Influence of Genipin Crosslinking on the Properties of Chitosan-Based Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
2.2.1. Preparation of Chitosan Films
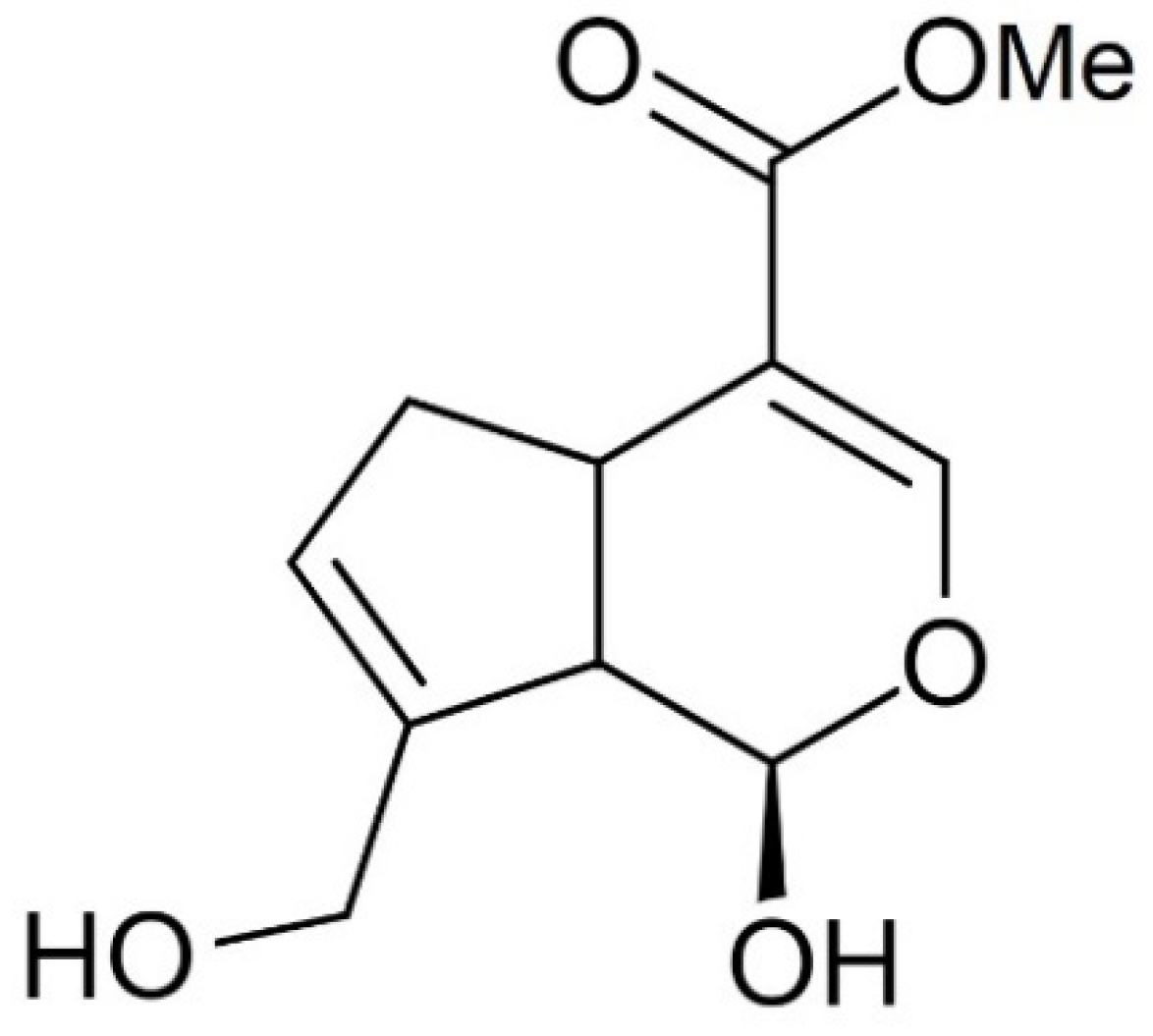
2.2.2. Preparation of Genipin-Crosslinked Films (Сhitosan-Gp)
2.2.3. Spectrophotometric Studies
2.2.4. Swelling Studies
2.2.5. Sorption Measurements
2.2.6. Mechanical Property Measurements
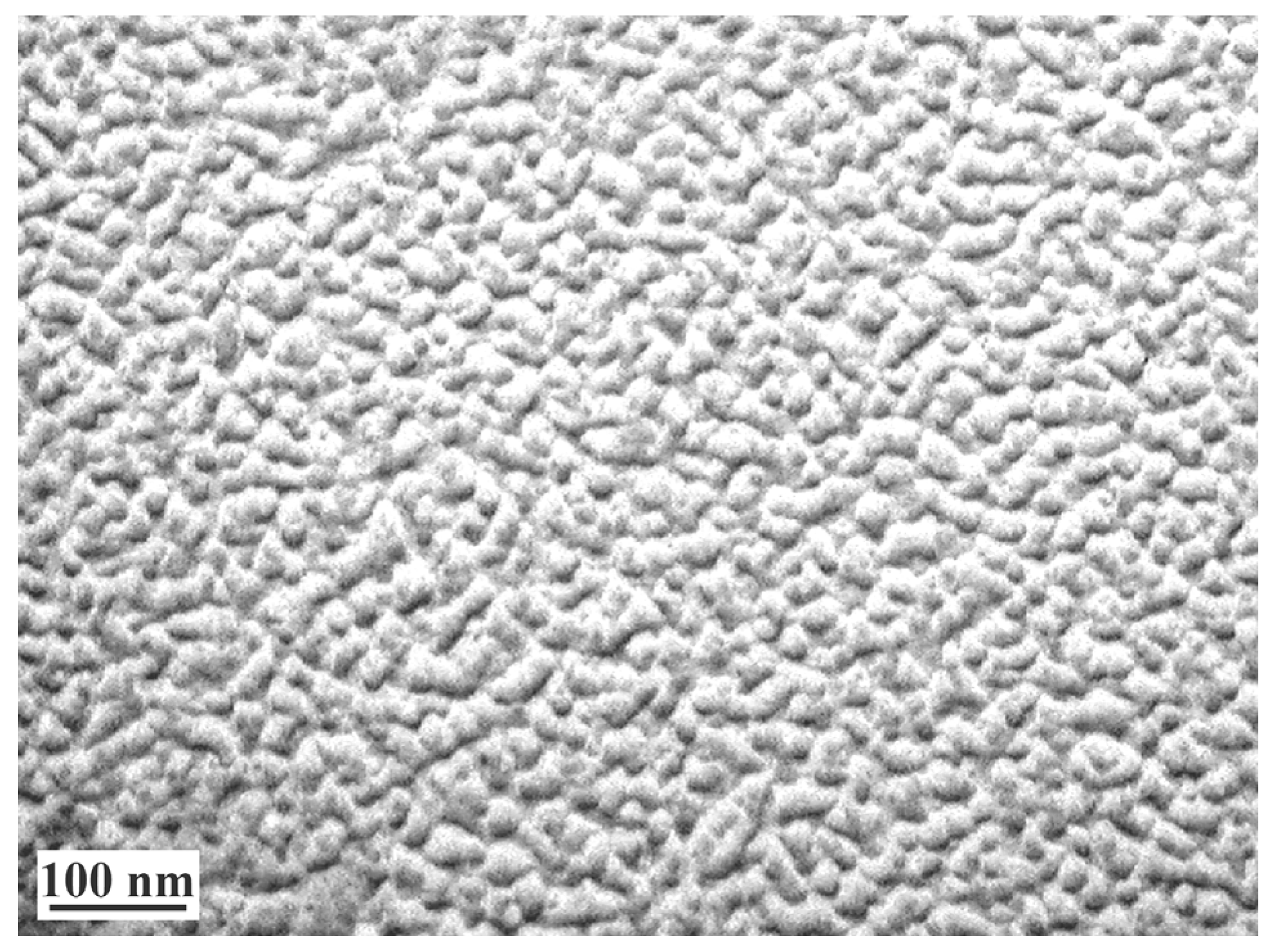
2.2.7. Structural-Morphological Studies
3. Results and Discussion
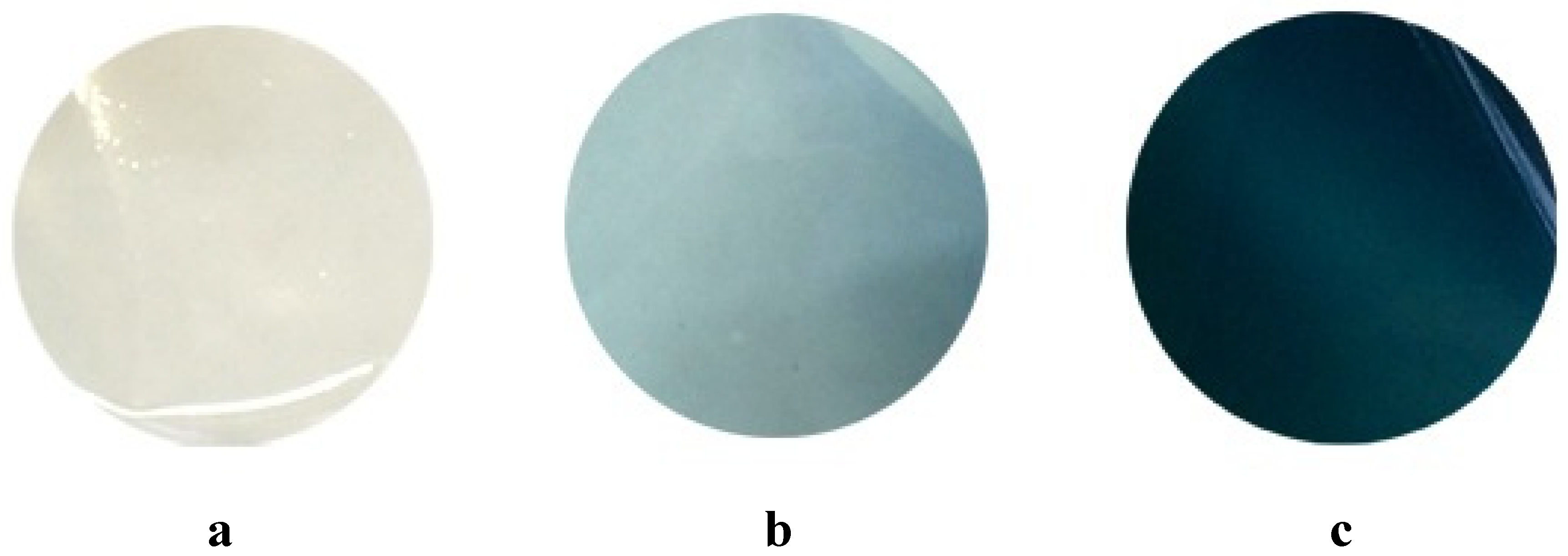
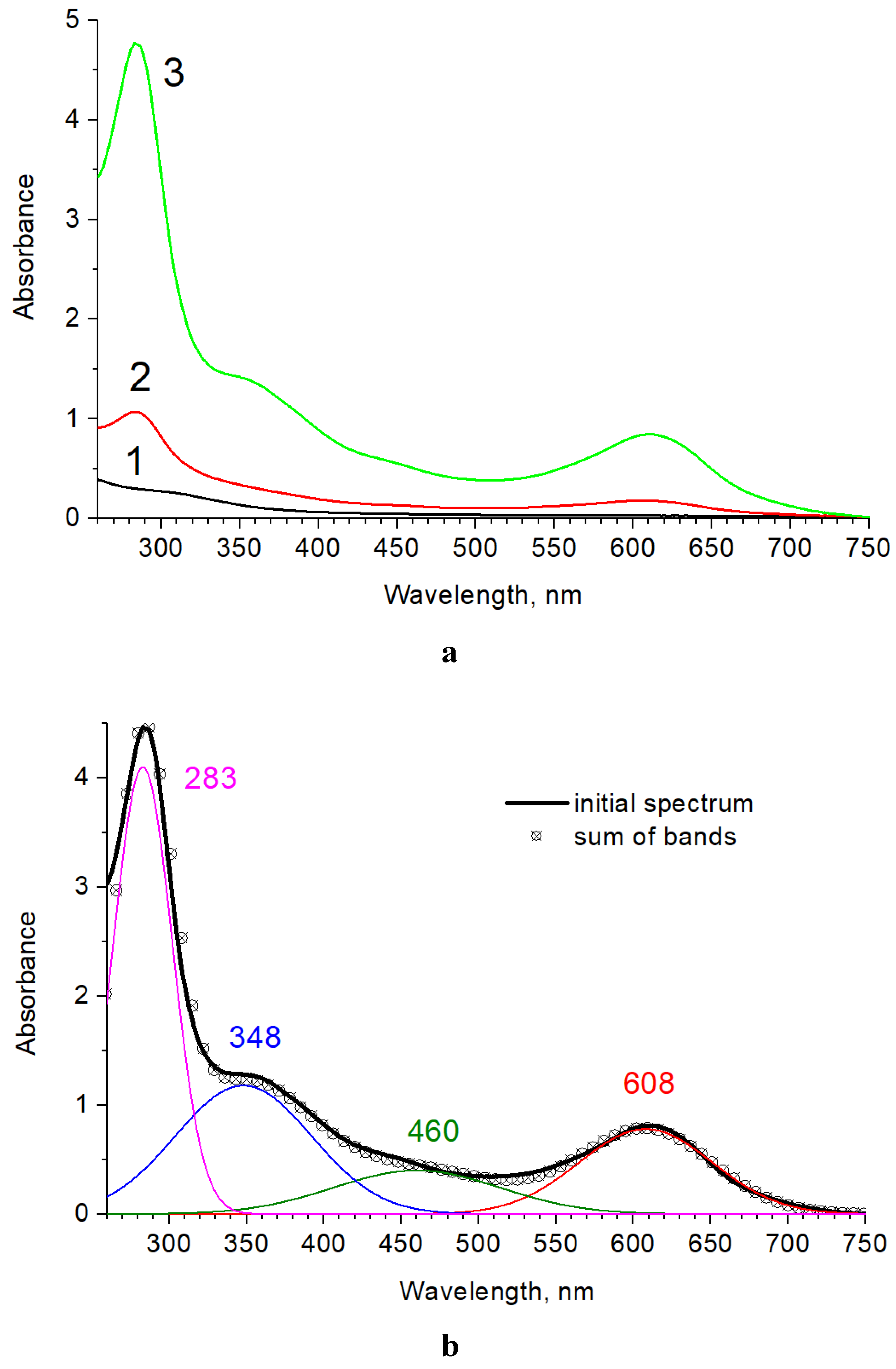
3.1. Optical Properties of the Gp Crosslinked Chitosan Films
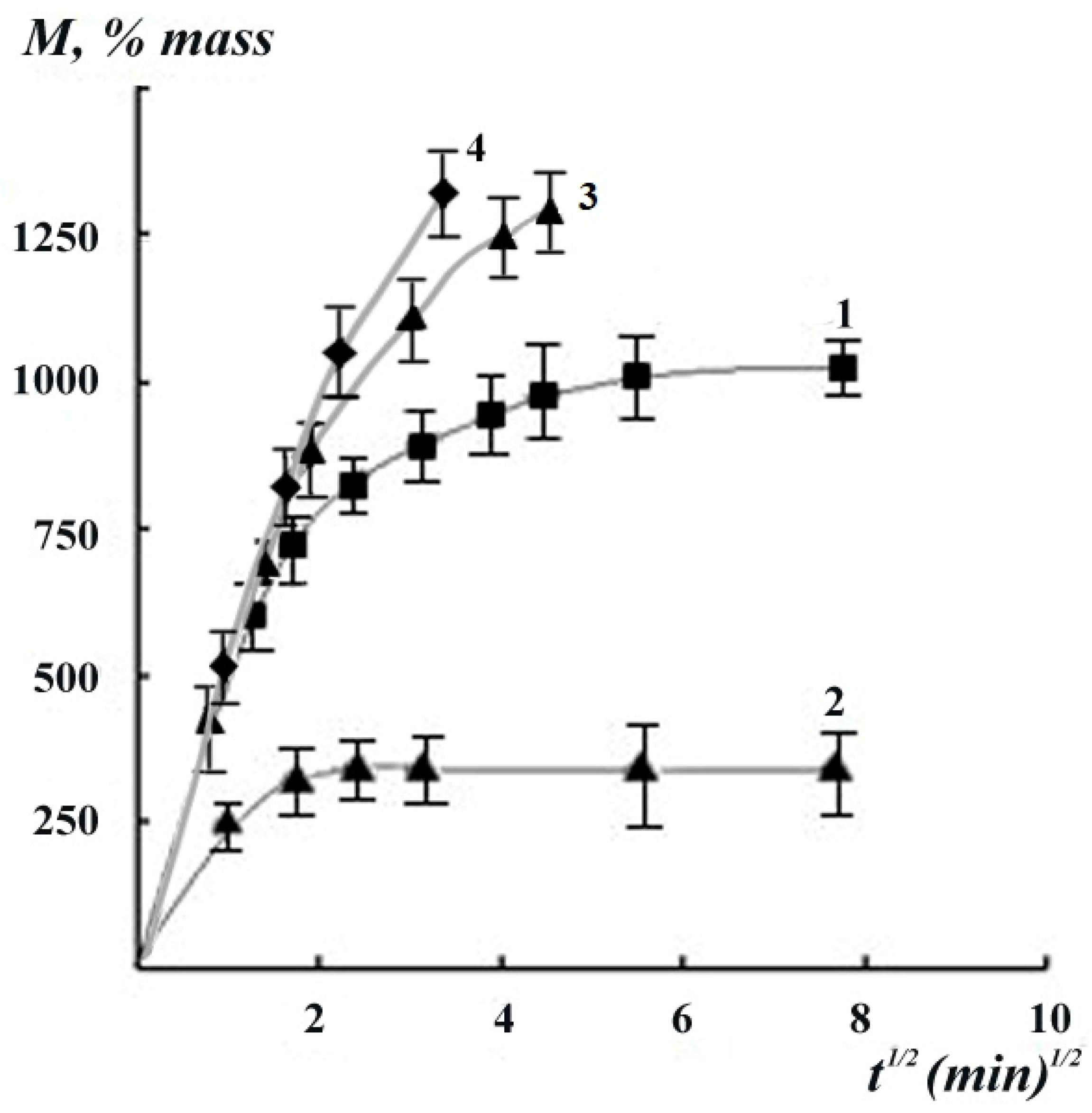
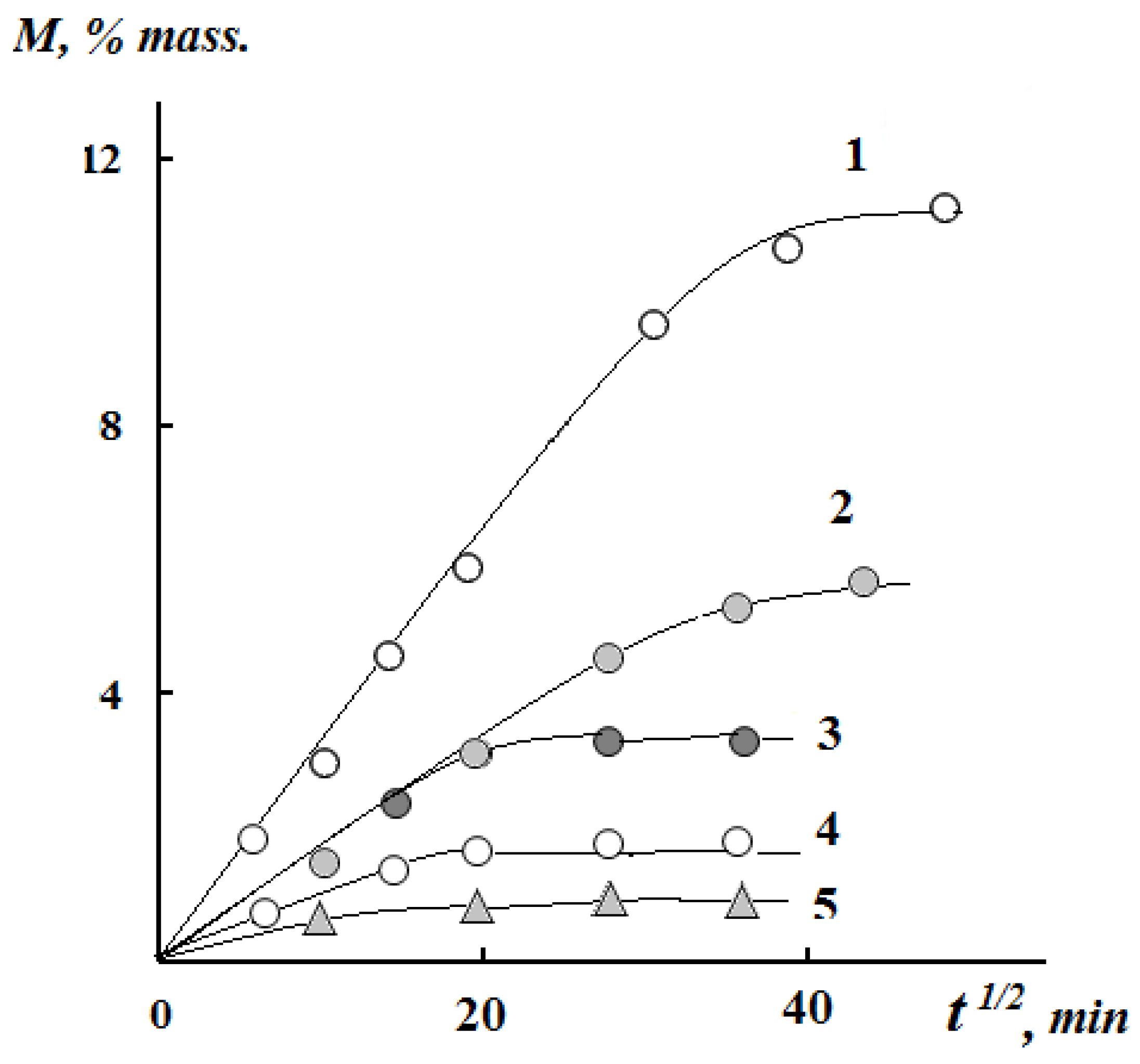
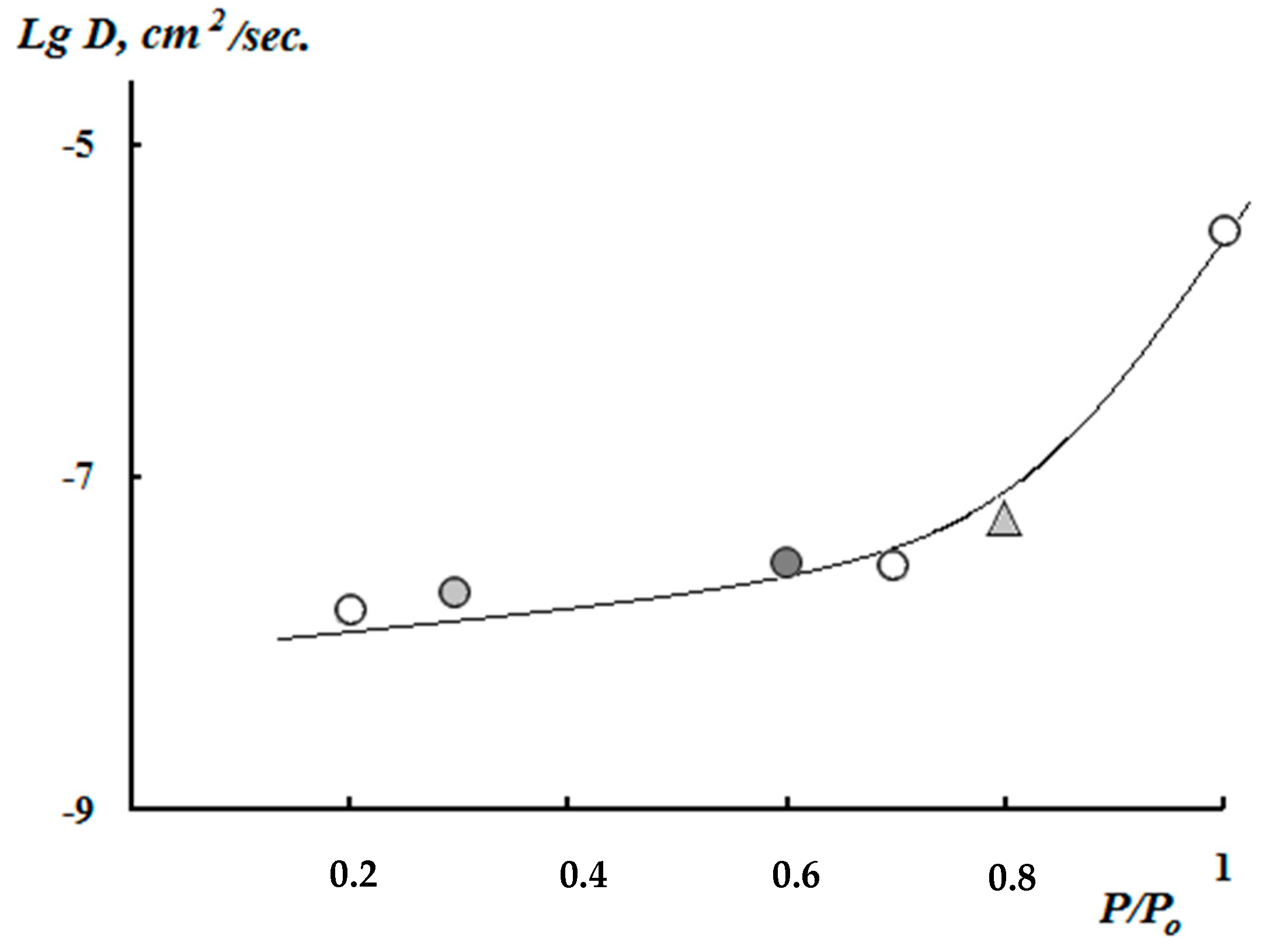
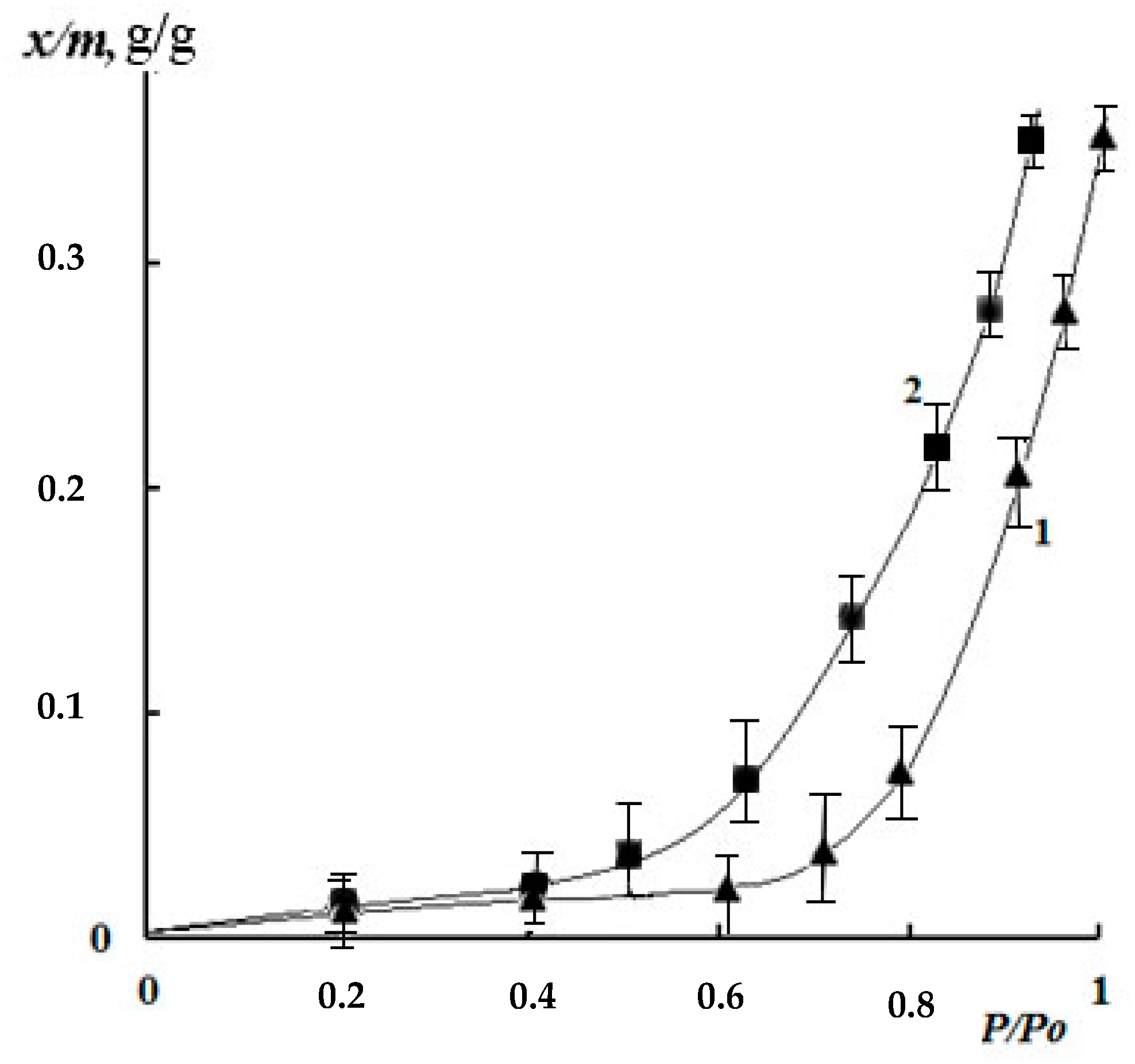
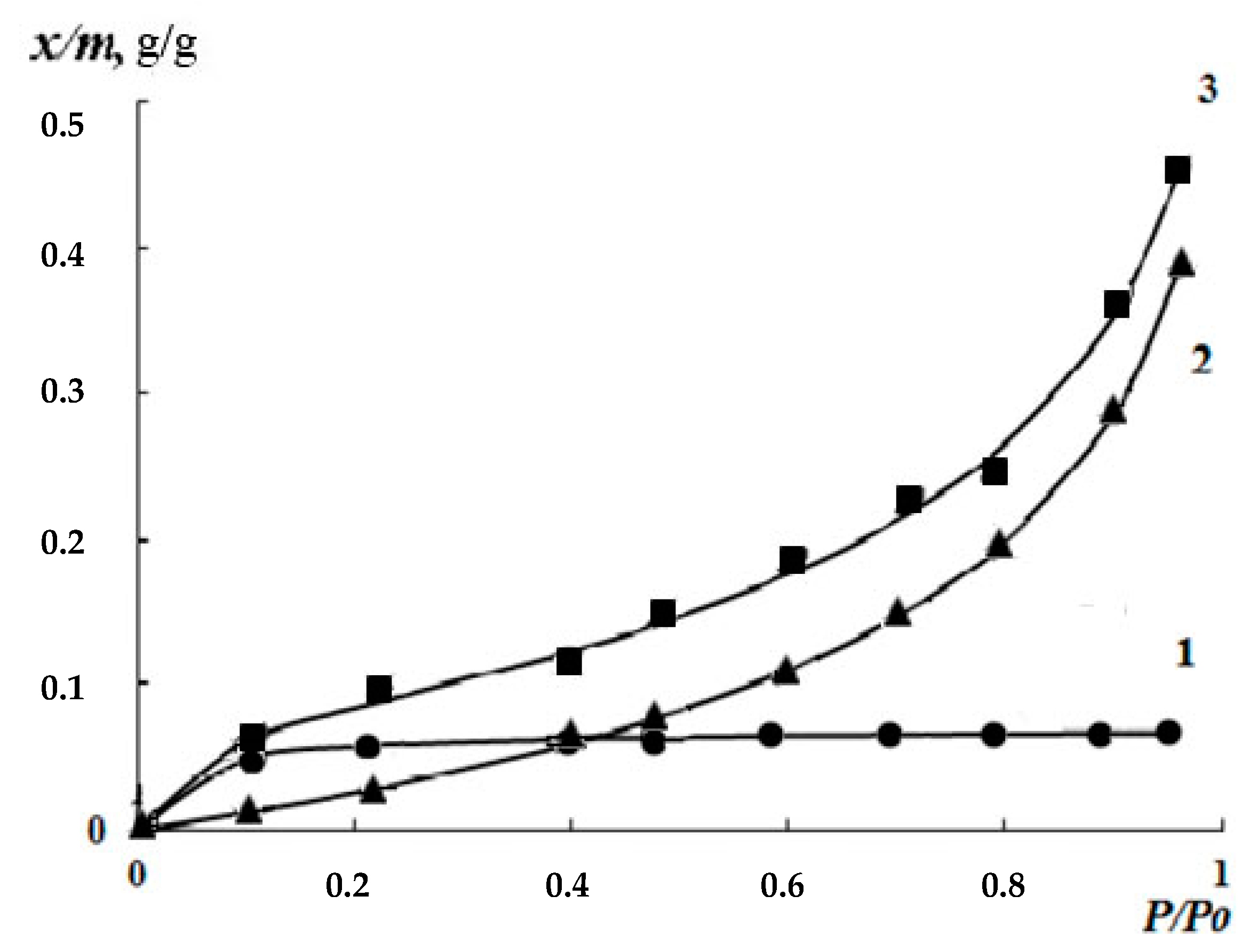
3.2. Kinetics of the Swelling and Water Vapor Sorption by the Films
3.3. Mechanical Properties of Gp-Crosslinked Chitosan Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jung, W.-J.; Park, R.-D. Bioproduction of chitooligosaccharides. Review. Mur. Drugs 2014, 12, 5328–5356. [Google Scholar] [CrossRef]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. Lwt-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, R.A.J.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Bhattacharya, S.; Nandy, A.; Bhattacharyya, A.; Mishra, R.; Kundu, P.P. Assessment of in vivo chronic toxicity of chitosan and its derivates used as oral insulin carriers. Toxicol. Res. 2015, 4, 281–290. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide Based Scaffolds for Soft Tissue Engineering Applications. Polymers 2019, 11, 1. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Delivery Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J. Trauma 2006, 60, 655–658. [Google Scholar] [CrossRef]
- Strand, S.P.; Tømmeraas, K.; Vårum, K.M.; Østgaard, K. Electrophoretic light scattering studies of chitosans with different degrees of N-acetylation. Biomacromolecules 2001, 2, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Preparation and evaluation of ammonia-treated collagen/chitosan matrices for liver tissue engineer-ing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kil’deeva, N.R.; Kasatkina, M.A.; Mikhailov, S.N. Peculiarities of obtaining biocompatible films based on chitosan cross linked by genipin. Polym. Sci. Ser. D 2017, 10, 189–193. [Google Scholar] [CrossRef]
- Kildeeva, N.R.; Perminov, P.A.; Vladimirov, L.V.; Novikov, V.V.; Mikhailov, S.N. About mechanism of chitosan crosslinking with glutaraldehyde. Russ. J. Bioorganic Chem. 2009, 35, 360–369. [Google Scholar] [CrossRef]
- Devi, D.A.; Smitha, B.; Sridhar, S.; Aminabhavi, T.M. Pervaporation separation of isopropanol/water mixtures through crosslinked chitosan membranes. J. Membr. Sci. 2005, 262, 91–99. [Google Scholar] [CrossRef]
- Zeng, Х.; Ruckenstein, E. Crosslinked macroporous chitosan anion-exchange membranes for protein separations. J. Membr. Sci. 1998, 148, 195–205. [Google Scholar] [CrossRef]
- Vieira, R.S.; Beppu, M.M. Interaction of natural and crosslinked chitosan membranes with Hg (II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 196–207. [Google Scholar] [CrossRef]
- Lai, J.Y.; Li, Y.T.; Wang, T.P. In vitro response of retinal pigment epithelial cells exposed to chitosan materials prepared with different crosslinkers. Interna-Tional J. Mol. Sci. 2010, 11, 5256–5272. [Google Scholar] [CrossRef]
- Kil’deeva, N.R.; Kasatkina, M.A.; Drozdova, M.G.; Demina, T.S.; Uspenskii, S.A.; Mikhailov, S.N.; Markvicheva, E.A. Biodegradable scaffolds based on chitosan: Preparation, properties, and use for the cultivation of animal cells. Appl. Biochem. Microbiol. 2016, 52, 515–524. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Butler, M.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual Crosslinked Collagen/Chitosan Film for Potential Biomedical Applications. Polymers 2019, 11, 2094. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppa, N.A. Gurny. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Sazhnev, N.A.; Drozdova, M.G.; Rodionov, I.A.; Kil’deeva, N.R.; Balabanova, T.V.; Markvicheva, E.A.; Lozinsky, V.I. Preparation of chitosan cryostructurates with controlled porous morphology and their use as 3D-scaffolds for the cultivation of animal cells. Appl. Biochem. Microbiol. 2018, 54, 459–467. [Google Scholar] [CrossRef]
- Matcham, K. Novakovic. Fluorescence Imaging in Genipin Crosslinked Chitosan–Poly(vinyl pyrrolidone) Hydrogels. Polymers 2016, 8, 385. [Google Scholar] [CrossRef]
- Jin, J.J.; Song, M.; Hourston, D.J. Novel Chitosan-Based Films Crosslinked by Genipin with Improved Physical Properties. Biomacromolecules 2004, 5, 162–168. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; da Silva, J.A.L.; Coimbra, M.A. Chitosan–caffeic acid–genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Mi, F.-L.; Tan, Y.-C.; Liang, H.-C.; Huang, R.-N.; Sung, H.-W. In vitro evaluation of a chitosan membrane crosslinked with genipin. J. Biomater. Sci. Polym. Ed. 2001, 12, 835–850. [Google Scholar] [CrossRef]
- Frick, J.M.; Ambrosi, A.; Pollo, L.D.; Tessaro, I.C. Influence of Glutaraldehyde Crosslinking and Alkaline Post-treatment on the Properties of Chitosan-Based Films. J. Polym. Environ. 2018, 26, 2748–2757. [Google Scholar] [CrossRef]
- Silva, R.M.; Silva, G.A.; Coutinho, O.P.; Mano, J.F.; Reis, R.L. Preparation and characterisation in simulated body conditions of glutaraldehyde crosslinked chitosan membranes. J. Mater. Sci. Mater. Med. 2004, 15, 1105–1112. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, E.; Faridi-Majidi, R.; Shokrgozar, M.A.; Paskiabi, F.A. Genipin cross-linked electrospun chitosan-based nanofibrous mat as tissue engineering scaffold. Nanomed. J. 2014, 1, 137–146. [Google Scholar]
- Pomari, A.A.d.N.; Montanheiro, T.L.d.A.; de Siqueira, C.P.; Silva, R.S.; Tada, D.B.; Lemes, A.P. Chitosan Hydrogels Crosslinked by Genipin and Reinforced with Cellulose Nanocrystals: Production and Characterization. J. Compos. Sci. 2019, 3, 84. [Google Scholar] [CrossRef]
- González, A.; Strumia, M.C.; Igarzabal, C.I.A. Cross-linked soy protein as material for biodegradable films: synthesis, characterization and biodegradation. J. Food Eng. 2011, 106, 331–338. [Google Scholar] [CrossRef]
- Chalykh, A.E.; Petrova, T.F.; Khasbiullin, R.R.; Ozerin, A.N. Water sorption on and water diffusion in chitin and chitosan. Polym. Sci. Ser. A 2014, 56, 614–622. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Peng, C.K. Characterization of ring-opening polymerization of genipin and pH-dependent crosslinking reactions between chitosan and genipin. J. Polym. Sci. Part A 2005, 43, 1985–2000. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; p. 688. [Google Scholar]
| Ratio Gp/NH2, mol/mole | Genipin Solution Concentration, % | Genipin Content of the Molding Solution, % of Chitosan Mass |
|---|---|---|
| 0.02 | 0.24 | 1.5 |
| 0.003 | 0.036 | 0.225 |
| 0.0025 | 0.03 | 0.187 |
| 0.002 | 0.024 | 0.15 |
| Molar Ratio Gp/NH2 | Equilibrium Swelling Degree, % | Paired Interaction Parameter χ | Molecular Weight of the Chitosan Chains between the Mesh Nodes Мс, g/mole | Number of Elementary Links of Chitosan between Mesh Nodes nc | Number of Meshes per 100 Elementary Links of Chitosan |
|---|---|---|---|---|---|
| 0.003 | 1025 | 0.85 | 6934 | 42 | 2.7 |
| 0.02 | 340 | 0.99 | 1015 | 6 | 18.8 |
| Gp/NH2 Molar Ratio | Breaking Load, MPa | Breaking Elongation, % |
|---|---|---|
| 0 | 73 | 33 |
| 0.02 | 65 | 33 |
| 0.0030 | 55 | 25 |
| 0.0025 | 90 | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kildeeva, N.; Chalykh, A.; Belokon, M.; Petrova, T.; Matveev, V.; Svidchenko, E.; Surin, N.; Sazhnev, N. Influence of Genipin Crosslinking on the Properties of Chitosan-Based Films. Polymers 2020, 12, 1086. https://doi.org/10.3390/polym12051086
Kildeeva N, Chalykh A, Belokon M, Petrova T, Matveev V, Svidchenko E, Surin N, Sazhnev N. Influence of Genipin Crosslinking on the Properties of Chitosan-Based Films. Polymers. 2020; 12(5):1086. https://doi.org/10.3390/polym12051086
Chicago/Turabian StyleKildeeva, Nataliya, Anatoliy Chalykh, Mariya Belokon, Tatyana Petrova, Vladimir Matveev, Evgeniya Svidchenko, Nikolay Surin, and Nikita Sazhnev. 2020. "Influence of Genipin Crosslinking on the Properties of Chitosan-Based Films" Polymers 12, no. 5: 1086. https://doi.org/10.3390/polym12051086
APA StyleKildeeva, N., Chalykh, A., Belokon, M., Petrova, T., Matveev, V., Svidchenko, E., Surin, N., & Sazhnev, N. (2020). Influence of Genipin Crosslinking on the Properties of Chitosan-Based Films. Polymers, 12(5), 1086. https://doi.org/10.3390/polym12051086







