Effects of Magnesium Oxide (MgO) Shapes on In Vitro and In Vivo Degradation Behaviors of PLA/MgO Composites in Long Term
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
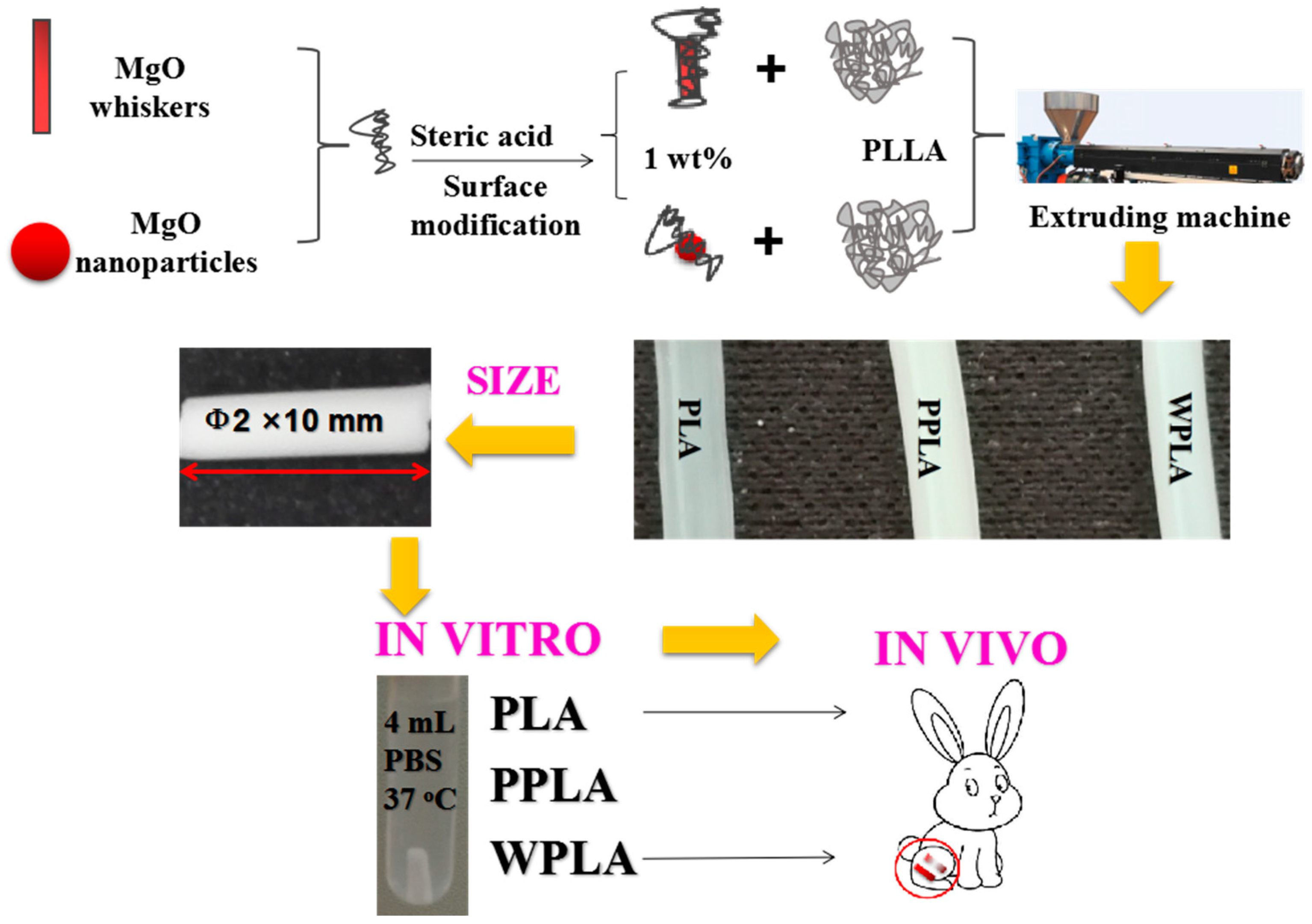
2.2. Preparations and Modifications of pMgO and wMgO
2.3. Preparation of PLA/pMgO and PLA/wMgO Composites
2.4. In Vitro Degradation in PBS
2.5. Characterization
2.6. In Vivo Experiment
2.6.1. Animal Models
2.6.2. Routine Pathological Examinations
3. Results
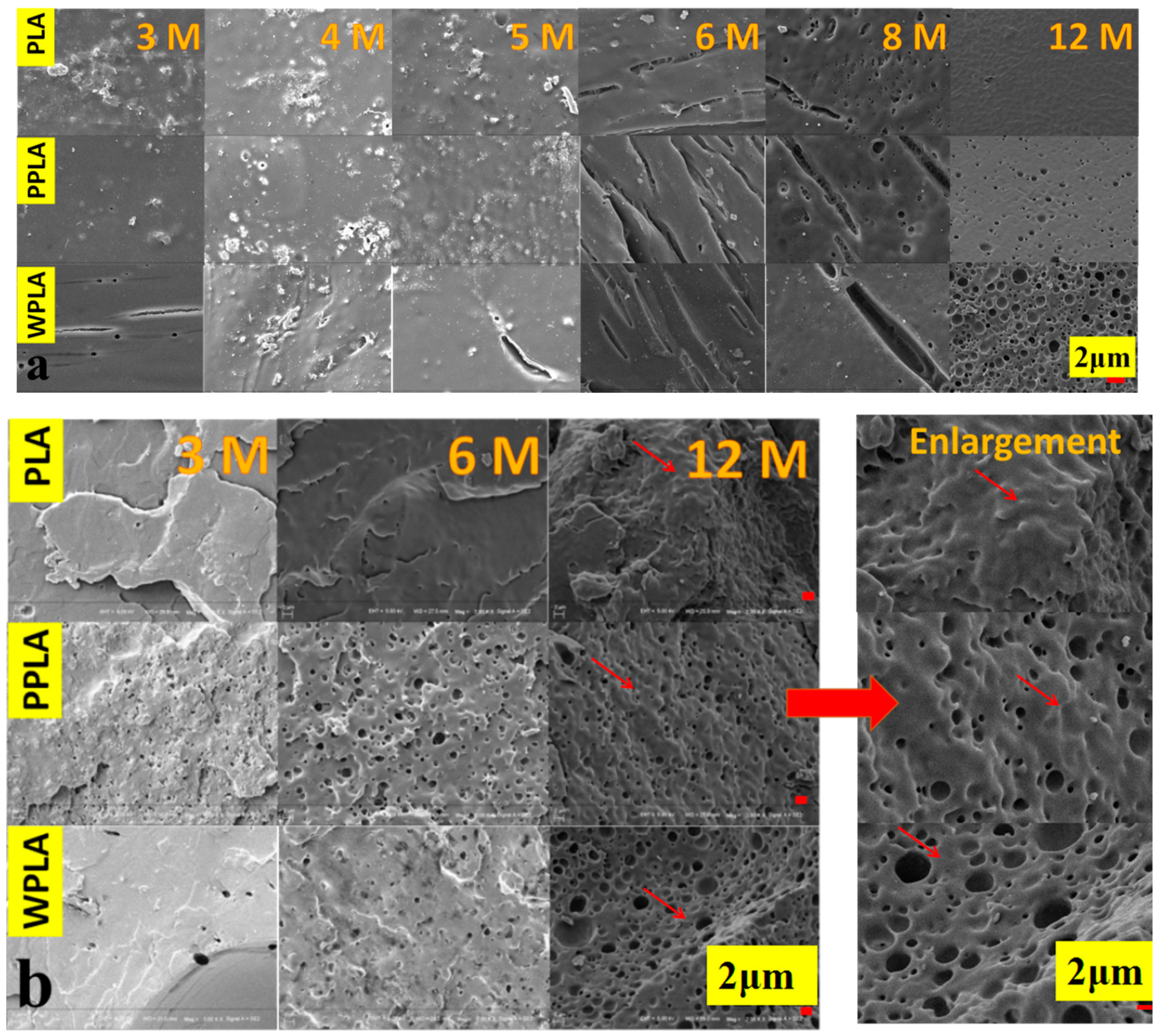
3.1. Microstructures of In Vitro Degradation of PLA and Composites
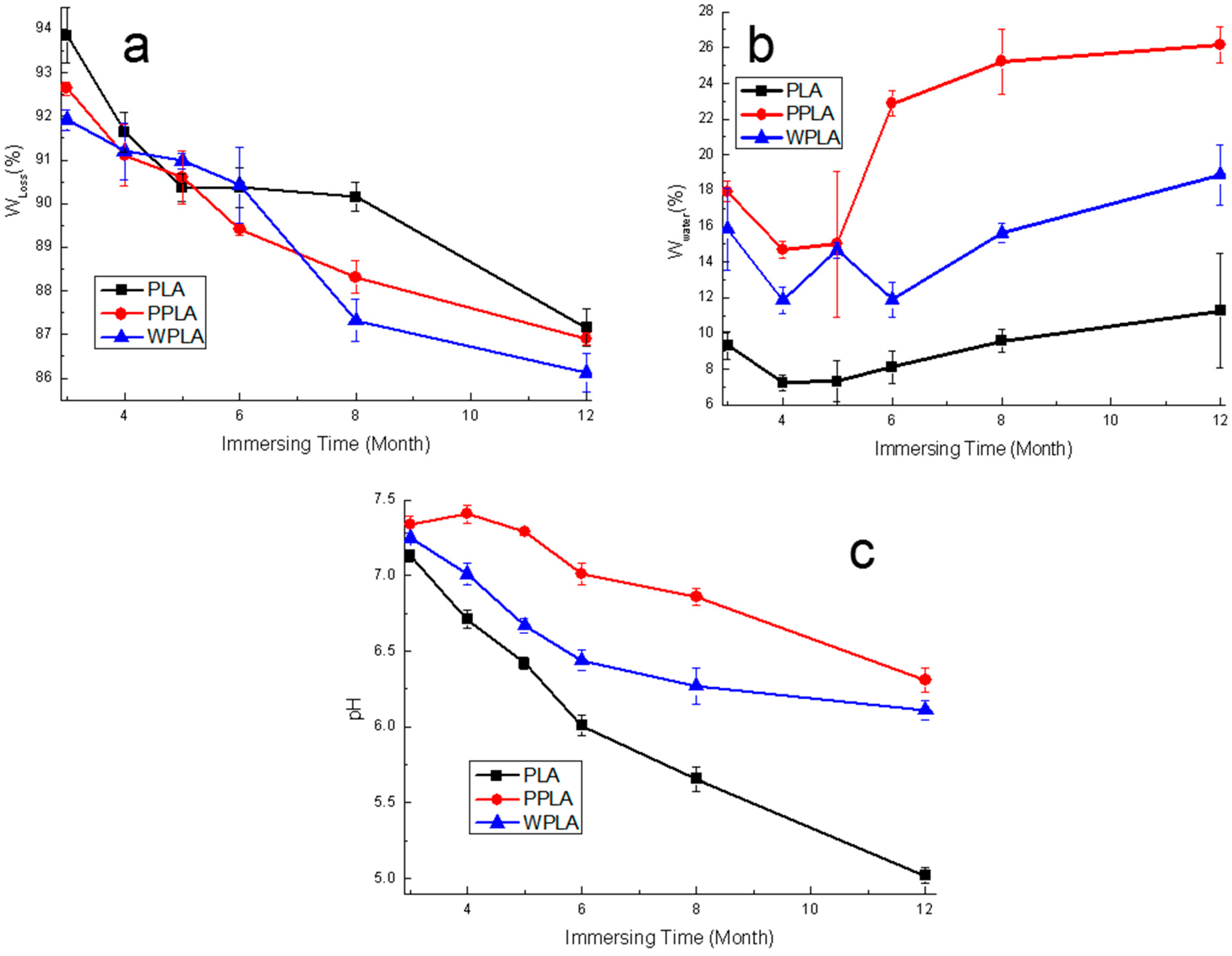
3.2. Weight Loss, Water Intake and pH Value
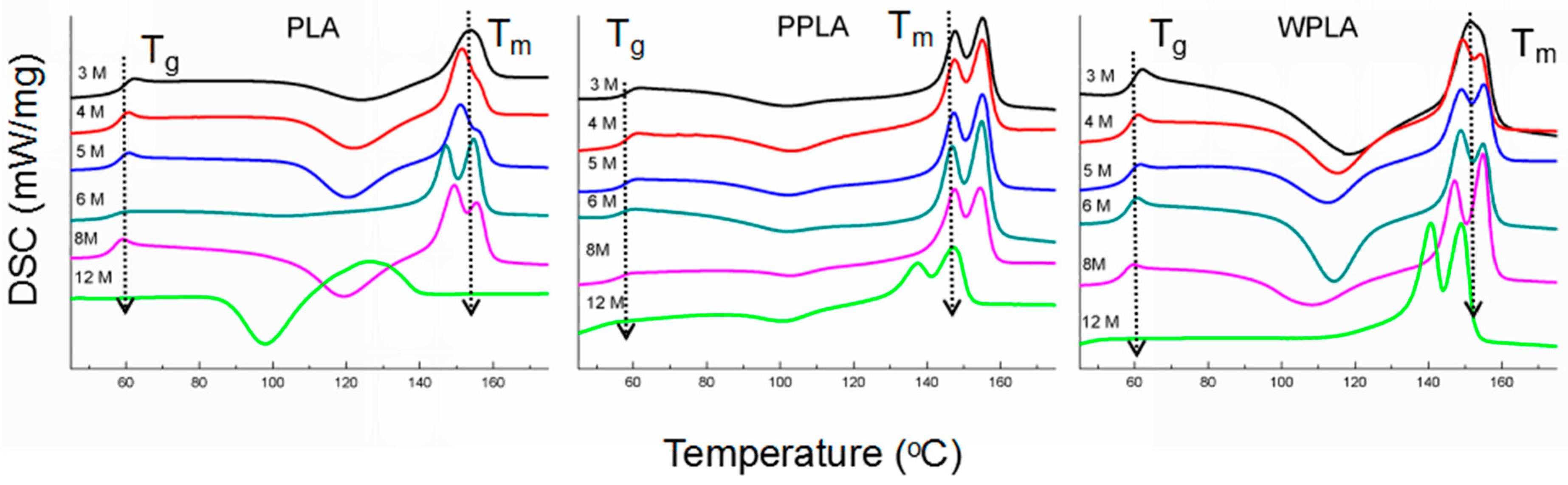
3.3. GPC and DSC Results
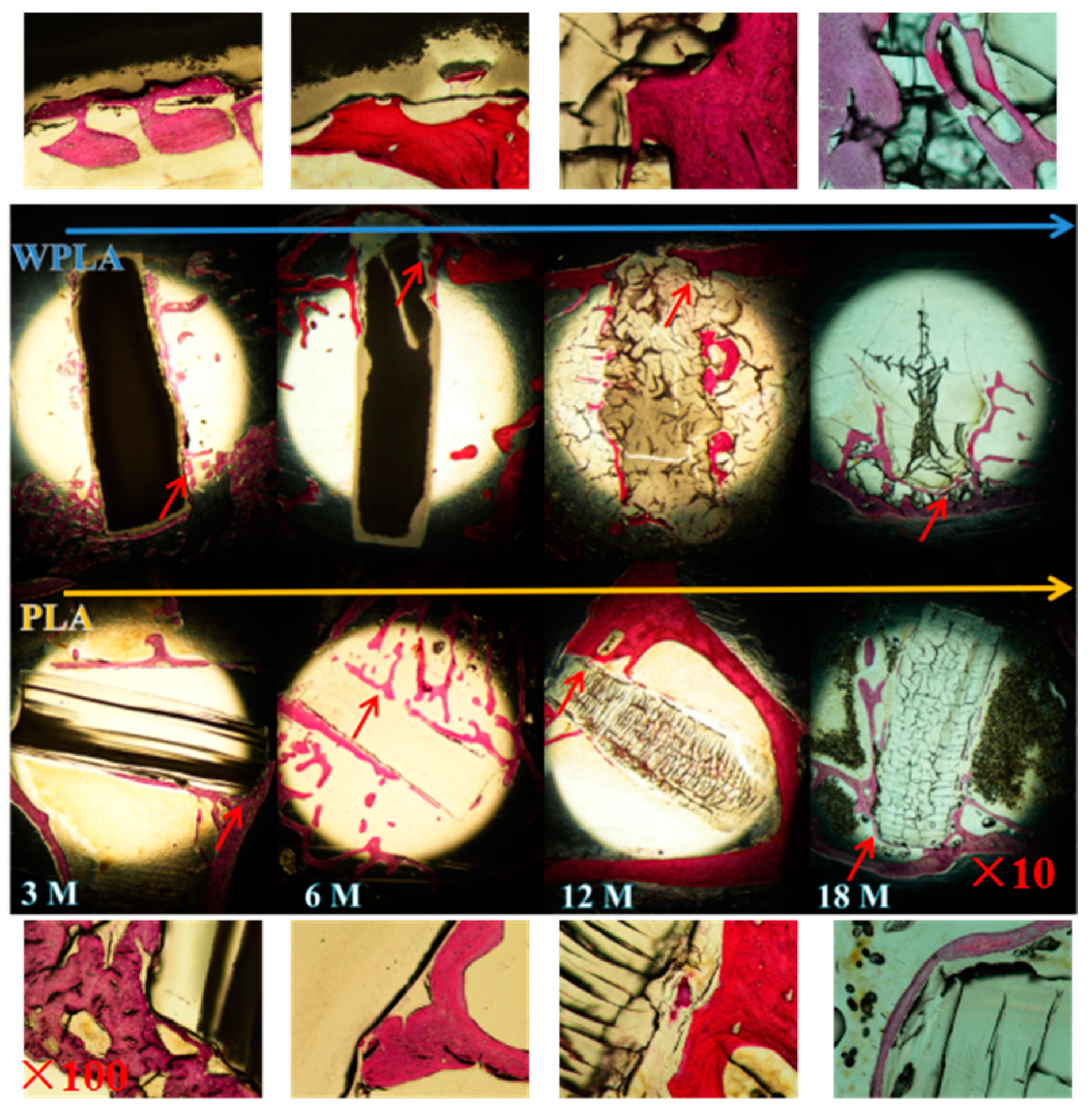
3.4. Dying of In Vivo Degradation
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alsberg, E.; Kong, H.J.; Hirano, Y.; Smith, M.K.; Albeiruti, A.; Mooney, D.J. Regulating bone formation via controlled scaffold degradation. J. Dent. Res. 2003, 82, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Kakinoki, S.; Uchida, S.; Ehashi, T.; Murakami, A.; Yamaoka, T. Surface Modification of Poly (L-lactic acid) Nanofiber with Oligo (D-lactic acid) Bioactive-Peptide Conjugates for Peripheral Nerve Regeneration. Polymers 2011, 3, 820–832. [Google Scholar] [CrossRef]
- Rogina, A.; Pribolšan, L.; Hanžek, A.; Gómez-Estrada, L.; Ferrer, G.G.; Marijanovi’c, I.; Ivankovi´c, M.; Ivankovi´c, H. Macroporous poly (lactic acid) construct supporting the osteoinductive porous chitosan-based hydrogel for bone tissue engineering. Polymer 2016, 98, 172–181. [Google Scholar] [CrossRef]
- Wang, X.J.; Song, G.J.; Lou, T. Fabrication and characterization of nano composite scaffold of poly (L-lactic acid)/hydroxyapatite. J. Mater. Sci. 2010, 21, 183–188. [Google Scholar] [CrossRef]
- Park, J.E.; Todo, M. Development and characterization of reinforced poly(L-lactide) scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 1171–1182. [Google Scholar] [CrossRef]
- Li, X.; Chu, C.L.; Liu, L.; Liu, X.K.; Bai, J.; Guo, C.; Xue, F.; Lin, P.H.; Chu, P.K. Biodegradable poly-lactic acidbased-composite reinforced unidirectionally with high-strength magnesium alloy wires. Biomaterials 2015, 49, 135–144. [Google Scholar] [CrossRef]
- Wen, W.; Zou, Z.P.; Luo, B.H.; Zhou, C.R. In vitro degradation and cytocompatibility of g-MgO whiskers/PLLA composites. J. Mater. Sci. 2017, 52, 2329–2344. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Böstman, O.M.; Pihlajamäki, H.K. Adverse tissue reactions to bioabsorbable fixation devices. Clin. Orthop. Relat. Res. 2000, 371, 216–227. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Xiong, C.D.; Jiang, L.X.; Xu, L.J. Effect of hydroxyapatite with different morphology on the crystallization behavior, mechanical property and in vitro degradation of hydroxyapatite/poly (lactic-co-glycolic) composite. Compos. Sci. Technol. 2014, 93, 61–67. [Google Scholar]
- Shalumon, K.T.; Sheu, C.; Fong, Y.T.; Liao, H.T.; Chen, J.P. Microsphere-Based Hierarchically Juxtapositioned Biphasic Scaffolds Prepared from Poly(Lactic-co-Glycolic Acid) and Nanohydroxyapatite for Osteochondral Tissue Engineering. Polymers 2016, 8, 429. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; Shaw, M.T.; Wei, M. Biodegradable HA-PLA 3-D porous scaffolds: Effect of nano-sized filler content on scaffold properties. Acta Biomater. 2005, 1, 653–662. [Google Scholar] [CrossRef]
- Ma, F.Q.; Lu, X.L.; Wang, Z.M.; Sun, Z.J.; Zhang, F.F.; Zheng, Y.F. Nanocomposites of poly(L-lactide) and surface modified magnesia nanoparticles: Fabrication, mechanical property and biodegradability. J. Phys. Chem. Solids 2011, 72, 111–116. [Google Scholar] [CrossRef]
- Kum, C.H.; Cho, Y.; Seo, S.H.; Joung, Y.K.; Ahn, D.J.; Han, D.K. A poly (lactide) stereocomplex structure with modified magnesium oxide and its effects in enhancing the mechanical properties and suppressing inflammation. Small 2014, 10, 3783–3794. [Google Scholar] [CrossRef]
- Yang, J.J.; Cao, X.X.; Zhao, Y.; Wang, L.; Liu, B.; Jia, J.P.; Liang, H.; Chen, M.F. Enhanced pH stability, cell viability and reduced degradation rate of poly(L-lactide)-based composite in vitro: Effect of modified magnesium oxide nanoparticles. J. Biomater. Sci. Polym. Ed. 2017, 28, 486–503. [Google Scholar] [CrossRef]
- Wetteland, C.L.; Nguyen, N.T.; Liu, H.N. Concentration-dependent behaviors of bone marrow derived mesenchymal stem cells and infectious bacteria toward magnesium oxide nanoparticles. Acta Biomater. 2016, 35, 341–356. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; You, C.; Chen, M.F. Effects of MgO whiskers on mechanical properties and crystallization behavior of PLLA/MgO composites. Mater. Des. 2016, 89, 573–581. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; Gavilán, R.; Lieblich, M.; Benavente, R.; González-Carrasco, J.L. In vitro degradation of biodegradable polylactic acid/magnesium composites: Relevance of Mg particle shape. Acta Biomater. 2016, 32, 348–357. [Google Scholar] [CrossRef]
- Wen, W.; Luo, B.; Qin, X.; Li, C.; Liu, M.; Ding, S.; Zhou, C. Strengthening and toughening of poly(L-lactide) composites by surface modified MgO whiskers. Appl. Surf. Sci. 2015, 332, 215–223. [Google Scholar] [CrossRef]
- Chen, H.M.; Shen, Y.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y.; Zhou, Z.W. Molecular ordering and α’-form formation of poly(L-lactide) during the hydrolytic degradation. Polymer 2013, 54, 6644–6653. [Google Scholar] [CrossRef]
- Chen, H.M.; Feng, C.X.; Zhang, W.B.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y. Hydrolytic degradation behavior of poly(L-lactide)/carbon nanotubes nanocomposites. Polym. Degrad. Stab. 2013, 98, 198–208. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Yang, J.J.; Jia, J.J.; You, C.; Chen, M.F. Effects of modifying agents on surface modifications of magnesium oxide whiskers. Appl. Surf. Sci. 2016, 388A, 370–375. [Google Scholar] [CrossRef]
- Jia, J.P.; Yang, J.J.; Zhao, Y.; Liang, H.; Chen, M.F. The crystallization behaviors and mechanical properties of poly(L-lactic acid)/magnesium oxide nanoparticle composites. RSC Adv. 2016, 6, 43855–43863. [Google Scholar] [CrossRef]
- Liu, Y.L.; Shao, J.; Sun, J.R.; Bian, X.C.; Li, D.; Xiang, F.S.; Sun, B.; Chen, Z.; Li, G.; Chen, X.S. Improved mechanical and thermal properties of PLLA by solvent blending with PDLA-b-PEG-b-PDLA. Polym. Degrad. Stab. 2014, 101, 10–17. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.; Senapati, S.; Singh, A.P.; Ray, B.; Maiti, P. Controlled drug release through regulated biodegradation of poly(lactic acid) using inorganic salts. Int. J. Biol. Macromol. 2017, 104, 487–497. [Google Scholar] [CrossRef]
- Larrañaga, A.; Aldazabal, P.; Martin, F.J.; Sarasua, J.R. Hydrolytic degradation and bioactivity of lactide and caprolactone based sponge-like scaffolds loaded with bioactive glass particles. Polym. Degrad. Stab. 2014, 110, 121–128. [Google Scholar] [CrossRef]
- Lizundia, E.; Mateos, P.; Vilas, J.L. Tuneable hydrolytic degradation of poly(L-lactide) scaffolds triggered by ZnO nanoparticles. Mat. Sci. Eng. C 2017, 75, 714–720. [Google Scholar] [CrossRef]
- Wu, Y.H.; Li, N.; Cheng, Y.; Zheng, Y.F.; Han, Y. In vitro study on biodegradable AZ31 magnesium alloy fibers reinforced PLGA composite. J. Mater. Sci. Technol. 2013, 29, 545–550. [Google Scholar] [CrossRef]
- Qu, M.; Tu, H.L.; Amarante, M.; Song, Y.Q.; Zhu, S.S. Zinc Oxide Nanoparticles Catalyze Rapid Hydrolysis of Poly(lactic acid) at Low Temperatures. J. Appl. Polym. Sci. 2014, 131, 40287. [Google Scholar] [CrossRef]
- Ning, N.Y.; Fu, S.R.; Zhang, W.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Realizing the enhancement of interfacial interaction in semicrystalline polymer/filler composites via interfacial crystallization. Prog. Polym. Sci. 2012, 37, 1425–1455. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, J.; Shao, L.N.; Yang, J.H.; Huang, T.; Zhang, N.; Wang, Y. Comparative Study of Poly(L-lactide) Nanocomposites with Organic Montmorillonite and Carbon Nanotubes. J. Polym. Sci. Pol. Phys. 2013, 51, 183–196. [Google Scholar] [CrossRef]
- Von Burkersroda, F.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
| Sample | SpMgO Content/PLA Content (w/w) | SwMgO Content/PLA Content (w/w) |
|---|---|---|
| PLA | 0 | 0 |
| PPLA | 1/100 | 0 |
| WPLA | 0 | 1/100 |
| Time/Months | PLA | PPLA | WPLA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mw | Mn | PI | Mw | Mn | PI | Mw | Mn | PI | |
| 3 | 135,777 | 85,630 | 1.59 | 124,969 | 79,492 | 1.57 | 142,721 | 87,717 | 1.63 |
| 4 | 122,638 | 78,164 | 1.57 | 119,200 | 74,087 | 1.61 | 128,695 | 78,720 | 1.63 |
| 5 | 113,343 | 70,064 | 1.62 | 105,310 | 65,020 | 1.62 | 111,342 | 75,066 | 1.48 |
| 6 | 84,728 | 55,543 | 1.53 | 77,485 | 50,884 | 1.52 | 103,873 | 67,122 | 1.55 |
| 8 | 51,364 | 28,036 | 1.83 | 64,290 | 41,053 | 1.57 | 68,602 | 44,645 | 1.54 |
| 12 | 4017 | 3365 | 1.22 | 9798 | 16,703 | 1.70 | 21,573 | 13,011 | 1.66 |
| Time(Months)/Samples | Tm1 (°C) | Tm2 (°C) | ΔHcc (J/g) | ΔHm (J/g) | Xc (%) | |
|---|---|---|---|---|---|---|
| 3 | PLA | 153.9 | - | −12.8 | 25.5 | 40.8 |
| PPLA | 147.7 | 155.2 | −8.37 | 35.3 | 46.6 | |
| WPLA | 151.7 | 154.5 | −23.6 | 37.7 | 65.4 | |
| 4 | PLA | 151.5 | 156.6 | −21.0 | 33.1 | 57.8 |
| PPLA | 147.6 | 155.2 | −10.9 | 37.5 | 51.6 | |
| WPLA | 149.4 | 154.2 | −22.1 | 34.3 | 60.2 | |
| 5 | PLA | 151.4 | 156.8 | −25.0 | 29.6 | 58.4 |
| PPLA | 147.3 | 155.1 | −6.3 | 38.9 | 48.4 | |
| WPLA | 149.1 | 155.1 | −22.1 | 30.6 | 56.2 | |
| 6 | PLA | 147.2 | 154.7 | −5.0 | 39.6 | 47.6 |
| PPLA | 146.8 | 154.9 | −5.9 | 43.0 | 52.2 | |
| WPLA | 148.8 | 154.8 | −30.3 | 32.9 | 67.5 | |
| 8 | PLA | 149.4 | 155.6 | −26.5 | 45.6 | 77.1 |
| PPLA | 147.7 | 154.6 | −4.8 | 35.8 | 43.3 | |
| WPLA | 147.1 | 154.9 | −16.8 | 43.0 | 63.8 | |
| 12 | PLA | 126.9 | - | −33.0 | 24.9 | 61.9 |
| PPLA | 137.5 | 147.2 | −6.8 | 28.7 | 38.0 | |
| WPLA | 140.6 | 148.9 | −4.0 | 43.1 | 50.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liang, H.; Zhang, S.; Qu, S.; Jiang, Y.; Chen, M. Effects of Magnesium Oxide (MgO) Shapes on In Vitro and In Vivo Degradation Behaviors of PLA/MgO Composites in Long Term. Polymers 2020, 12, 1074. https://doi.org/10.3390/polym12051074
Zhao Y, Liang H, Zhang S, Qu S, Jiang Y, Chen M. Effects of Magnesium Oxide (MgO) Shapes on In Vitro and In Vivo Degradation Behaviors of PLA/MgO Composites in Long Term. Polymers. 2020; 12(5):1074. https://doi.org/10.3390/polym12051074
Chicago/Turabian StyleZhao, Yun, Hui Liang, Shiqiang Zhang, Shengwei Qu, Yue Jiang, and Minfang Chen. 2020. "Effects of Magnesium Oxide (MgO) Shapes on In Vitro and In Vivo Degradation Behaviors of PLA/MgO Composites in Long Term" Polymers 12, no. 5: 1074. https://doi.org/10.3390/polym12051074
APA StyleZhao, Y., Liang, H., Zhang, S., Qu, S., Jiang, Y., & Chen, M. (2020). Effects of Magnesium Oxide (MgO) Shapes on In Vitro and In Vivo Degradation Behaviors of PLA/MgO Composites in Long Term. Polymers, 12(5), 1074. https://doi.org/10.3390/polym12051074




