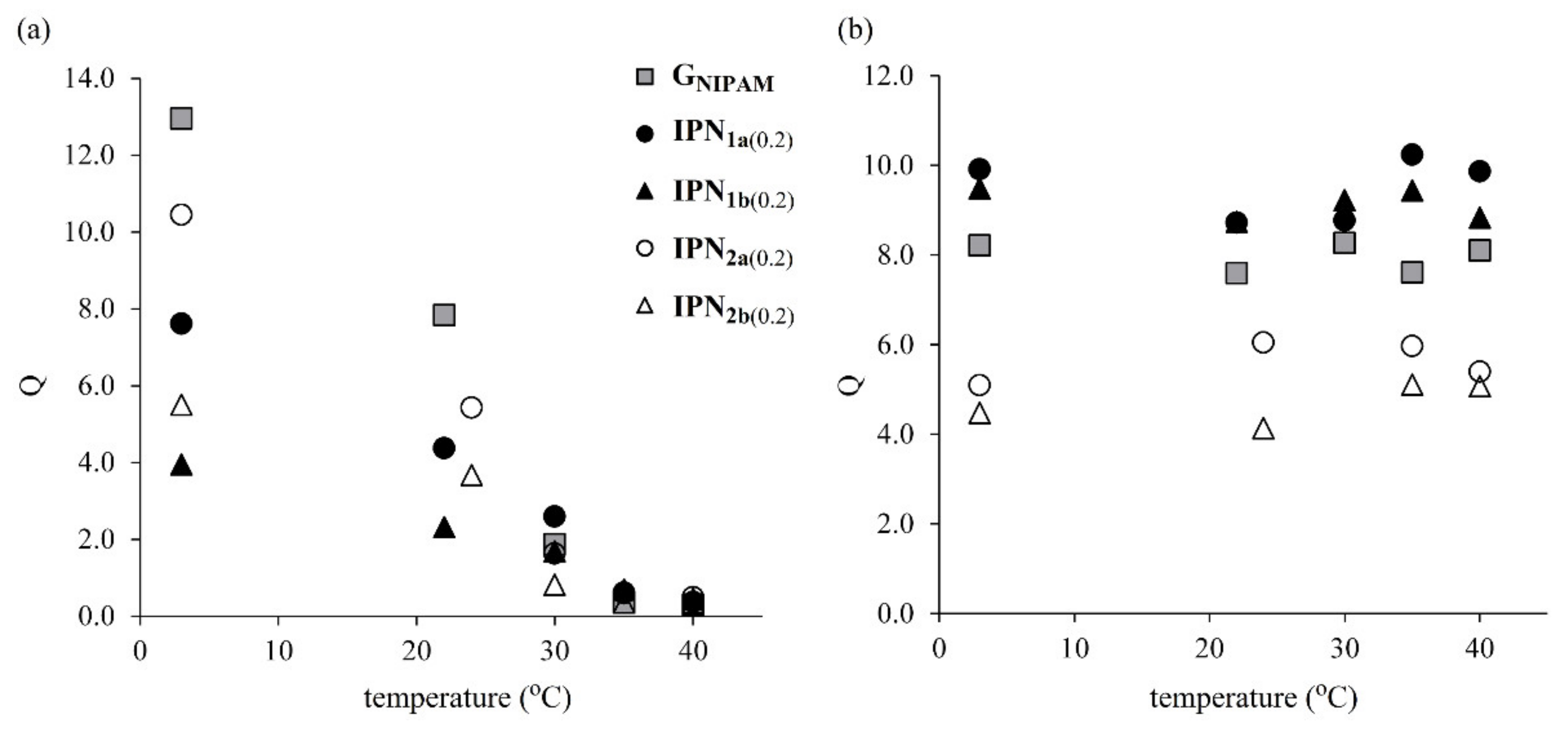
3.3. Swelling Properties of the Obtained IPNs
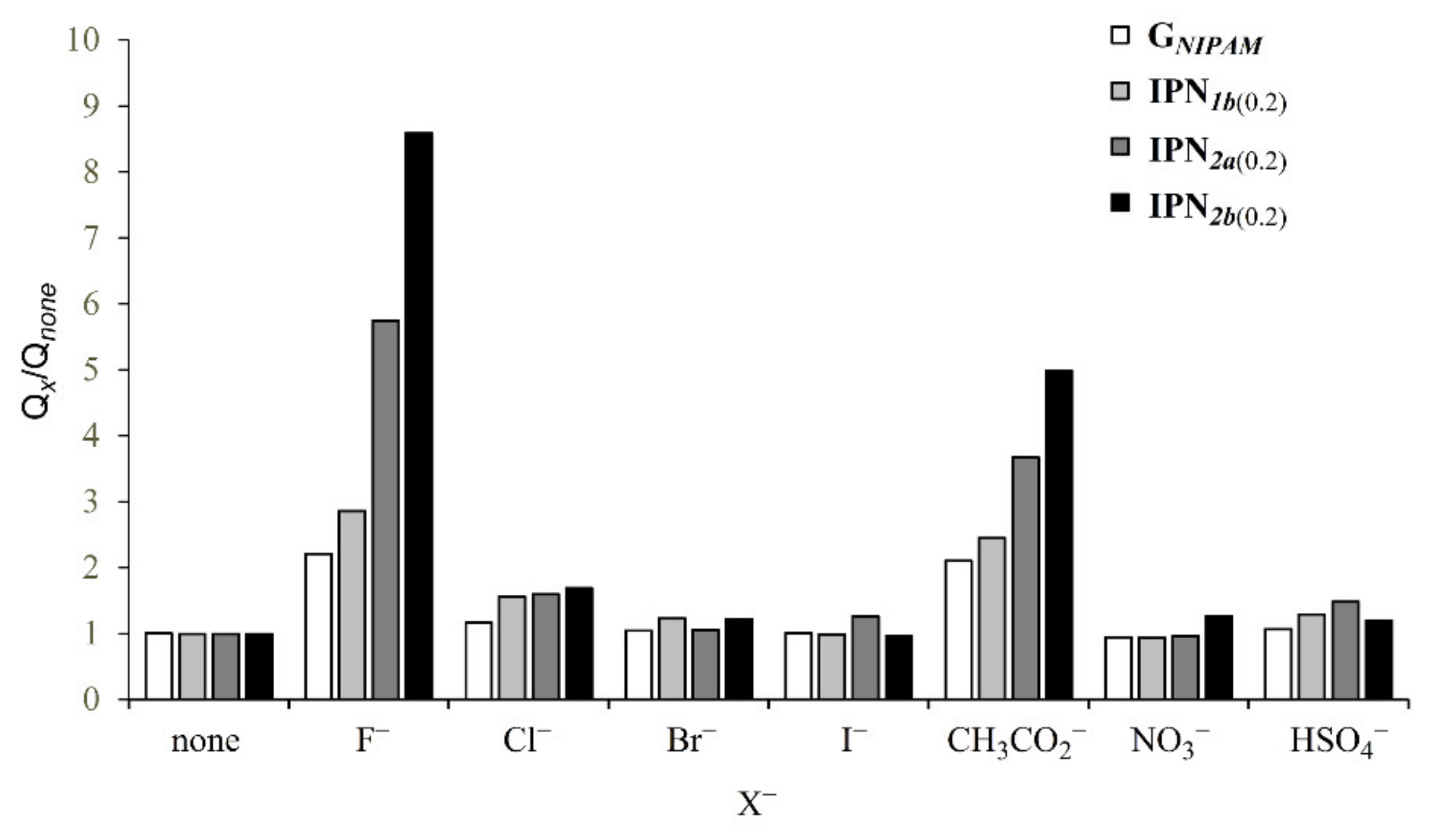
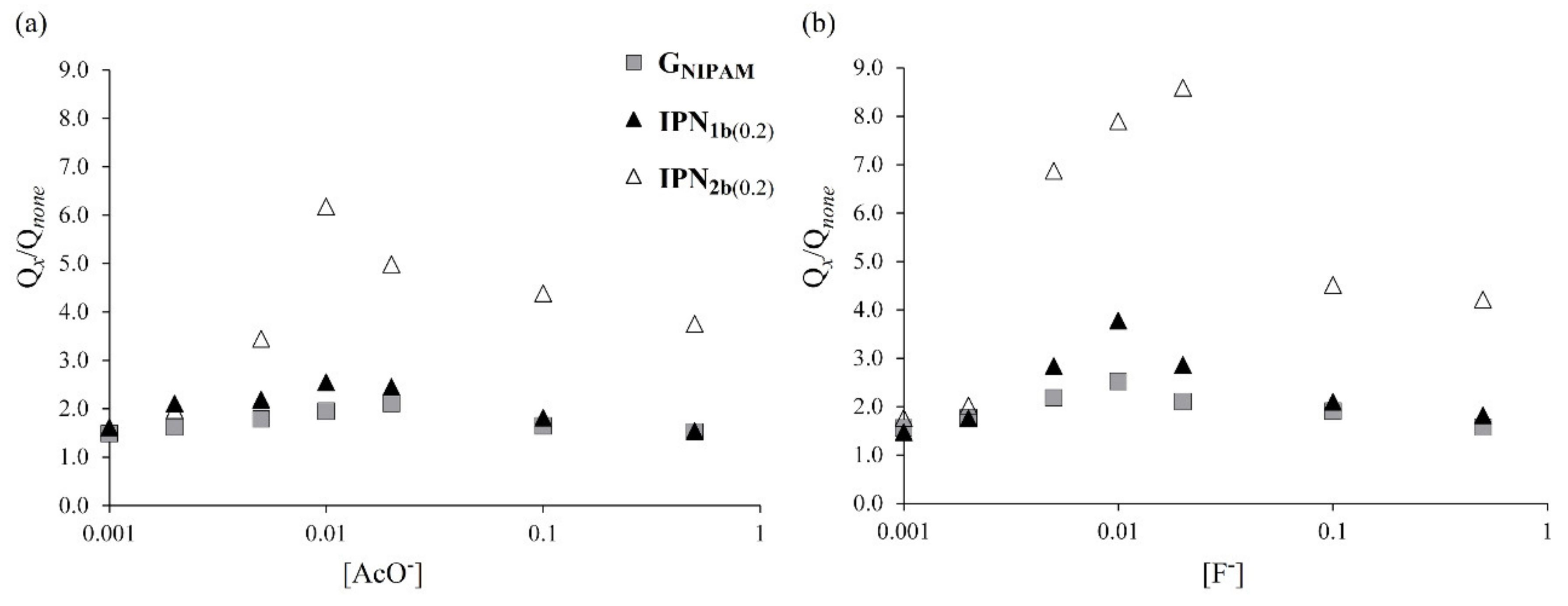
3.4. Behaviors of the Obtained IPNs to Anion Stimuli
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sperling, J.H.; Mishra, V. The current status of interpenetrating polymer networks. Polym. Adv. Technol. 1996, 7, 197–208. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Singh, S.; Frisch, H.L.; Ghiradella, H. Poly(2,6-dimethyl-1,4-phenylene oxide)-poly(methyl methacrylate) interpenetrating polymer networks. Macromolecules 1990, 23, 375–377. [Google Scholar] [CrossRef]
- Wang, M.; Fang, Y.; Hu, D. Preparation and properties of chitosan-poly(N-isopropylacrylamide) full-IPN hydrogels. React. Funct. Polym. 2001, 48, 215–221. [Google Scholar] [CrossRef]
- Chen, P.; Wu, R.; Wang, J.; Liu, Y.; Ding, C.; Xu, S. One-pot preparation of ultrastrong double network hydrogels. J. Polym. Res. 2012, 19, 9825. [Google Scholar] [CrossRef]
- Wu, W.; Wang, D. A fast pH-responsive IPN hydrogel: Synthesis and controlled drug delivery. React. Funct. Polym. 2010, 70, 684–691. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, R.P.; Mitchell, G.R.; Vasile, C. Multi-responsive hydrogels based on N-isopropylacrylamide and sodium alginate. Polym. Int. 2011, 60, 222–233. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. A one-step strategy for thermal- and pH-responsive graphene oxide interpenetrating polymer hydrogel networks. J. Mater. Chem. 2011, 20, 4095–4097. [Google Scholar] [CrossRef]
- Dragan, E.S.; Cocatra, A.I. Smart macroporous IPN hydrogels responsive to pH, temperature, and ionic strength: Synthesis, characterization, and evaluation of controlled release of drugs. ACS Appl. Mater. Interfaces 2016, 8, 12018–12030. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Cai, P.; Xiao, H. Dual-responsive IPN hydrogel based on sugarcane bagasse cellulose as drug carrier. Int. J. Bio. Maclomol. 2018, 118, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Sano, J.; Habaue, S. Synthesis of interpenetrating polymer networks using silicone polymer with silanol residue. React. Funct. Polym. 2019, 137, 21–26. [Google Scholar] [CrossRef]
- Abe, Y.; Misono, T. Preparation of polysiloxanes from silicic acid. II. Esterification of silicic acid with various alcohols and isolation of esterification products by silylation. J. Polym. Sci. Polym. Lett. Ed. 1982, 20, 205–210. [Google Scholar] [CrossRef]
- Gunji, T.; Nagao, Y.; Misono, T.; Abe, Y. Condensation and structure of silicic acid in tetrahydrofuran. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1779–1787. [Google Scholar] [CrossRef]
- Habaue, S.; Hirasa, T.; Akagi, Y.; Yamashita, K.; Kajiwara, M. Synthesis and property of silicone polymer from chrysotile asbestos by acid-Leaching and silylation. J. Inorg. Organomet. Polym. Mater. 2006, 16, 155–160. [Google Scholar] [CrossRef]
- Habaue, S.; Sato, K.; Yamashita, K.; Shimamura, T.; Kaito, M.; Masuda, T.; Kajiwara, M. Polysiloxanes derived from chrysotile asbestos via acid-leaching and silylation processes. J. Appl. Polym. Sci. 2008, 110, 2891–2897. [Google Scholar] [CrossRef]
- Habaue, S.; Takahashi, Y.; Suzuki, H.; Takamushi, Y. Formation of polyurethane film containing silicone polymer with silanol residue. Int. J. Biotechnol. Wellness Ind. 2017, 6, 48–54. [Google Scholar] [CrossRef]
- Kato, R.; Nishizawa, S.; Hayashita, T.; Teramae, N. A thiourea-based chromoionophore for selective binding and sensing of acetate. Tetrahedron Lett. 2001, 42, 5053–5056. [Google Scholar] [CrossRef]
- Jose, D.A.; Kumar, D.K.; Ganguly, B.; Das, A. Efficient and simple colorimetric fluoride ion sensor based on receptors having urea and thiourea binding sites. Org. Lett. 2004, 6, 3445–3448. [Google Scholar] [CrossRef]
- Gunnlaugsson, T.; Kruger, P.E.; Jensen, P.; Tiernery, J.; Ali, H.D.P.; Hussey, G.M. Colorimetric “naked eye” sensing of anions in aqueous solution. J. Org. Chem. 2005, 70, 10875–10878. [Google Scholar] [CrossRef]
- Ono, T.; Sugimoto, T.; Shinkai, S.; Sada, K. Molecular design of superabsorbent polymers for organic solvents by crosslinked lipophilic polyelectrolytes. Adv. Funct. Mater. 2008, 18, 3936–3940. [Google Scholar] [CrossRef]
- Krishnamurthi, J.; Ono, T.; Amemori, S.; Komatsu, H.; Shinkai, S.; Sada, K. Thiourea-tagged poly(octadecyl acrylate) gels as fluoride and acetate responsive polymer gels through selective complexation. Chem. Commun. 2001, 47, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Erbil, C.; Kazancıoğlu, E.; Uyanık, N. Synthesis, characterization and thermoreversible behaviours of poly(dimethyl siloxane)/poly(N-isopropyl acrylamide) semi-interpenetrating networks. Euro. Polym. J. 2004, 40, 1145–1154. [Google Scholar] [CrossRef]


| Run | Silylation Reagent (Equiv.) | PSO-TMS | PSOL | ||
|---|---|---|---|---|---|
| Unit Ratio (R/MeO/TMS) 1 | Abbreviation | Silanol Ratio (%) 2 | |||
| 1 | 1a | 0.1 | 59/2/39 | PSOL1a(0.1) | 18 |
| 2 | 0.2 | 65/3/32 | PSOL1a(0.2) | 14 | |
| 3 | 0.4 | 69/2/29 | PSOL1a(0.4) | 12 | |
| 4 | 1b | 0.1 | 62/2/36 | PSOL1b(0.1) | 16 |
| 5 | 0.2 | 64/1/35 | PSOL1b(0.2) | 15 | |
| 6 | 2a | 0.1 | 57/3/40 | PSOL2a(0.1) | 19 |
| 7 | 0.2 | 60/3/37 | PSOL2a(0.2) | 17 | |
| 8 | 2b | 0.1 | 52/3/45 | PSOL2b(0.1) | 23 |
| 9 | 0.2 | 54/2/44 | PSOL2b(0.2) | 22 | |
| Run | Silicone Polymer | Gel Abbreviation | Yield (g) 1 | Q2 |
|---|---|---|---|---|
| 1 | none | GNIPAM | 0.72 | 13.0 |
| 2 | PSOL1a(0.1) | IPN1a(0.1) | 0.75 | 9.9 |
| 3 | PSOL1a(0.2) | IPN1a(0.2) | 0.81 | 7.6 |
| 4 | PSOL1a(0.4) | IPN1a(0.4) | 0.84 | 7.0 |
| 5 | PSOL1b(0.1) | IPN1b(0.1) | 0.75 | 6.2 |
| 6 | PSOL1b(0.2) | IPN1b(0.2) | 0.68 | 3.9 |
| 7 | PSOL2a(0.1) | IPN2a(0.1) | 0.83 | 8.8 |
| 8 | PSOL2a(0.2) | IPN2a(0.2) | 0.83 | 10.4 |
| 9 | PSOL2b(0.1) | IPN2b(0.1) | 0.76 | 6.3 |
| 10 | PSOL2b(0.2) | IPN2b(0.2) | 0.69 | 5.5 |
| X− | Qx | |||
|---|---|---|---|---|
| GNIPAM | IPN1b(0.2) | IPN2a(0.2) | IPN2b(0.2) | |
| none | 8.0 | 8.7 | 5.3 | 4.2 |
| F− | 17.7 | 24.9 | 30.5 | 36.1 |
| Cl− | 9.4 | 13.6 | 8.5 | 7.1 |
| Br− | 8.3 | 10.7 | 5.6 | 5.1 |
| I− | 8.0 | 8.5 | 6.7 | 4.0 |
| CH3CO2− | 16.8 | 21.3 | 19.5 | 20.9 |
| NO3− | 7.5 | 8.1 | 5.1 | 5.3 |
| HSO4− | 8.6 | 11.2 | 7.9 | 5.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sano, J.; Habaue, S. Multi-Responsive Polysiloxane/Poly(N-isopropylacrylamide) Interpenetrating Networks Containing Urea and Thiourea Groups. Polymers 2020, 12, 1175. https://doi.org/10.3390/polym12051175
Sano J, Habaue S. Multi-Responsive Polysiloxane/Poly(N-isopropylacrylamide) Interpenetrating Networks Containing Urea and Thiourea Groups. Polymers. 2020; 12(5):1175. https://doi.org/10.3390/polym12051175
Chicago/Turabian StyleSano, Junta, and Shigeki Habaue. 2020. "Multi-Responsive Polysiloxane/Poly(N-isopropylacrylamide) Interpenetrating Networks Containing Urea and Thiourea Groups" Polymers 12, no. 5: 1175. https://doi.org/10.3390/polym12051175
APA StyleSano, J., & Habaue, S. (2020). Multi-Responsive Polysiloxane/Poly(N-isopropylacrylamide) Interpenetrating Networks Containing Urea and Thiourea Groups. Polymers, 12(5), 1175. https://doi.org/10.3390/polym12051175