Preparation of Tri(alkenyl)functional Open-Cage Silsesquioxanes as Specific Polymer Modifiers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
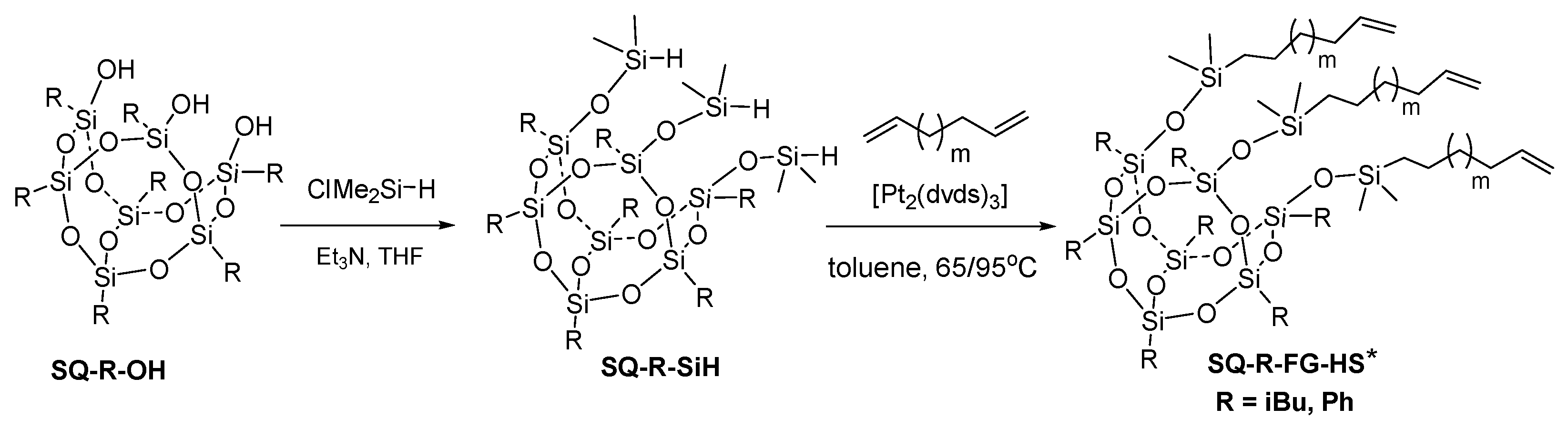
2.3. Synthetic Procedures for Preparation of SQ-R-SiH R = iBu, Ph
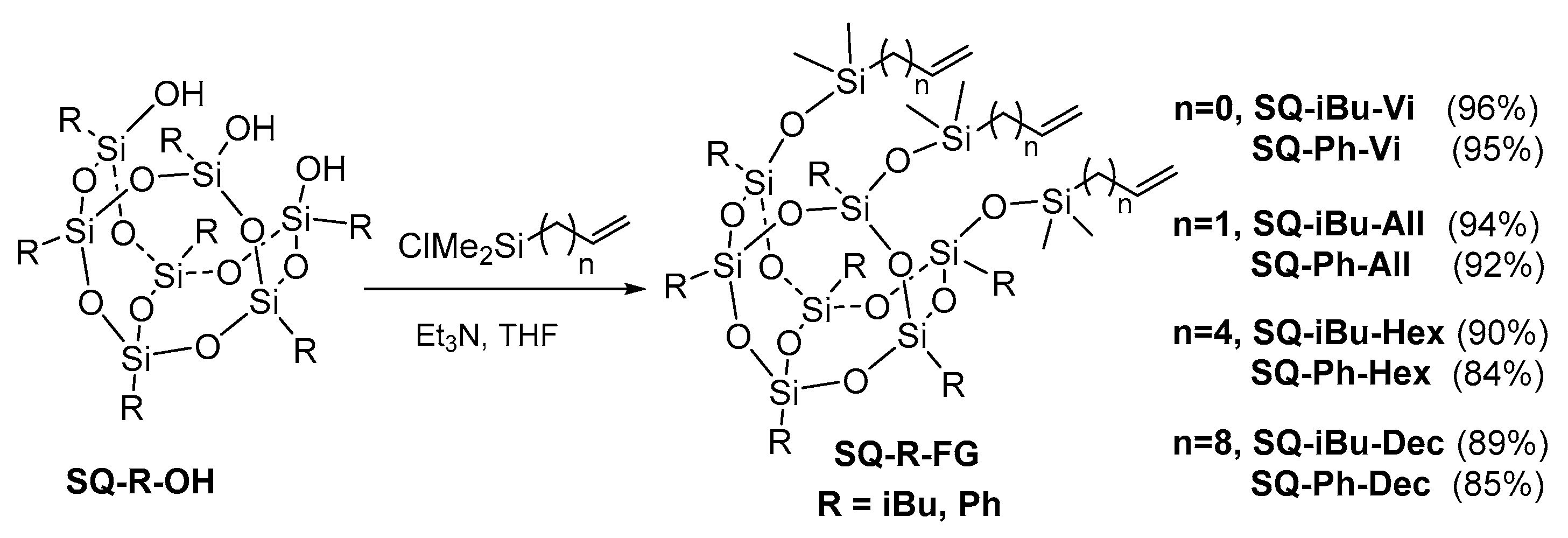
2.4. General Procedure for Preparation of tri(alkenyl)functional SQs (SQ-R-FG; R = iBu, Ph; FG = Vi, All, Hex, Dec)
2.4.1. Procedure for Preparation of SQ-R-FG (R = iBu, Ph; FG = Vi, All, Hex, Dec) Compounds via a Hydrolytic Condensation Reaction
2.4.2. Procedure for the Hydrosilylation of Dienes by SQ-R-SiH (R = iBu, Ph; diene = 1,5-hexadiene, 1,9-decadiene)
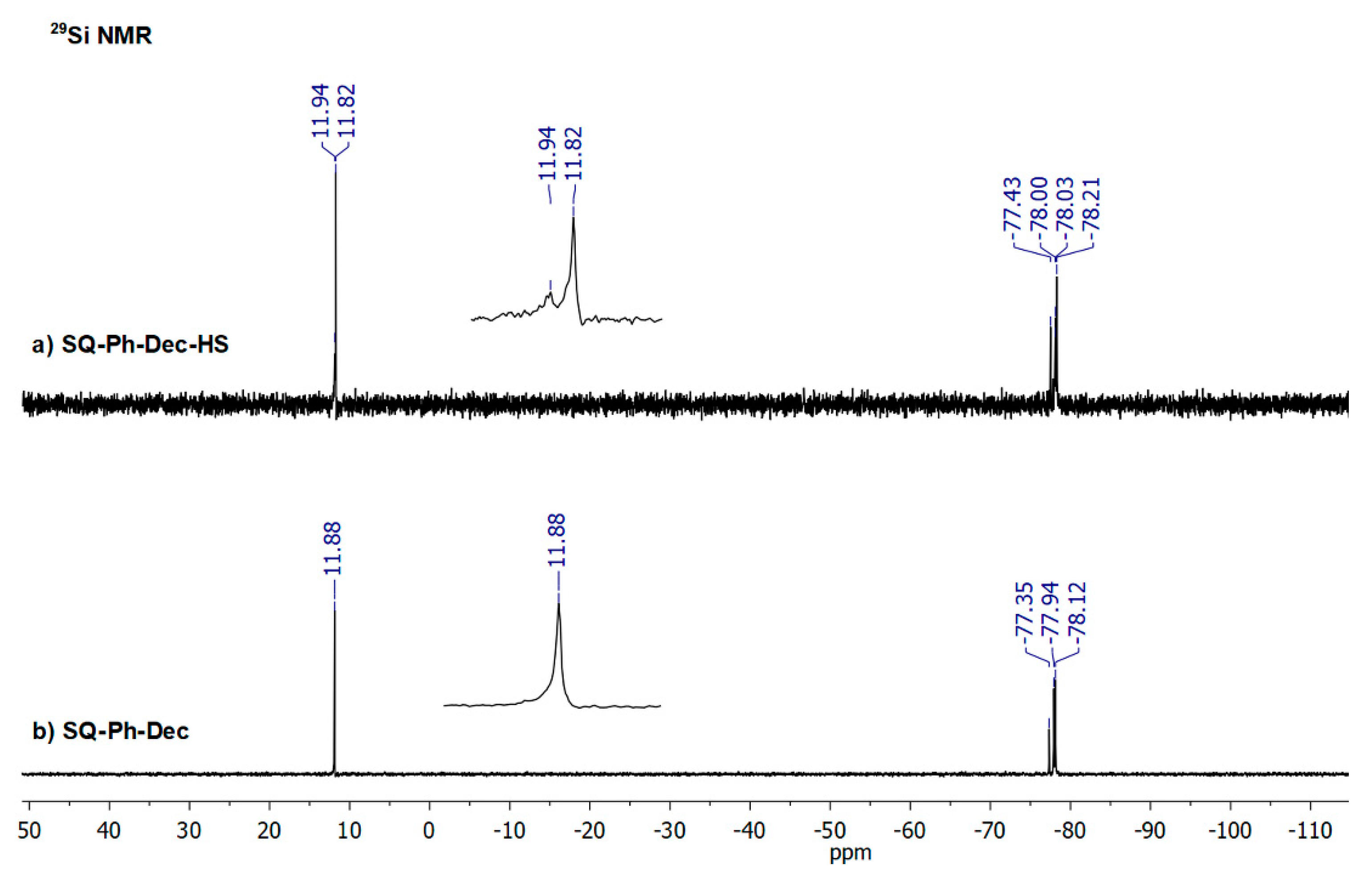
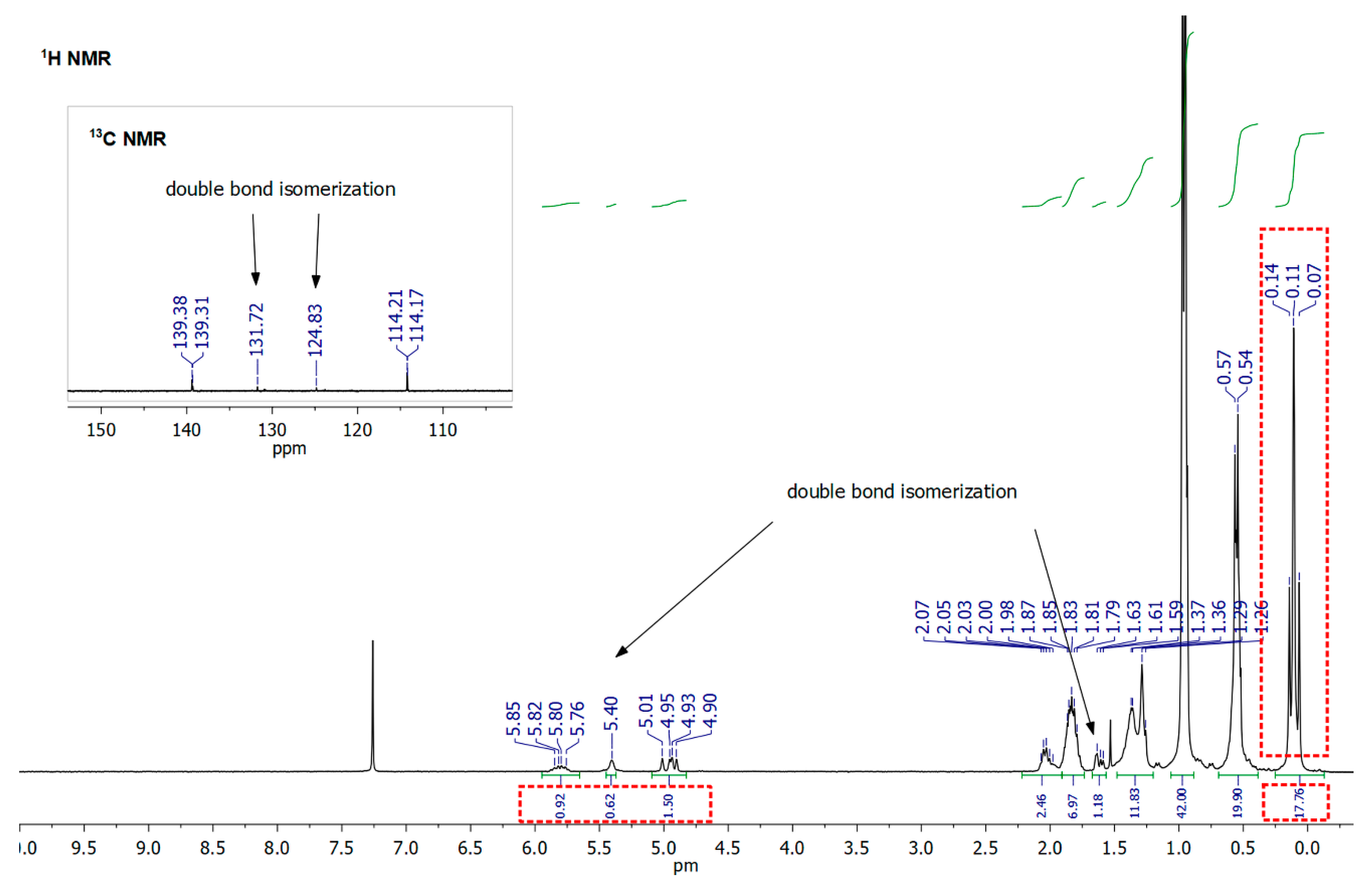
3. Results and Discussion
TG and DSC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Applications of Polyhedral Oligomeric Silsesquioxanes; Hartmann-Thompson, C., Ed.; Springer: London, UK; New York, NY, USA, 2011; ISBN 978−90−481−3786−2. [Google Scholar]
- Du, Y.; Liu, H. Cage-like Silsesquioxanes-based Hybrid Materials. Dalt. Trans. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dudziec, B.; Marciniec, B. Double-decker Silsesquioxanes: Current Chemistry and Applications. Curr. Org. Chem. 2017, 21, 2794–2813. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, H.; Xu, J. Cubic polyhedral oligomeric silsesquioxane based functional materials: Synthesis, assembly, and applications. Chem. Asian J. 2016, 11, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, S.; Sato, Y.; Imoto, H.; Naka, K. Fabrication of composite films with poly(methyl methacrylate) and incompletely condensed cage-silsesquioxane fillers. J. Appl. Polym. Sci. 2018, 135, 46033. [Google Scholar] [CrossRef]
- Lee, D.W.; Kawakami, Y. Incompletely Condensed Silsesquioxanes: Formation and Reactivity. Polym. J. 2007, 39, 230–238. [Google Scholar] [CrossRef]
- Li, Z.; Kawakami, Y. Formation of Incompletely Condensed Oligosilsesquioxanes by Hydrolysis of Completely Condensed POSS via Reshuffling. Chem. Lett. 2008, 37, 804–805. [Google Scholar] [CrossRef]
- Katoh, R.; Imoto, H.; Naka, K. One-pot strategy for synthesis of open-cage silsesquioxane monomers. Polym. Chem. 2019, 10, 2223–2229. [Google Scholar] [CrossRef]
- Wada, S.; Imoto, H.; Naka, K. Palladium-Catalyzed Arylation of Open-Cage Silsesquioxanes toward Thermally Stable and Highly Dispersible Nanofillers. Bull. Chem. Soc. Jpn. 2019, 92, 989–994. [Google Scholar] [CrossRef]
- Dutkiewicz, M.; Karasiewicz, J.; Rojewska, M.; Skrzypiec, M.; Dopierała, K.; Prochaska, K.; Maciejewski, H. Synthesis of an Open-Cage Structure POSS Containing Various Functional Groups and Their Effect on the Formation and Properties of Langmuir Monolayers. Chem. Eur. J. 2016, 22, 13275–13286. [Google Scholar] [CrossRef] [PubMed]
- Imoto, H.; Nakao, Y.; Nishizawa, N.; Fujii, S.; Nakamura, Y.; Naka, K. Tripodal polyhedral oligomeric silsesquioxanes as a novel class of three-dimensional emulsifiers. Polym. J. 2015, 47, 609–615. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Kuciński, K.; Hreczycho, G. Highly Efficient Catalytic Route for the Synthesis of Functionalized Silsesquioxanes. Inorg. Chem. 2017, 56, 9337–9342. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Kuciński, K.; Stachowiak, H.; Hreczycho, G. Introduction of Boron Functionalities into Silsesquioxanes - Novel Independent Methodologies. Chem. Eur. J. 2018, 24, 2509–2514. [Google Scholar]
- Blanco, I.; Bottino, F.A.; Bottino, P. Influence of Symmetry/Asymmetry of the Nanoparticles Structure on the Thermal Stability of Polyhedral Oligomeric Silsesquioxane/Polystyrene Nanocomposites. Polym. Compos. 2012, 33, 1903–1910. [Google Scholar] [CrossRef]
- Lorenz, V.; Spoida, M.; Fischer, A.; Edelmann, F.T. Silsesquioxane chemistry: Part 5. New silyl-functionalized silsesquioxanes. J. Organomet. Chem. 2001, 625, 1–6. [Google Scholar] [CrossRef]
- Yuasa, S.; Sato, Y.; Imoto, H.; Naka, K. Thermal properties of open-cage silsesquioxanes: The effect of substituents at the corners and opening moieties. Bull. Chem. Soc. Jpn. 2019, 92, 127–132. [Google Scholar] [CrossRef]
- Liu, H.; Kondo, S. ichi; Tanaka, R.; Oku, H.; Unno, M. A spectroscopic investigation of incompletely condensed polyhedral oligomeric silsesquioxanes (POSS-mono-ol, POSS-diol and POSS-triol): Hydrogen-bonded interaction and host-guest complex. J. Organomet. Chem. 2008, 693, 1301–1308. [Google Scholar] [CrossRef]
- Duchateau, R. Incompletely condensed silsesquioxanes: Versatile tools in developing silica-supported olefin polymerization catalysts. Chem. Rev. 2002, 102, 3525–3542. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Basset, J.M. On silsesquioxanes’ accuracy as molecular models for silica-grafted complexes in heterogeneous catalysis. Coord. Chem. Rev. 2010, 254, 707–728. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Yalymov, A.I.; Kulakova, A.N.; Petrov, A.A.; Bilyachenko, A.N. Cage-like metallasilsesquioxanes in catalysis: A review. J. Mol. Catal. A Chem. 2017, 426, 297–304. [Google Scholar] [CrossRef]
- Wada, K.; Yamasaki, T.; Kondo, T.; Mitsudo, T. Synthesis of Novel Organic–Inorganic Hybrid Cages via Cobalt-catalyzed Cyclotrimerization of Dimethylethynylsilyl Groups on a Silsesquioxane. Chem. Lett. 2004, 33, 1218–1219. [Google Scholar] [CrossRef]
- Yusa, S.; Ohno, S.; Honda, T.; Imoto, H.; Nakao, Y.; Naka, K.; Nakamura, Y.; Fujii, S. Synthesis of silsesquioxane-based element-block amphiphiles and their self-assembly in water. RSC Adv. 2016, 6, 73006–73012. [Google Scholar] [CrossRef]
- Imoto, H.; Katoh, R.; Honda, T.; Yusa, S.I.; Naka, K. Self-association behavior of amphiphilic molecules based on incompletely condensed cage silsesquioxanes and poly(ethylene glycol)s. Polym. J. 2018, 50, 337–345. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Sun, Y.; Xie, W. POSS dental nanocomposite resin: Synthesis, shrinkage, double bond conversion, hardness, and resistance properties. Polymers 2018, 10, 369. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Yu, J.; Sun, Y.; Xie, W. Study of POSS on the properties of novel inorganic dental composite resin. Polymers 2020, 12, 478. [Google Scholar] [CrossRef]
- Celso, F.; Mikhailenko, S.D.; Rodrigues, M.A.S.; Mauler, R.S.; Kaliaguine, S. Electrical conductivity of sulfonated poly(ether ether ketone) based composite membranes containing sulfonated polyhedral oligosilsesquioxane. J. Power Sources 2016, 305, 54–63. [Google Scholar] [CrossRef]
- Gupta, D.; Madhukar, A.; Choudhary, V. Effect of functionality of polyhedral oligomeric silsesquioxane [POSS] on the properties of sulfonated poly(ether ether ketone) [SPEEK] based hybrid nanocomposite proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 12817–12829. [Google Scholar] [CrossRef]
- Chang, Y.W.; Shin, G. Crosslinked poly(ethylene glycol) (PEG)/sulfonated polyhedral oligosilsesquioxane (sPOSS) hybrid membranes for direct methanol fuel cell applications. J. Ind. Eng. Chem. 2011, 17, 730–735. [Google Scholar] [CrossRef]
- Chang, Y.W.; Wang, E.; Shin, G.; Han, J.-E.; Mather, P.T. Poly(vinyl alcohol) (PVA)/sulfonated polyhedral oligosilsesquioxane (sPOSS) hybrid membranes for direct methanol fuel cell applications. Polym. Adv. Technol. 2007, 18, 535–543. [Google Scholar] [CrossRef]
- Imoto, H.; Katoh, R.; Naka, K. Open-cage silsesquioxane necklace polymers having closed-cage silsesquioxane pendants. Polym. Chem. 2018, 9, 4108–4112. [Google Scholar] [CrossRef]
- Yuasa, S.; Imoto, H.; Naka, K. Synthesis and properties of hyperbranched polymers by polymerization of an AB3-type incompletely condensed cage silsesquioxane (IC-POSS) monomer. Polym. J. 2018, 50, 879–887. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wei, H.; Zheng, J.; Huang, G. Effect of incompletely condensed tri-silanol-phenyl-POSS on the thermal stability of silicone rubber. Polym. Bull. 2019, 76, 2835–2850. [Google Scholar] [CrossRef]
- Turgut, G.; Dogan, M.; Tayfun, U.; Ozkoc, G. The effects of POSS particles on the flame retardancy of intumescent polypropylene composites and the structure-property relationship. Polym. Degrad. Stab. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Mituła, K.; Dudziec, B. Multi-Alkenylsilsesquioxanes as Comonomers and Active Species Modifiers of Metallocene Catalyst in Copolymerization with Ethylene. Polymers 2018, 10, 223. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, J.; Yu, Y.; Kong, J. Preparation of POSS/Quartz Fibers/Cyanate Ester Resins Laminated Composites. Polym. Compos. 2014, 36, 2017–2021. [Google Scholar] [CrossRef]
- Smith, E.R.; Howlin, B.J.; Hamerton, I. Using POSS reagents to reduce hydrophobic character in polypropylene nanocomposites. J. Mater. Chem. A 2013, 1, 12971–12980. [Google Scholar] [CrossRef][Green Version]
- Raimondo, M.; Russo, S.; Guadagno, L.; Longo, P.; Chirico, S.; Mariconda, A.; Bonnaud, L.; Murariu, O.; Dubois, P. Effect of incorporation of POSS compounds and phosphorous hardeners on thermal and fire resistance of nanofilled aeronautic resins. RSC Adv. 2015, 5, 10974–10986. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K.; Białek, M.; Man, D. Tri-alkenyl polyhedral oligomeric silsesquioxanes as comonomers and active center modifiers in ethylene copolymerization catalyzed by bis(phenoxy- imine) Ti, Zr, V and V salen-type complexes. Appl. Catal. A Gen. J. 2018, 567, 122–131. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Zeng, X.; Yi, L.; Xu, H.; Mao, Z. Effect of trisilanolphenyl-POSS on rheological, mechanical, and flame-retardant properties of poly(ethylene terephthalate)/cyclotriphosphazene systems. J. Appl. Polym. Sci. 2017, 45912. [Google Scholar] [CrossRef]
- Gârea, S.A.; Nicolescu, A.; Deleanu, C.; Iovu, H. New nanocomposites based on epoxy resins reinforced with modified montmorillonite. Mater. Plast. 2008, 45, 414–420. [Google Scholar] [CrossRef]
- Miyasaka, M.; Fujiwara, Y.; Kudo, H.; Nishikubo, T. Synthesis and characterization of hyperbranched polymer consisting of silsesquioxane derivatives. Polym. J. 2010, 42, 799–803. [Google Scholar] [CrossRef]
- Bian, Y.; Mijović, J. Effect of side chain architecture on dielectric relaxation in polyhedral oligomeric silsesquioxane/polypropylene oxide nanocomposites. Polymer (Guildf). 2009, 50, 1541–1547. [Google Scholar] [CrossRef]
- Zeng, F.; Liu, Y.; Sun, Y.; Hu, E.; Zhou, Y. Nanoindentation, nanoscratch, and nanotensile testing of poly(vinylidene fluoride)-polyhedral oligomeric silsesquioxane nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1597–1611. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S.; Nie, K. Morphology and Thermomechanical Properties of Organic - Inorganic Hybrid Composites Involving Epoxy Resin and an Incompletely Condensed Polyhedral Oligomeric Silsesquioxane. Macromolecules 2005, 38, 5088–5097. [Google Scholar] [CrossRef]
- Mituła, K.; Dutkiewicz, M.; Dudziec, B.; Marciniec, B.; Czaja, K. A library of monoalkenylsilsesquioxanes as potential comonomers for synthesis of hybrid materials. J. Therm. Anal. Calorim. 2018, 132, 1545–1555. [Google Scholar] [CrossRef]
- Marciniec, B.; Maciejewski, H.; Pietraszuk, C.; Pawluć, P. Hydrosilylation and Related Reactions of Silicon Compounds. In Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook; Cornils, B., Herrmann, W.A., Beller, M., Paciello, R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA.: Dresden, Germany, 2018; pp. 491–512. ISBN 9783527618231. [Google Scholar]
- Saiki, T. Effect of ether on regioselectivity in hydrosilylation of 1,5-hexadiene with chlorohydrosilanes catalyzed by homogeneous platinum catalysts. Phosphorus, Sulfur Silicon Relat. Elem. 2008, 183, 1556–1563. [Google Scholar] [CrossRef]

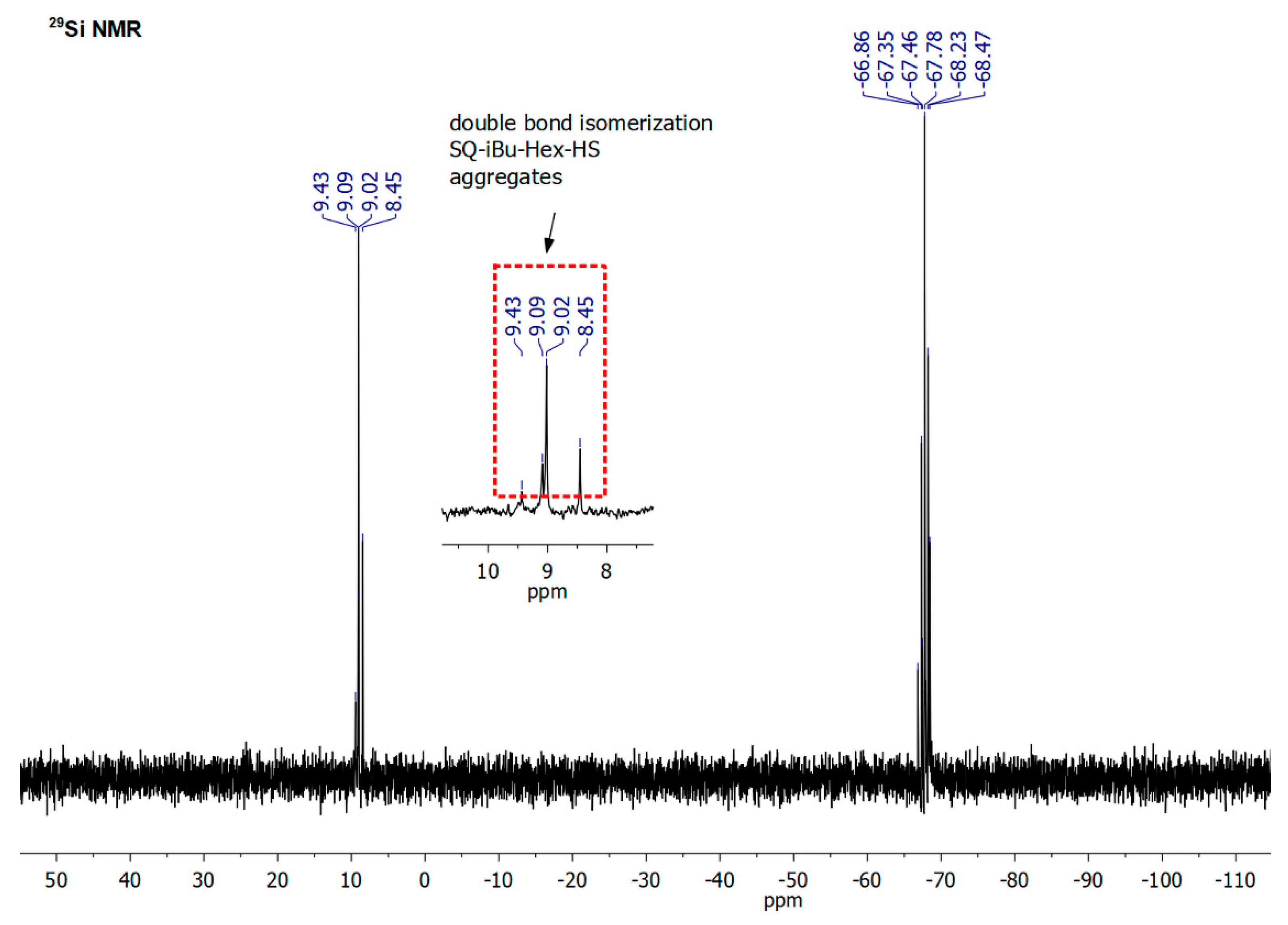
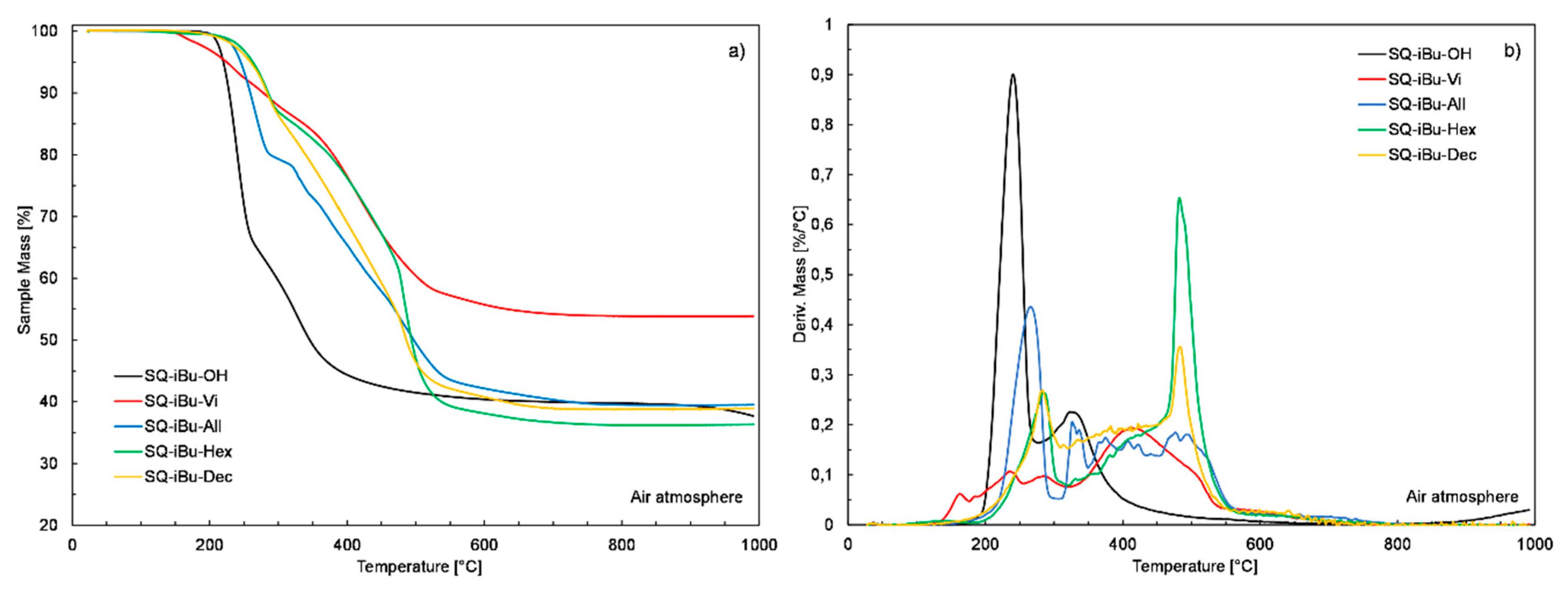
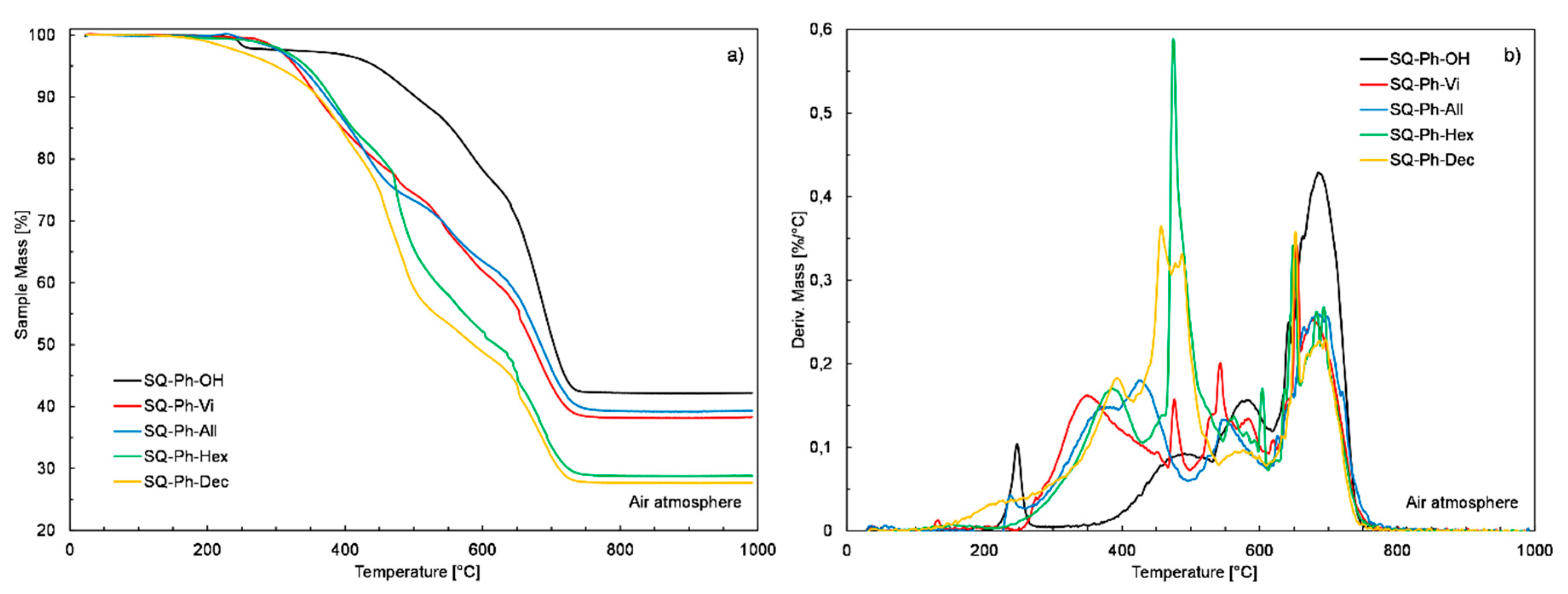
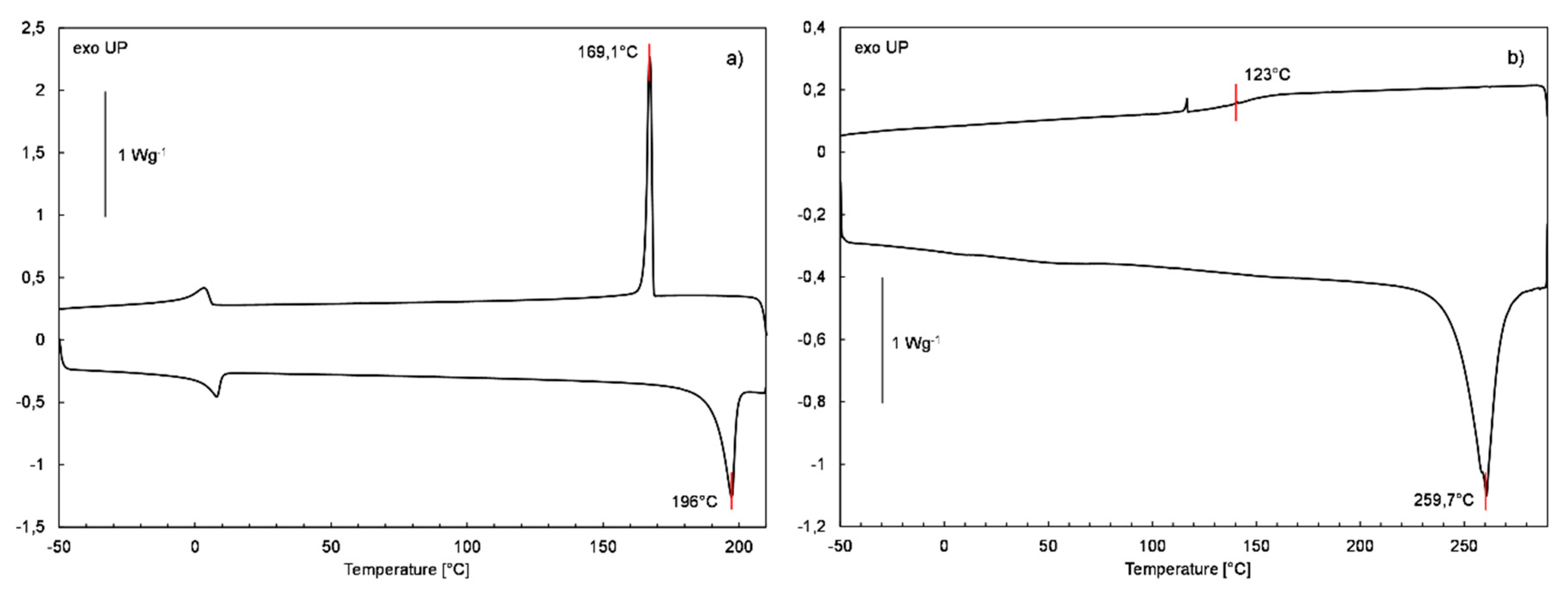

| Sample | Mass Loss Temperature [°C] | Tonset [°C] | CHAR Yield [%] a | ||
|---|---|---|---|---|---|
| 1% | 5% | 10% | |||
| SQ-iBu-OH | 206.6 | 220 | 240.4 | 218.2 | 37.7 |
| SQ-iBu-Vi | 165.9 | 223.2 | 392 | 147.5 | 54.2 |
| SQ-iBu-All | 218.1 | 245.2 | 288.2 | 236.4 | 39.5 |
| SQ-iBu-Hex | 220 | 262.8 | 374 | 250.2 | 36.3 |
| SQ-iBu-Dec | 210.8 | 259 | 339.2 | 245.9 | 38.9 |
| SQ-Ph-OH | 223.4 | 451.8 | 590.2 | 426.3 | 42.5 |
| SQ-Ph-Vi | 281.4 | 327.9 | 442.6 | 300.9 | 38.3 |
| SQ-Ph-All | 267.7 | 334.9 | 435.7 | 307.2 | 39.3 |
| SQ-Ph-Hex | 267.6 | 343.5 | 454.1 | 327.2 | 28.8 |
| SQ-Ph-Dec | 198.6 | 299.9 | 424.1 | 305.8 | 27.7 |
| Sample | Melting | Reaction | Crystallization | Glass Transition [°C] | |||
|---|---|---|---|---|---|---|---|
| Temp. [°C] | ΔH [Jg−1] | Temp. [°C] | ΔH [Jg−1] | Temp. [°C] | ΔH [Jg−1] | ||
| SQ-iBu-OH | 196 | −32.3 | - | - | 169.1 | 26 | - |
| SQ-iBu-Vi | - | - | 159.5 189.8 | 275.9 180.3 | - | - | - |
| SQ-iBu-All | - | - | 164.7 | 114.9 | - | - | - |
| SQ-iBu-Hex | - | - | 203.7 | 15.1 | - | - | - |
| SQ-iBu-Dec | - | - | 195.6 | 8.8 | - | - | - |
| SQ-Ph-OH | 259.7 | −64.8 | - | - | - | - | 123 |
| SQ-Ph-Vi | 132.1 139.5 | −8.2 −17.1 | - | - | 107.3 | 18 | - |
| SQ-Ph-All | 63.7 | −26.8 | - | - | −1.1 | 6.2 | - |
| SQ-Ph-Hex | 76.7 | −22.2 | - | - | 20.2 | 15.5 | - |
| SQ-Ph-Dec | 18.6 44.1 73.3 | −1.3 −9.8 −0.4 | - | - | - | - | - * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mituła, K.; Dutkiewicz, M.; Duszczak, J.; Rzonsowska, M.; Dudziec, B. Preparation of Tri(alkenyl)functional Open-Cage Silsesquioxanes as Specific Polymer Modifiers. Polymers 2020, 12, 1063. https://doi.org/10.3390/polym12051063
Mituła K, Dutkiewicz M, Duszczak J, Rzonsowska M, Dudziec B. Preparation of Tri(alkenyl)functional Open-Cage Silsesquioxanes as Specific Polymer Modifiers. Polymers. 2020; 12(5):1063. https://doi.org/10.3390/polym12051063
Chicago/Turabian StyleMituła, Katarzyna, Michał Dutkiewicz, Julia Duszczak, Monika Rzonsowska, and Beata Dudziec. 2020. "Preparation of Tri(alkenyl)functional Open-Cage Silsesquioxanes as Specific Polymer Modifiers" Polymers 12, no. 5: 1063. https://doi.org/10.3390/polym12051063
APA StyleMituła, K., Dutkiewicz, M., Duszczak, J., Rzonsowska, M., & Dudziec, B. (2020). Preparation of Tri(alkenyl)functional Open-Cage Silsesquioxanes as Specific Polymer Modifiers. Polymers, 12(5), 1063. https://doi.org/10.3390/polym12051063







