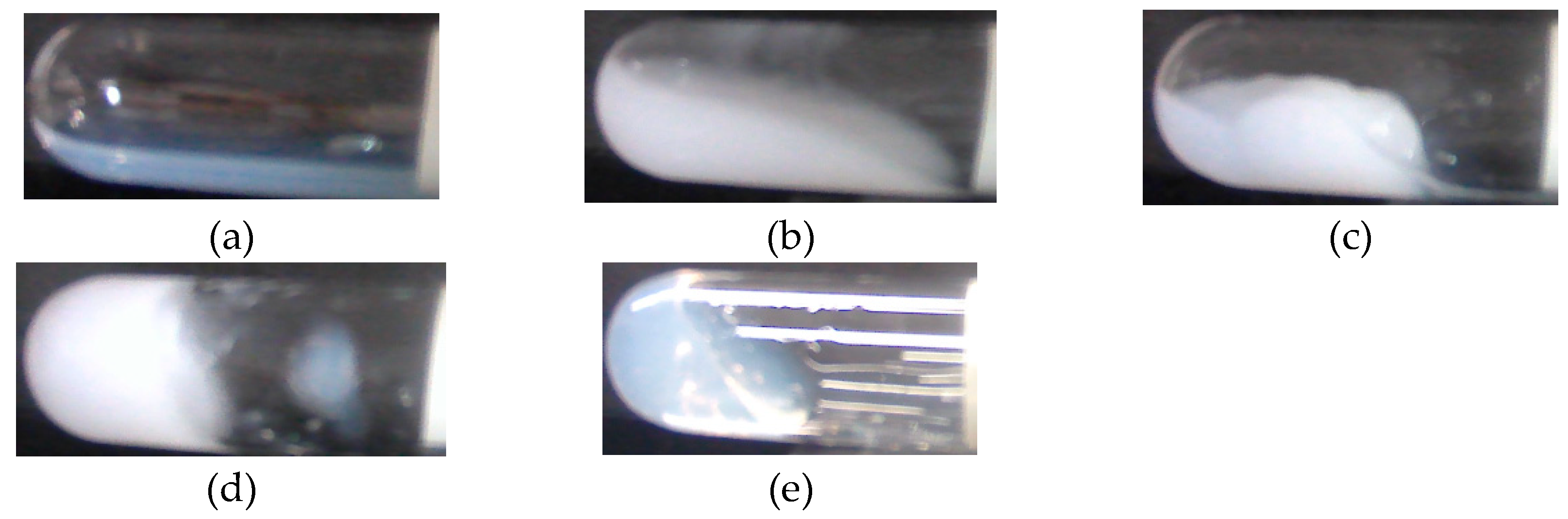
3.1. State Diagram
The silica-PEO suspensions changed their state before and after shaking by the test tube mixer. Representative states of the suspensions are shown in
Figure 1.
Figure 1a is the suspensions before shaking. It is translucent and behaves as sol.
Figure 1b–e are suspensions after shaking.
Figure 1b shows a completely turbid sol, which is stated as cloudy. For gelling suspensions, some of the gels turn back to sol state. We call such gels ‘shake-gel’ (
Figure 1c). Some gels keep their gel state for a long time, say, more than several months. Such gels are called ‘permanent gel’ (
Figure 1d). Furthermore, some suspensions keep translucent and viscous sols after shaking (
Figure 1e).
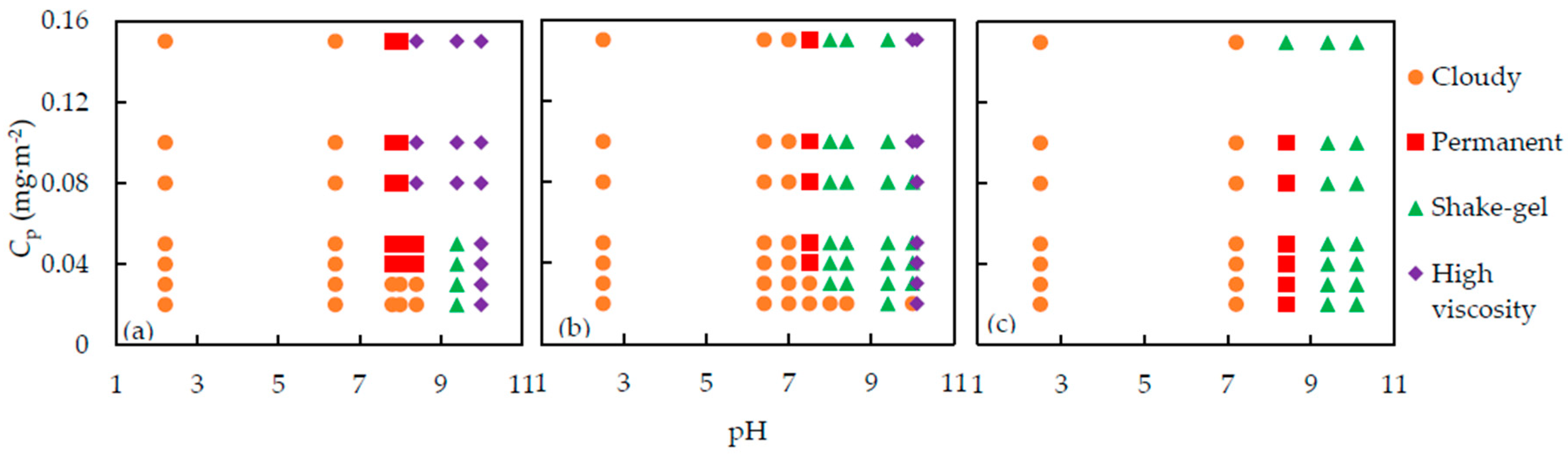
The results of the state of the suspensions are summarized in
Figure 2, in which the symbols stand for different states, cloudy, permanent gel, shake-gel, and high viscosity sol, observed at various pH and
Cp.
From
Figure 2, we can find out that pH,
Cp and molecular weight of PEO are the factors which influence the state of silica-PEO suspensions. The shake-gel phenomena occur in a pH range of 8 to 9.4. If pH is lower than 7, the suspensions turn to cloudy sols, meaning the aggregation of the silica nanoparticles. For pH above 10, almost all the suspensions are translucent high viscosity sol, except the suspensions consisting of 4000 kDa PEO, indicating that no silica flocs appeared.
Cp is a parameter to evaluate the dose of PEO per silica surface area. With increasing
Cp, the suspensions changed in the order of cloudy, permanent gel, shake-gel, and high viscosity sol [
9,
10,
14,
22]. We also observed a similar tendency around pH 8. However, it seems that the effect of pH is more significant than
Cp. Consequently, we found that at low pH, say below pH 7, no matter how
Cp changes, the suspensions are cloudy, and almost all the suspensions at high pH are high viscosity sols. Moreover, we can find that the gelled area, permanent gel and shake-gel, of 4000 kDa PEO suspensions, is the largest. Thus, we consider that the high molecular weight of PEO promotes the formation of gel.
As a mechanism of gelation, we consider that the bridging effect through hydrogen bonds between PEO chains and silica particles is the primary prerequisite of shake-gel, as mentioned in previous studies [
9,
10,
11,
14,
18]. The states change due to shear can be explained by the PEO conformation. Added PEO can be adsorbed to the silanol groups on the silica surface by hydrogen bonds [
15]. Even though the enthalpy of displacement of water by PEO is not high [
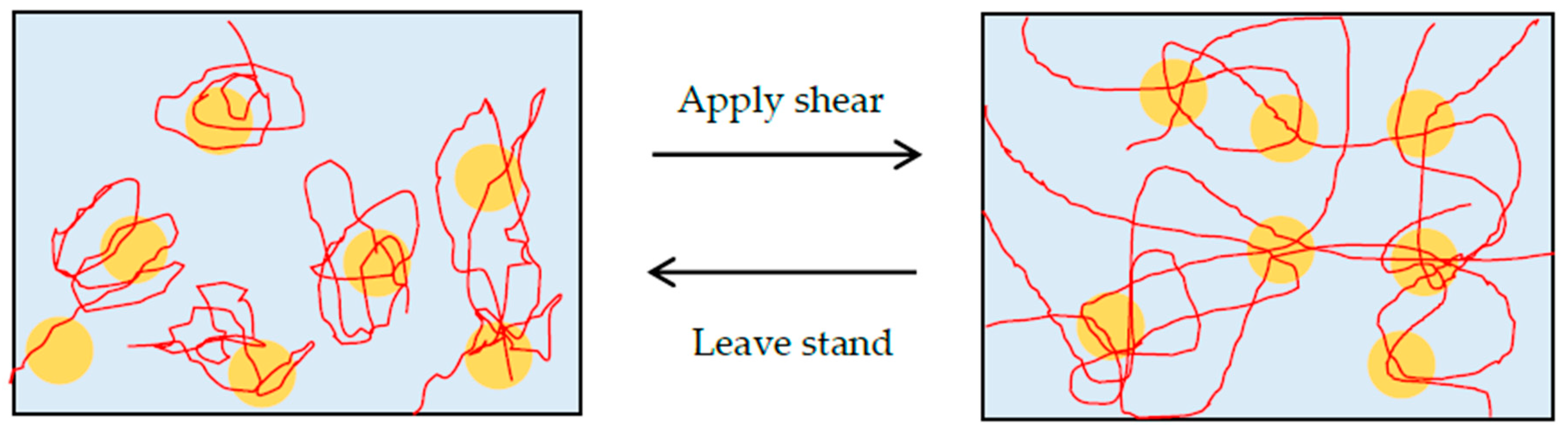
23], so many monomers of PEO can bind to the silica surface. Before shaking, the PEO is adsorbed to the surface of silica, and the conformation remains random-coil-liked. Once a shear flow is applied to the suspension, the PEO is stretched to linear-liked or extended conformation by the shear flow. Stretched PEO polymers can adsorb to other silica particles. Consequently, transient PEO bridges between silica particles and whole network are formed (
Figure 3). We discuss the effect of
Cp, pH, and molecular weight of PEO to the transient PEO bridges below in more detail.
pH changes the surface charge and zeta potential of silica via the protonation and de-protonation of silanol groups on the silica surface [
24,
25,
26]. The isoelectric point (IEP) of silica is about pH 2. Silica particles have low negative zeta potential around pH 2, and the magnitude of zeta potential increases with increasing pH. At IEP, no electrical repulsion exist between silica particles, and the adsorbed PEO amount is high [
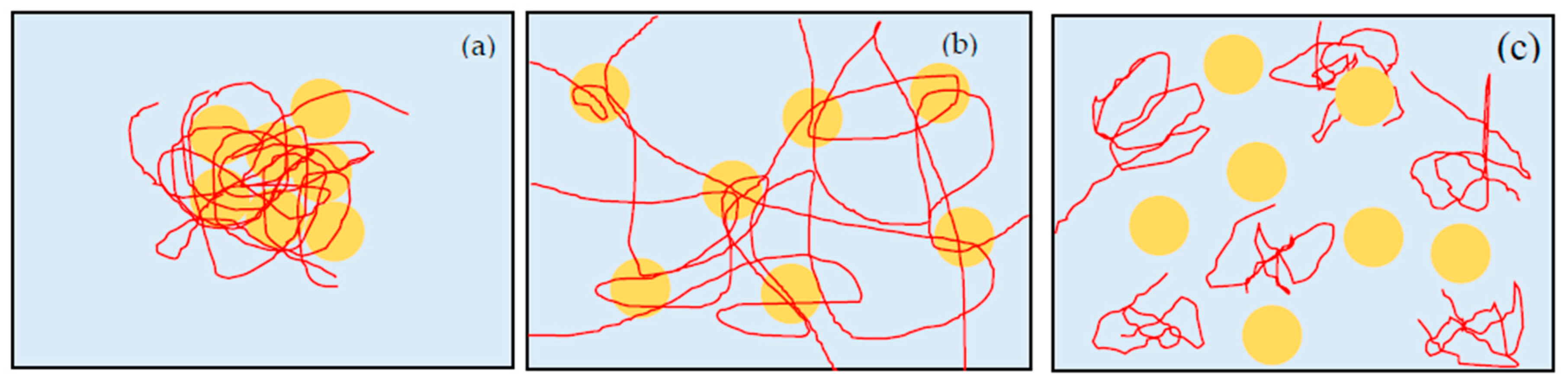
15]. Therefore, PEO binds the particles strongly, and the particles get together to make dense flocs (
Figure 4a). We can see large whity flocs in this suspension. For suspensions at pH below 7, even though the zeta potential magnitude and electrical repulsion increase with pH, the repulsion is smaller than the bridging attraction, and is not enough to form gel state [
18]. When pH increases to about 8, the electrical repulsion increases further. Therefore, silica particles do not form dense flocs but keep an appropriate distance each other. At this condition, PEO polymers make bridges among the particles to create gel network, and the suspension turn to the gel state. However, the adsorption of PEO is still strong and the gel network is too stable to be broken. Therefore, once the suspension turns to gel state, it cannot go back to sol state anymore. At about pH 9.4, the electrical repulsion increases more and the adsorbed amount of PEO decreases. Shear flow stretches the PEO polymers. However, once the shear flow disappears, PEO opts to return to random coil. Then, the gels return to sols because of the desorption of PEO and have a state of shake-gel (
Figure 4b). When pH increases above 10, the electrical repulsion is large and PEO adsorbed amount is quite low [
11,
15]. Hence, the suspension has a state of high viscosity sol, probably due to the increased free PEO concentration (
Figure 4c).
Cp is the dose of PEO polymer and also determines the state of suspensions. When we apply the shear flow to silica-PEO suspension, absorbed PEO is stretched and binds other silica particles by bridging effect. However, when
Cp is low, the amount of PEO chains is not enough to form a network in whole suspension to fix water molecules. Therefore, the suspension turns to the cloudy state after shaking (
Figure 5a). With the increase of the PEO, the number concentration of polymer bridges increases. PEO bridges make a strong network among the flocs, and the motion of water is limited in the network. Suspensions in this condition show a state of gel (
Figure 5b). If the PEO concentration increases further, the adsorbed amount of PEO increases and the adsorption sites of silica surface are occupied by PEO polymers. It means that the available adsorption site for bridging polymers on silica surface decreases, and PEO chains are difficult to make bridges between the particles. Therefore, neither flocs of particles nor the gel network is formed, and thus the suspension remains as high viscosity sol state (
Figure 5c). The viscosity is high because of the high mass fraction of PEO in the bulk [
27]. However, the effect of
Cp seems to be influenced by pH, as if pH is low, the suspension trends to get cloudy instead of gel or high viscosity sol, though
Cp is high. If pH is very high, almost all the suspensions tend to be high viscosity sol, regardless of
Cp. We consider that it is because pH influences the adsorbed amount of PEO. At low pH, even though the dose is high, almost the PEO adsorbed to silica surface strongly and form flocs, because the dose is smaller than the saturation [
15]. However, at very high pH, the adsorbed amount is low, regardless of
Cp. Therefore, the suspensions have a trend to be high viscosity sol.
The molecular weight of PEO relates to the number of monomers in one polymer, hereby influences the state of suspensions. At determined condition of pH and
Cp, the state of suspension changes from cloudy to gel, and then to high viscosity sol. For 400 kDa PEO, the number of monomers in a PEO polymer is low. Therefore, the number of hydrogen bonds between PEO and silica per polymer chain is low. The radius of gyration
Rg is 35.9 nm, which is almost the same as the size of the silica particle [
18,
28]. This makes one polymer able to bind only a few particles. Furthermore, short polymers are difficult to form network among the whole suspension, so the particles trend to aggregate to flocs, and the suspensions become cloudy. For 1000 and 4000 kDa PEO polymers, the
Rg is about 61.0 nm and 136.3 nm, respectively, and larger than silica particles size, so that one PEO can bind more particles. The number of hydrogen bonds per PEO chain is also high. Therefore, high molecular weight polymers promote the formation of gels and inhibit the relaxation of gels. Nevertheless, we should keep in mind the effect of pH. Too low pH may make the bridging effect too strong and induces cloudy suspensions. As a result, at most cases around pH 7.2, the suspensions with 1000 kDa PEO form permanent gels, but the suspensions with 4000 kDa PEO form cloudy sols.
3.2. Photos and the Relaxation Time of Suspensions
We took photos of the suspensions from immediately after the shaking to 24 h to confirm the temporal change of state of suspensions (
Figures S1–S5). The state of the suspensions can be roughly classified into sol and gel. In this paper, we focus on the direct observation of the gelled ones.
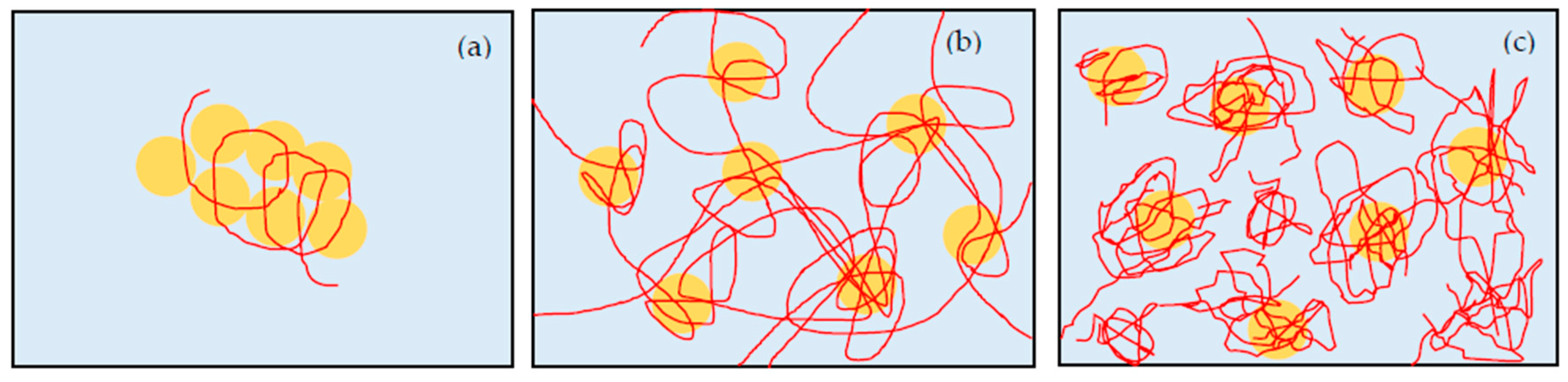
In the relaxation process, we can find some obvious changes in the states of suspensions. Immediately after the shaking, the suspensions show a state of gel. For gelled suspensions, the elasticity of gel can support their weight themselves, so the interface between gel and air can make a large angle or even be vertical to the ground (
Figure 6a). As time passes, the gels begin to relax, and the angle between interface and ground decreases. In this regime, the gels keep sticking to the upper wall of test tube (
Figure 6b). Then, the elasticity of gel decreases again in time, and the silica-PEO mixture becomes no longer able to support the weight. Therefore, gels slide or flow down from the upper wall of test tubes (
Figure 6c). After this regime, the angle between interface and ground decreases continuously and finally becomes horizontal (
Figure 6d). Measuring the relaxation time of the suspensions can help us to understand the effect of pH and
Cp. However, it is difficult to measure the time when the interface becomes horizontal correctly. Thus, in this study, we define the condition that the gel sticks to the half of the bottom of test tube as a relaxed condition (
Figure 6c). The time from finishing the shaking to the relaxed condition is defined as a relaxation time. For suspensions relaxing in 1 h, the time interval of photos is 3 s. For suspensions whose relaxation time is more than 1 h, the time interval of photo is 30 s. We obtained the relaxation time from these photos and summarized the results in
Figure 7 and
Figure 8.
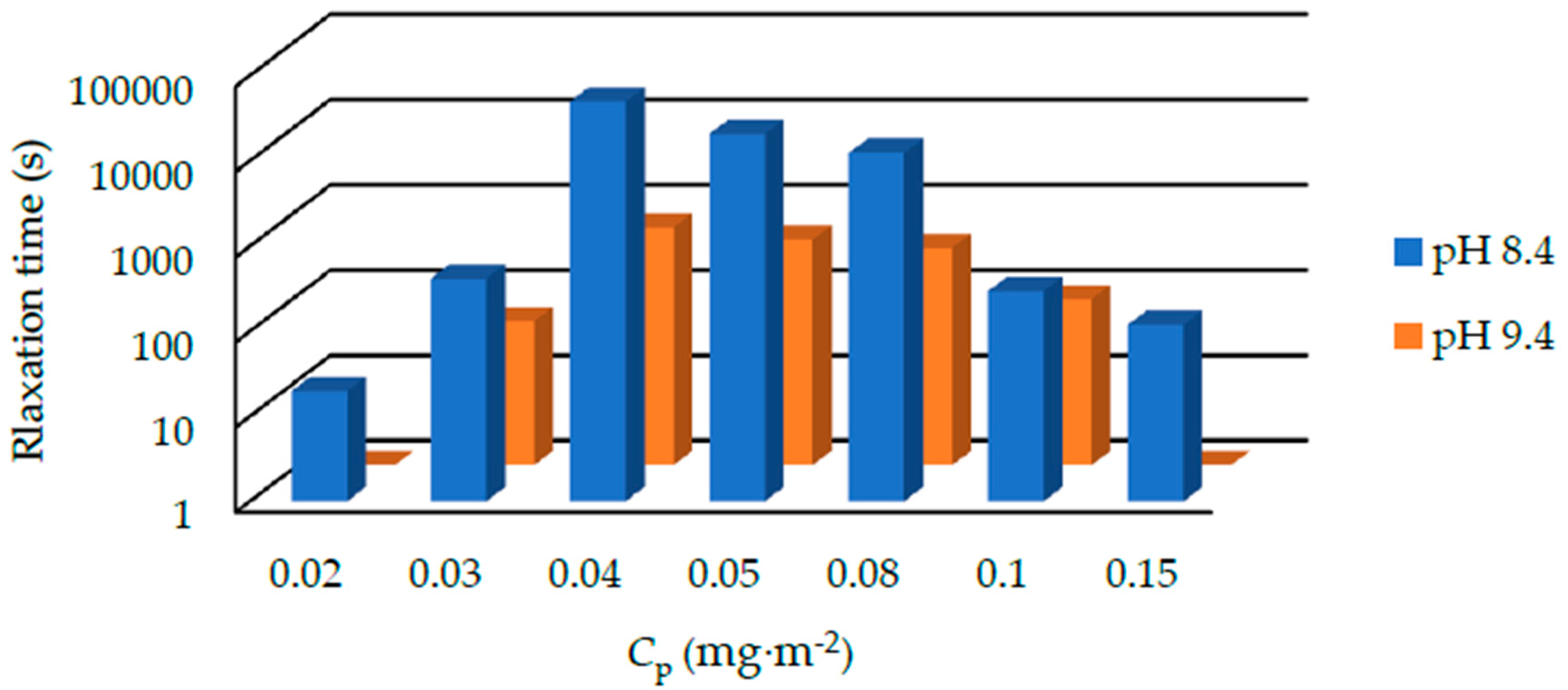
The relaxation time of suspensions with 1000 kDa PEO under the pH of 8.4 and 9.4 is shown in
Figure 7. The relaxation time varies from several minutes to several hours. Generally, the relaxation time at pH 9.4 is shorter than that at pH 8.4. We also summarized the results of suspensions containing 400, 1000, and 4000 kDa PEOs at pH 9.4 in
Figure 8. For 400 kDa silica-PEO suspensions, we see all the relaxation times are several seconds. It is too short to measure the relaxation time of most of the suspensions correctly. The relaxation time of suspensions with 1000 kDa PEO is longer. For 4000 kDa suspensions, the relaxation time is very long. In both the conditions of pH 8.4 and 9.4, and all the condition of suspensions with 400, 1000, and 4000 kDa PEOs, we can observe that the relaxation time increases to the maximum at first and then decreases with
Cp. This trend cannot be noted just from the state diagram.
As for a reason of the effect of pH on the relaxation time of shake-gel, we consider that this is due to the change in the adsorbed amount of PEO and electrical repulsion between silica nanoparticles. When pH is low, the adsorbed amount of PEO is high [
15] and the electrical repulsion between silica nanoparticles is low [
24]. Therefore, the bonds of PEO and silica are difficult to break up, and silica nanoparticles are hard to be dispersed. Consequently, the relaxation time at pH 8.4 is longer than that at pH 9.4.
Under the same pH and
Cp, we can see that the relaxation time of silica-PEO suspensions increases with the molecular weight of PEO. PEO with larger molecular weight has long loop and tail of adsorbed chains. Long loop and tails mean that the polymer can attach silica at more points and trap more particles, and a stronger network is formed. Therefore, the binding between PEO and silica nanoparticles and the gel network are more stable. Additionally, stretched chains with higher molecular weight under exerted force are calculated to relax slowly to equilibrium after the force is released [
29]. Adam et al. [
30] reported that large molecular weight polymers have a long rheological relaxation time from their experiments. These mean that large polymers need more time to return to random coil state. For these reasons, the suspensions with higher molecular weight PEO have a long relaxation time. On the contrary, the PEOs with molecular weight of 400 kDa have a shorter loop of monomers. Thus, the gel is difficult to form and the relaxation time is shorter.
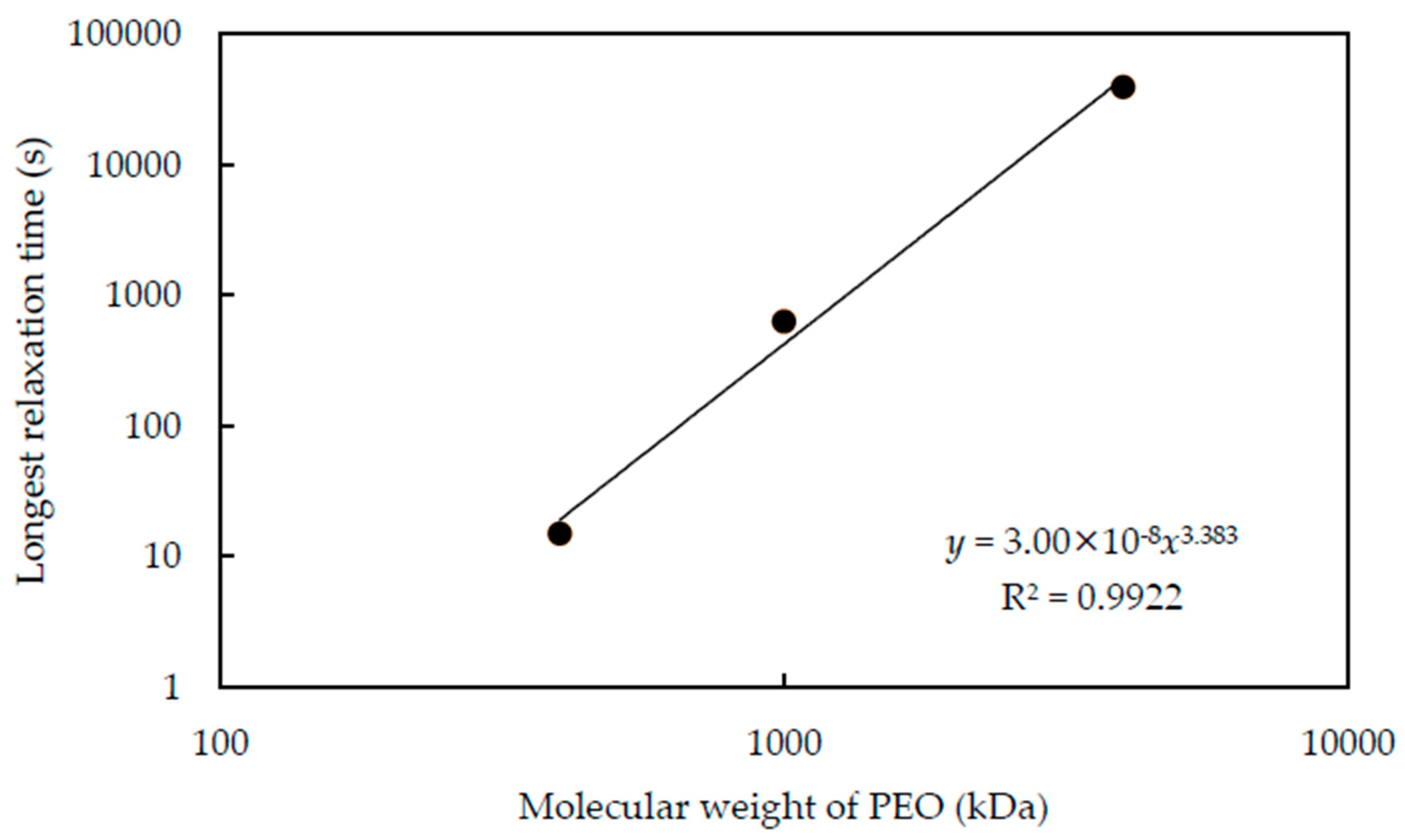
While the relaxation time changes with the pH and
Cp, it is interesting to discuss the relationship between longest relaxation time and molecular weight. We summarize the longest relaxation time of each molecular weight PEO at pH 9.4 in
Figure 9. We can confirm that the longest relaxation time increases with the increasing molecular weight of PEO and follows the power-law relationship. The power is 3.38, which is almost the same as the power for the relationship between molecular weight and the relaxation time of polymeric fluids [
31]. This means that the polymers are constrained by entanglement [
31] as well as adsorption.
Cp also influences the adsorbed amount and thus changes the amount of available adsorption site of silica surface. When Cp is low, increasing PEO dose promotes the bridges between particles. The gel structure gets more stable, and the relaxation time increases and reaches the maximum. If Cp increases further, the available adsorption sites on the silica surface decrease. This makes it difficult for each polymer to form bridges among many particles. In this condition, PEO polymers are easier to desorb from particle surface, because of fewer hydrogen bonds. Thus, the relaxation time decreases. Furthermore, we find that the longest relaxation time occurs at around Cp = 0.04–0.05 mg∙m−2 irrespective of pH and the molecular weight of PEO. This is such an interesting result, that PEO dose per surface area seems to be the most important factor to determine the relaxation of shake-gel. This also supports the hypothesis that a sufficient amount of the available adsorption site is necessary to build and keep the bridges between particles. Again, this discussion is now possible from our novel observation on the relaxation and was impossible from only the state diagram.
Moreover, we consider the measurement of viscoelasticity as a better method to evaluate the gelation and relaxation. We will conduct some experiments about viscoelasticity in future research. Before such measurements, our results are convenient as a fingerprint of the states.
The photos used to confirm the states and calculate the relaxation time are summarized in the Supporting Information (
Figures S1–S5).
3.3. The Results of Viscosity Measurement
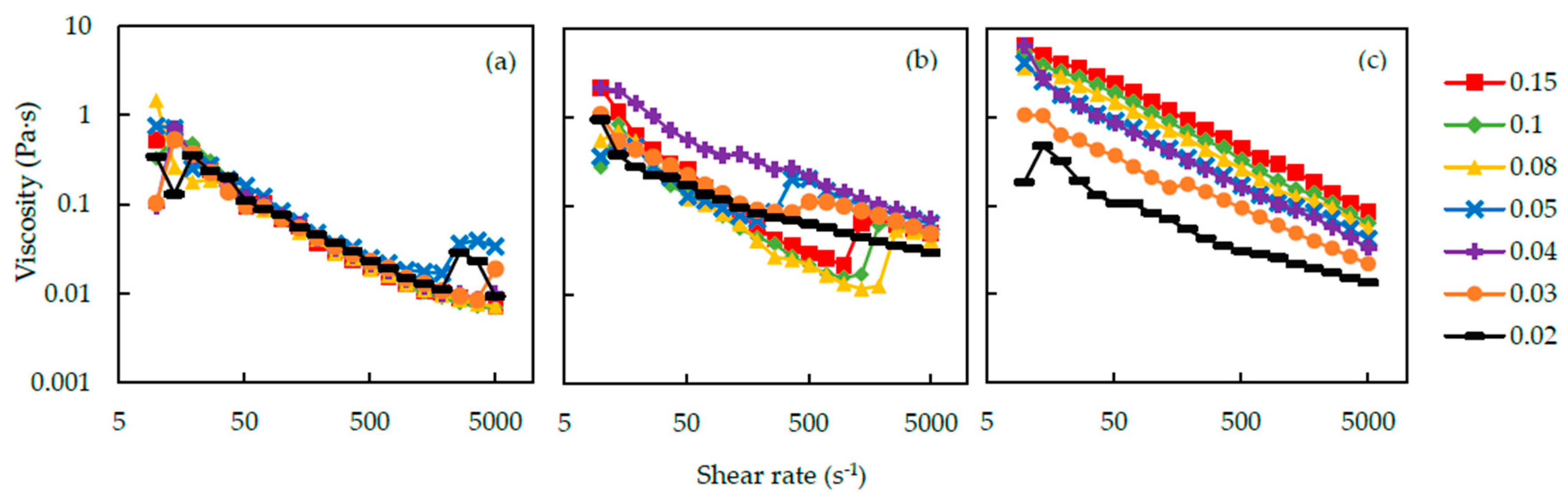
Figure 10 shows the relation between viscosity of the silica-PEO suspensions and shear rate. The conditions of these suspensions are (a) 400 kDa and pH 9.4, (b) 1000 kDa and pH 9.4 and (c) 1000 kDa and pH 10. In all of the figures, the viscosity decreases with the increasing shear rate at first. This phenomenon is called shear-thinning [
31]. Nevertheless, particular suspensions show an increase of viscosity in the process of increasing shear rate. We call this phenomenon shear-thickening. Because the viscosity increase occurs in a gelation process, we consider the shear-thickening phenomenon as a symbol of gelation induced by shear flow. Under the condition of PEO molecular weight 1000 kDa and pH 9.4 in
Figure 10(b), the suspensions seem to show the shear-thickening phenomenon. At first, the viscosity of the suspensions decreases with the increase of shear rate. At a critical shear rate, however, the viscosity of suspensions increases sharply, and at the same time, the gelation of the suspensions was observed. When the shear rate reaches the critical shear rate, it also takes some time for the suspensions to form gel [
11]. When the PEO concentration is not too high, shear flow stretches the PEO chains to adsorb to multiple particles, because of the large amount of bare silanol group on the surface of silica particle (
Figure 5b). Polymer bridges connect and trap the particles and increase the size of particles’ clusters. Therefore, the viscosity increases. After this increase in viscosity, high shear rate breaks the clusters and the viscosity decreases again. With increasing the concentration of PEO, more PEO polymers are adsorbed to the surface of silica. This induces the decrease of the available adsorption sites of silica. Therefore, PEO chains are difficult to bridge between silica particles, and a higher shear rate is needed to form a gel network (
Figure 5c).
If we look into the result with the PEO molecular weight to 400 kDa, we can observe obvious differences in the viscosity against the shear rate results (
Figure 10a). The suspensions with 400 kDa PEO have lower viscosities, and higher shear rate is needed to cause the shear-thickening. The chain length and
Rg of the 400 kDa polymer are smaller [
28]. Smaller polymers can only bind fewer particles. Particle clusters become smaller, and thus, the viscosity is lower than that of 1000 kDa silica-PEO suspensions. The viscosity of the suspension with 4000 kDa PEO could not be measured, because the gel was easily formed and could not be set up on the viscometer.
For the suspension with 1000 kDa PEO, when the pH of the suspension increased to 10, almost no shear-thickening phenomenon happened (
Figure 10c). At high pH, the adsorbed amount of PEO on silica decrease rapidly [
15] and PEO bridges over silica particles are difficult to form. Therefore, the shear-thickening did not occur, and no shake-gel phenomenon was observed at pH 10. The viscosity of suspensions increases with
Cp. Furthermore, compared to the viscosity at pH 9.4, the viscosity at pH 10 is higher overall. A previous study [
19] reported similar results and advocated that longer distance between bridged silica nanoparticles due to the strong electrical repulsion at high pH is the reason of this phenomenon. We also think that high viscosity at high pH is induced by the decrease in the adsorbed amount of PEO and a large amount of PEO polymers remained in the medium.
We also find that through the change of
Cp, the suspension at
Cp = 0.04 mg∙m
−2 has the highest viscosity among all of the shake-gelled suspensions. This agrees with the longest relaxation time in
Section 3.2 and may indicate that this is the best
Cp condition for stable gel. Furthermore, the suspensions gel in the viscosity measurement process, mainly after the increase of viscosity. Therefore, because of the slipping of gels on the plate of the viscometer, the viscosity is possible to be incorrect after the sudden increase of viscosity. We plan to measure the dynamic viscoelasticity of the gels in future studies to solve this problem.