Role of Organo-Modifier and Metal Impurities of Commercial Nanoclays in the Photo- and Thermo-Oxidation of Polyamide 11 Nanocomposites
Abstract
1. Introduction
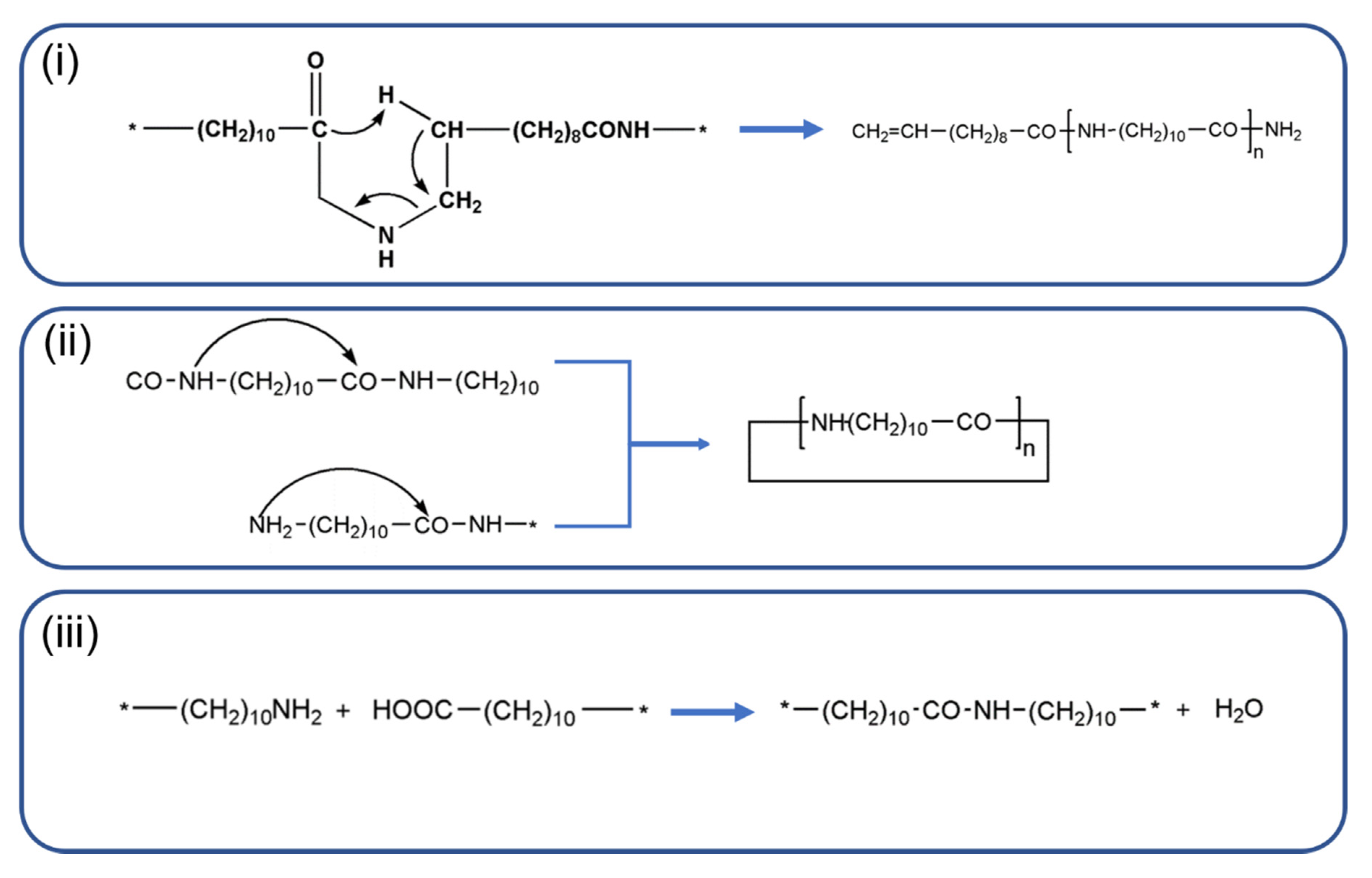
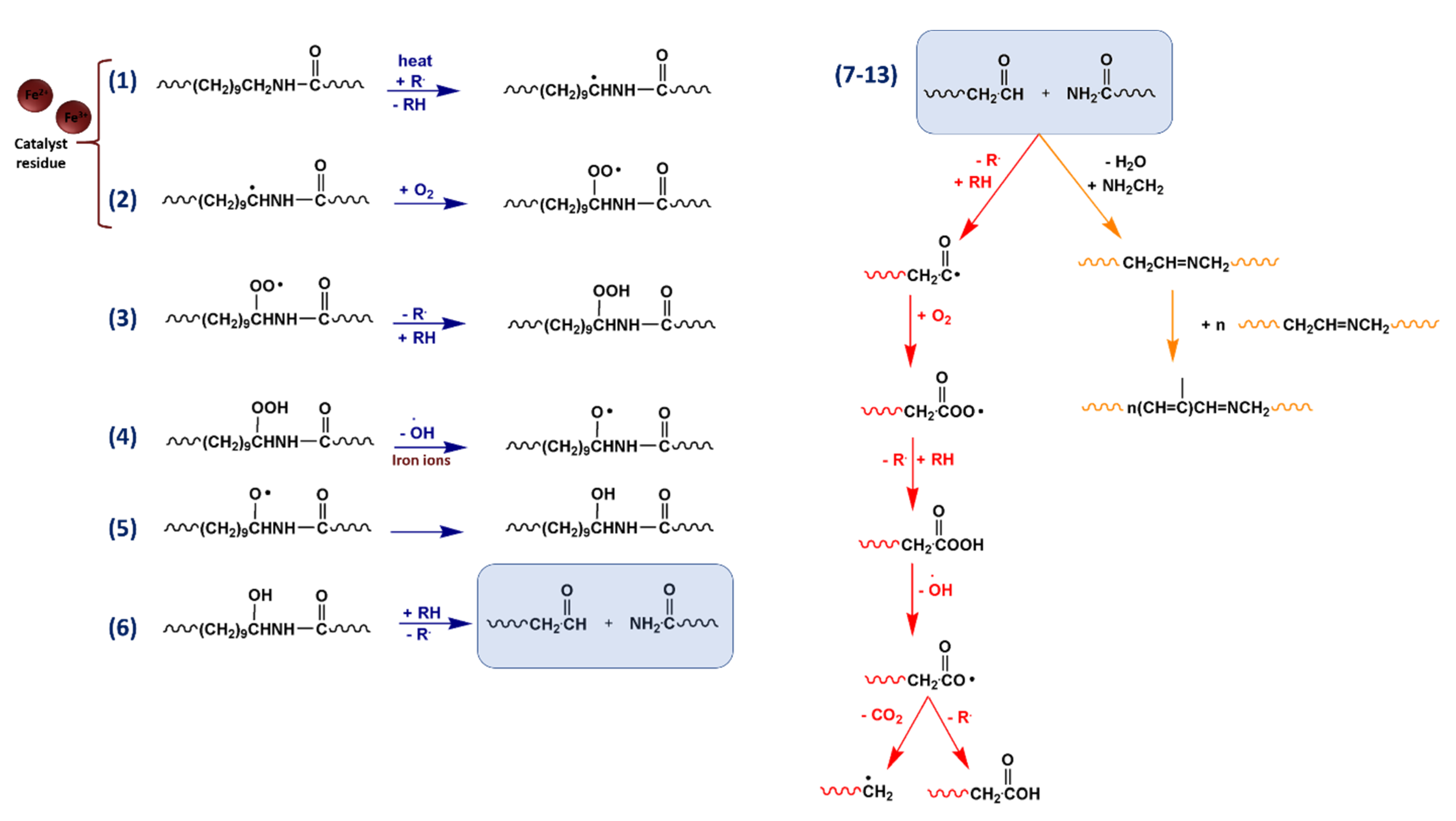
2. Background
3. Materials and Methods
3.1. Materials
3.2. PA11 Nanocomposites Preparation
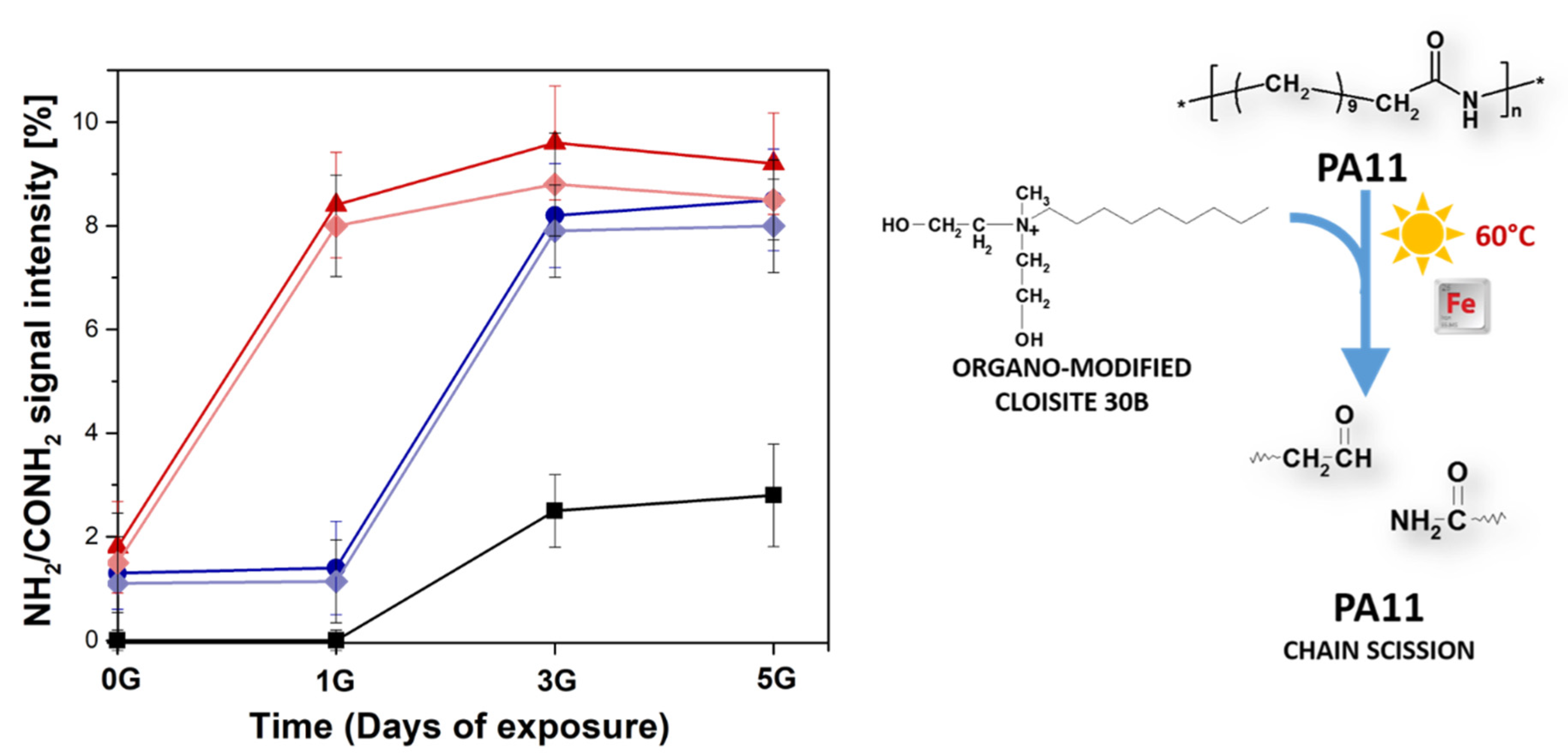
3.3. Photo-Oxidative Degradation of PA11 Samples
3.4. Molecular Characterization by SEC-MALS Chromatographic System
3.5. Thermogravimetric Analysis
3.6. Wide Angle X-Ray Diffractometry (WAXD)
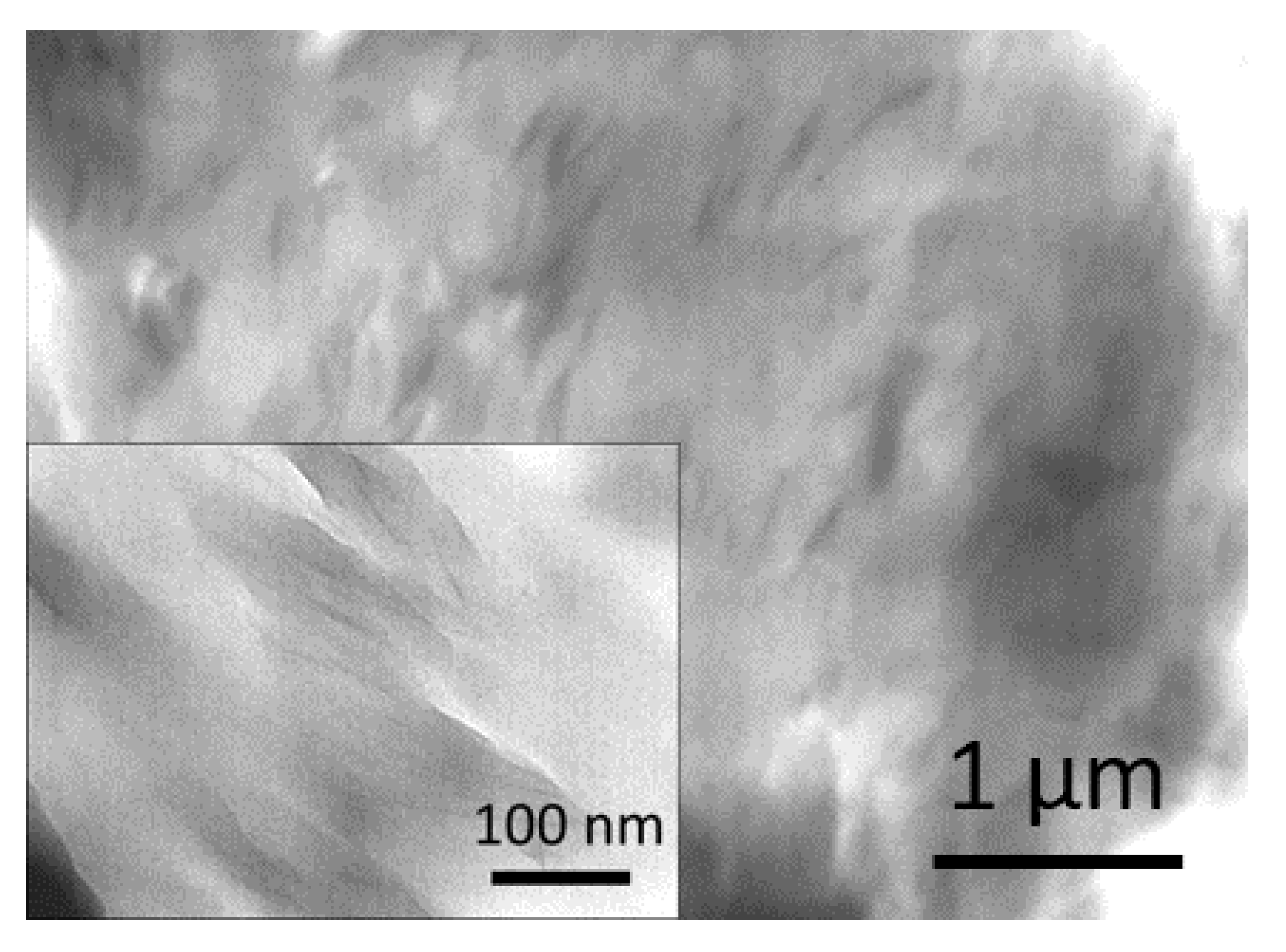
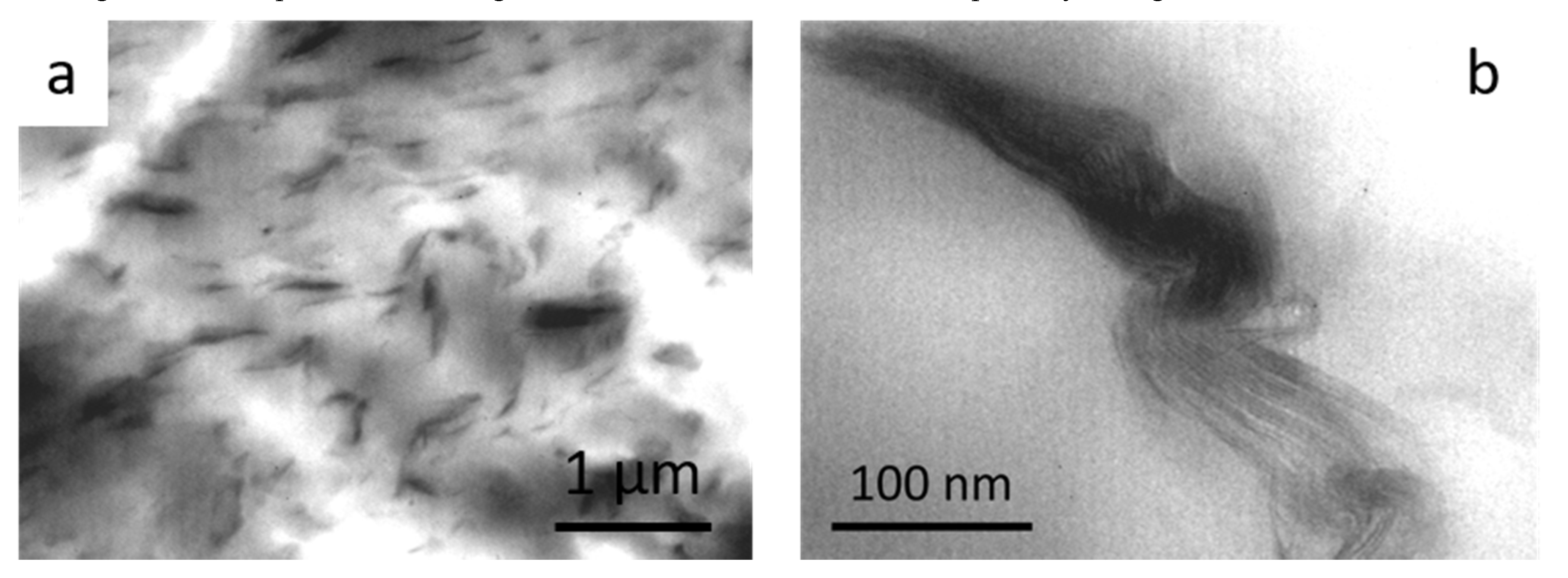
3.7. Transmission Electron Microscopy (TEM)
3.8. MALDI-TOF MS Analysis
3.9. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS)
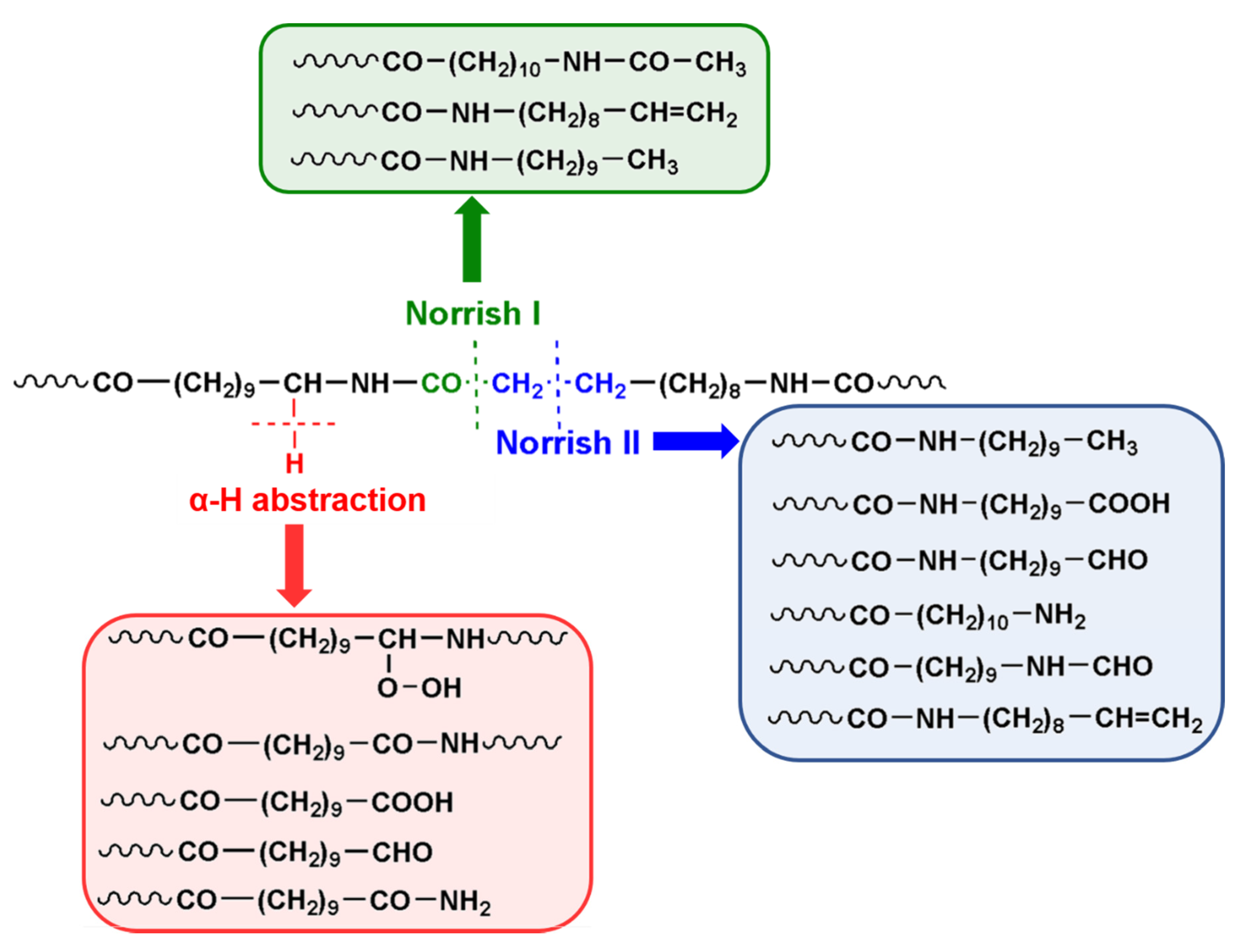
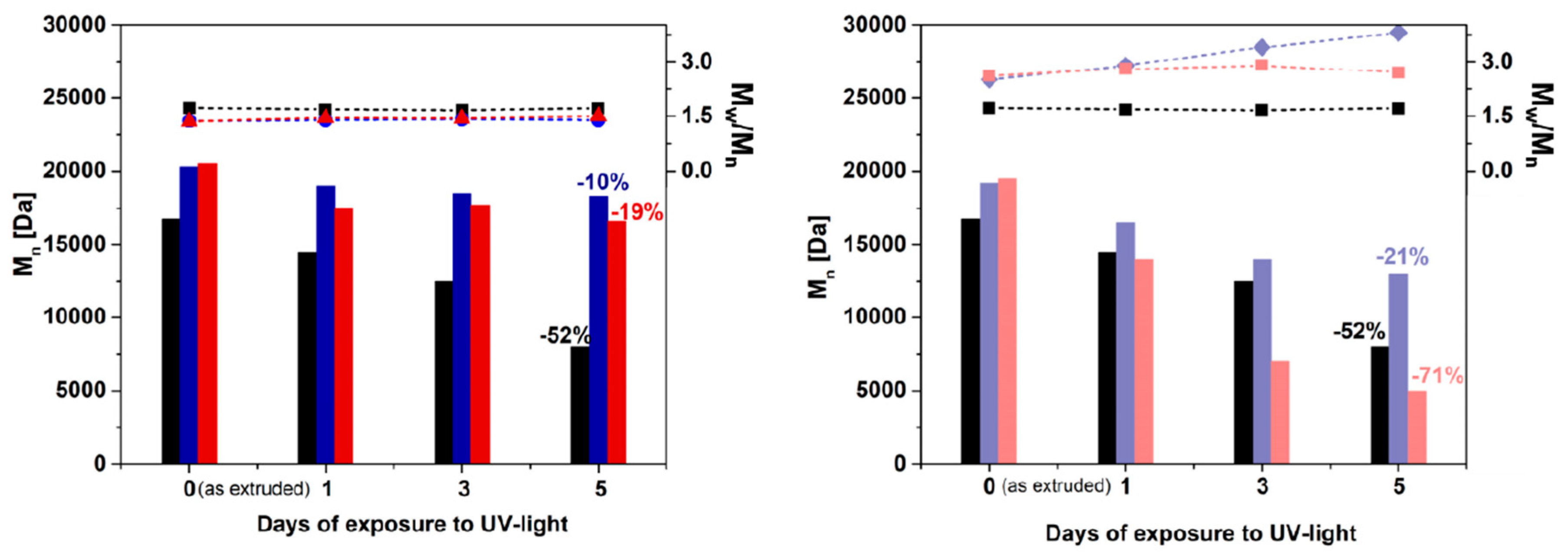
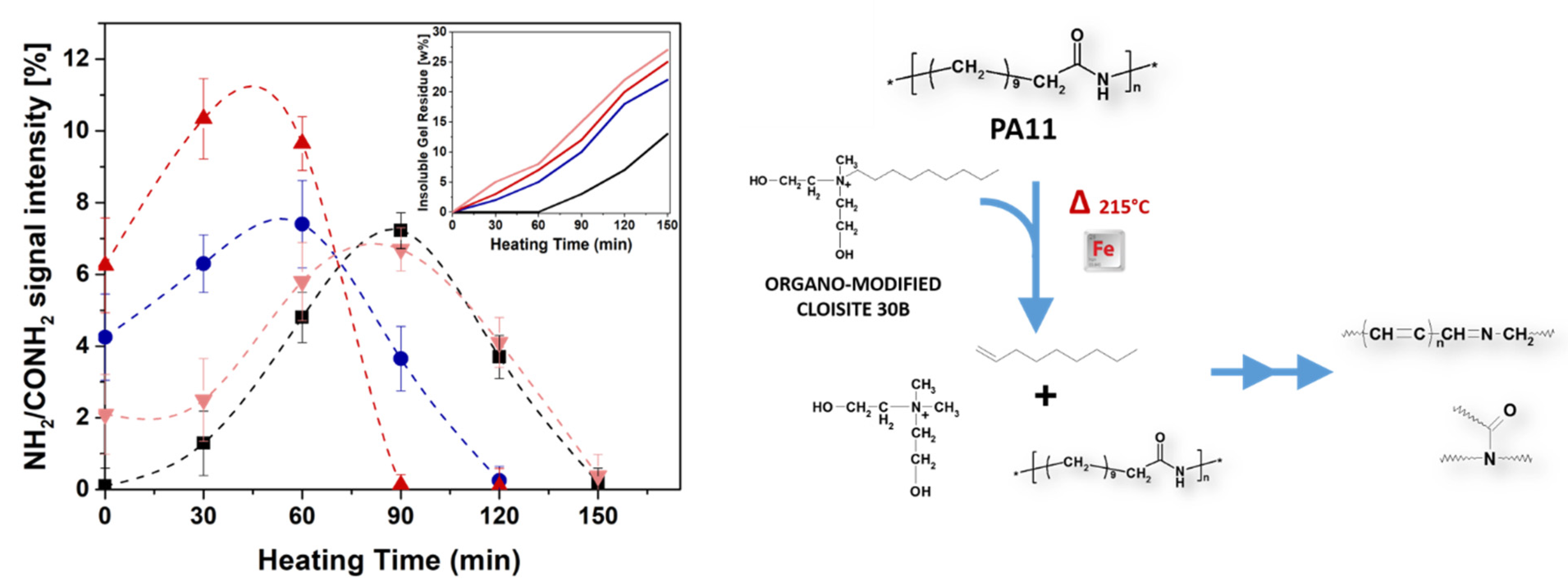
4. Results and Discussion
4.1. Molar Masses Determination
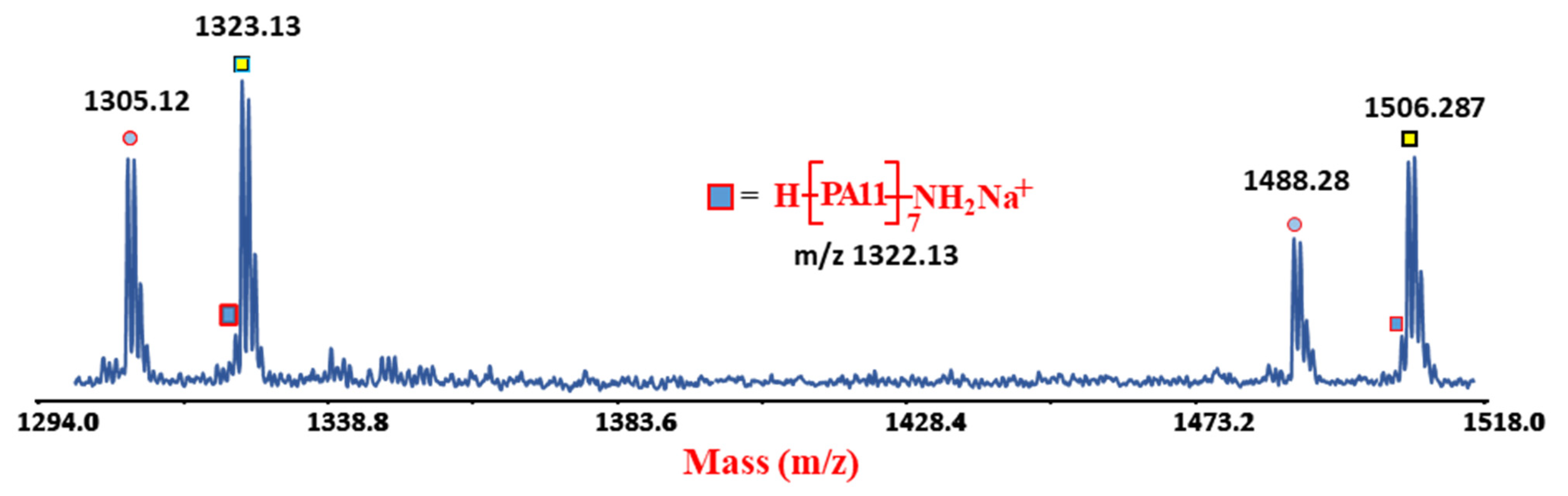
4.2. MALDI-TOF Analyses
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef]
- Richaud, E.; Diogo, O.; Fayolle, B.; Verdu, J.; Guilment, J.; Fernagut, F. Auto-oxidation of aliphatic polyamides. Polym. Degrad. Stab. 2013, 98, 1929–1939. [Google Scholar] [CrossRef]
- Oliveira, M.J.; Botelho, G. Degradation of polyamide 11 in rotational moulding. Polym. Degrad. Stab. 2008, 93, 139–146. [Google Scholar] [CrossRef]
- Filippone, G.; Carroccio, S.C.; Mendichi, R.; Gioiella, L.; Dintcheva, N.T.; Gambarotti, C. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt state—Part I: Thermal and thermo-oxidative degradation of polyamide 11. Polymer 2015, 72, 134–141. [Google Scholar] [CrossRef]
- Bai, J.; Yuan, S.; Shen, F.; Zhang, B.; Chua, C.K.; Zhou, K.; Wei, J. Toughening of polyamide 11 with carbon nanotubes for additive manufacturing. Virtual Phys. Prototyp. 2017, 12, 235–240. [Google Scholar] [CrossRef]
- Hocker, S.J.A.; Hudson-Smith, N.V.; Smith, P.T.; Komatsu, C.H.; Dickinson, L.R.; Schniepp, H.C.; Kranbuehl, D.E. Graphene oxide reduces the hydrolytic degradation in polyamide-11. Polymer 2017, 126, 248–258. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Singh Tomer, N.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization—Trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Ferry, L.; Sonnier, R.; Lopez-Cuesta, J.M.; Petigny, S.; Bert, C. Thermal degradation and flammability of polyamide 11 filled with nanoboehmite. J. Therm. Anal. Calorim. 2017, 129, 1029–1037. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Ussia, M.; Mendichi, R.; Zampino, D.; Puglisi, C.; Carroccio, S.C. Halloysite nanotubes and thymol as photo protectors of biobased polyamide 11. Polym. Degrad. Stab. 2018, 152, 43–51. [Google Scholar] [CrossRef]
- Hao, A.; Wong, I.; Wu, H.; Lisco, B.; Ong, B.; Sallean, A.; Butler, S.; Londa, M.; Koo, J.H. Mechanical, thermal, and flame-retardant performance of polyamide 11–halloysite nanotube nanocomposites. J. Mater. Sci. 2015, 50, 157–167. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwo, G. Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 2010, 20, 9306–9321. [Google Scholar] [CrossRef]
- Bussière, P.O.; Peyroux, J.; Chadeyron, G.; Therias, S. Influence of functional nanoparticles on the photostability of polymer materials: Recent progress and further applications. Polym. Degrad. Stab. 2013, 98, 2411–2418. [Google Scholar] [CrossRef]
- Rosu, D.; Visakh, P.M. (Eds.) Photochemical Behavior of Multicomponent Polymeric-Based Materials; Springer International Publishing: Cham, Switzerland, 2016; Volume 26, pp. 21–65. [Google Scholar]
- Morlat, S.; Mailhot, B.; Gonzalez, D.; Gardette, J.L. Photo-oxidation of polypropylene/montmorillonite nanocomposites. 1. Influence of nanoclay and compatibilizing agent. Chem. Mater. 2004, 16, 377–383. [Google Scholar] [CrossRef]
- Qin, H.L. UV photo aging of polyamide 6/montmorillonite nanocomposite. Chem. J. Chin. Univ. 2004, 25, 197–198. [Google Scholar]
- Morlat, S.; Mailhot, B.; Gonzalez, D.; Gardette, J.L. Photo-oxidation of polypropylene/montmorillonite nanocomposites. 2. Interactions with antioxidants. Chem. Mater. 2005, 17, 1072–1078. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Al-Malaika, S.; La Mantia, F.P. Effect of extrusion and photo-oxidation on polyehylene/clay nanocomposites. Polym. Degrad. Stab. 2009, 94, 1571–1588. [Google Scholar] [CrossRef]
- Qin, H.; Zhao, C.; Zhang, Z.; Chen, G.; Yang, M. Photo-oxidative degradation of polypropylene/montmorillonite nanocomposites. Polym. Degrad. Stab. 2003, 81, 497–500. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, Z.; Feng, M.; Gong, F.; Zhang, S.; Yang, M. The influence of interlayer cations on the photo-oxidative degradation of polyethylene/ montmorillonite composites. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3006–3012. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Kumar, A.P.; Singh, R.P. Photo-stabilization of EPDM-clay nanocomposites: Effect of antioxidant on the preparation and durability. Polym. Adv. Technol. 2007, 18, 891–900. [Google Scholar] [CrossRef]
- Morlat, S.; Mailhot, B.; Gardette, J.L.; Da Silva, C.; Haidar, B.; Vidal, A. Photo-oxidation of ethylene-propylene-diene/montmorillonite nanocomposites. Polym. Degrad. Stab. 2005, 90, 78–85. [Google Scholar] [CrossRef]
- Zaidi, L.; Kaci, M.; Bruzaud, S.; Bourmaud, A.; Grohens, Y. Effect of natural weather on the structure and properties of polylactide/ Cloisite 30 B nanocomposites. Polym. Degrad. Stab. 2010, 95, 527–535. [Google Scholar] [CrossRef]
- Larchè, J.-F.; Bussiere, P.O.; Gardette, J.L. Photo-oxidation of acrylic-urethane thermoset networks. Relating materials properties to changes of chemical structure. Polym. Degrad. Stab. 2011, 96, 1438–1444. [Google Scholar] [CrossRef]
- Filippone, G.; Carroccio, S.C.; Curcuruto, G.; Passaglia, E.; Gambarotti, C.; Dintcheva, N. Time-resolved rheology as a tool to monitor the progress of polymer degradation in the melt state—Part II: Thermal and thermo-oxidative degradation of polyamide. Polymer 2015, 73, 102–110. [Google Scholar] [CrossRef]
- Montaudo, G.; Carroccio, S.; Samperi, F.; Puglisi, C. Recent advances in MALDI mass spectrometry of polymers. Macromol. Symp. 2004, 218, 101–112. [Google Scholar] [CrossRef]
- Montaudo, G.; Samperi, F.; Montaudo, M.S.; Carroccio, S.C.; Puglisi, C. Current Trends in Matrix-Assisted Laser Desorption/Ionization of Polymeric Materials. Eur. J. Mass Spectrom. 2005, 11, 1–14. [Google Scholar] [CrossRef]
- Roger, A.; Lemaire, J.; Sallet, D. Photochemistry of aliphatic polyamides. 4. Mechanisms of photooxidation of polyamides 6, 11, and 12 at long wavelengths. Macromolecules 1986, 19, 579–584. [Google Scholar] [CrossRef]
- Lemaire, J.; Gardette, J.L.; Rivaton, A.; Roger, A. Dual photo-chemistries in aliphatic polyamides, bisphenol A polycarbonate and aromatic polyurethanes-A short review. Polym. Degrad. Stab. 1986, 15, 1–13. [Google Scholar] [CrossRef]
- Carroccio, S.C.; Puglisi, C.; Scaltro, G.; Ferreri, T.; Montaudo, G. Matrix-assisted laser desorption/ionization time-of-flight investigation of Nylon 6 and Nylon 66 thermo-oxidation products. Eur. J. Mass Spectrom. 2007, 13, 397–408. [Google Scholar] [CrossRef]
- Carroccio, S.C.; Puglisi, C.; Montaudo, G. New vistas in the photo-oxidation of nylon 6. Macromolecules 2003, 36, 7499–7507. [Google Scholar] [CrossRef]
- Carroccio, S.C.; Puglisi, C.; Montaudo, G. MALDI investigation of the photooxidation of nylon-66. Macromolecules 2004, 37, 6037–6049. [Google Scholar] [CrossRef]
- Karsters, T.; Rossbach, V. Thermo-oxidative degradation of polyamide 6 and 6,6. Structure of UV/Vis-active chromophores. Die Makromol. Chem. 1990, 191, 757–771. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Chun, D.-M. Fe2O3 as a photocatalytic material: A review. Appl. Cataly. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Carroccio, S.C.; Puglisi, C. Chapter 13: Polymer Degradation. In Mass Spectrometry in Polymer Chemistry; WILEY-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2011; p. 437. [Google Scholar]
- Xie, W.; Gao, Z.; Pan, W.P.; Hunter, D.; Singh, A.; Vaia, R. Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem. Mater. 2001, 13, 2979–2990. [Google Scholar] [CrossRef]
| Organoclay | Modifier Concentration | Specific Gravity (g/cc) | Particle Size (µm) | Interlayer Spacing (d001) |
|---|---|---|---|---|
| Cloisite®30B | 90 mEq/100 g clay | 1.98 | 13 | 1.85 |
| Cloisite Na+ | - | 2.86 | ≤2 | 1.17 |
| PA11-MMTC3 | PA11-MMTC9 | PA11-CC3 | PA11-CC9 | |
|---|---|---|---|---|
| Iron Content (ppb) | 105.0 | 183 | 104.5 | 181.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ussia, M.; Curcuruto, G.; Zampino, D.; Dintcheva, N.T.; Filippone, G.; Mendichi, R.; Carroccio, S.C. Role of Organo-Modifier and Metal Impurities of Commercial Nanoclays in the Photo- and Thermo-Oxidation of Polyamide 11 Nanocomposites. Polymers 2020, 12, 1034. https://doi.org/10.3390/polym12051034
Ussia M, Curcuruto G, Zampino D, Dintcheva NT, Filippone G, Mendichi R, Carroccio SC. Role of Organo-Modifier and Metal Impurities of Commercial Nanoclays in the Photo- and Thermo-Oxidation of Polyamide 11 Nanocomposites. Polymers. 2020; 12(5):1034. https://doi.org/10.3390/polym12051034
Chicago/Turabian StyleUssia, Martina, Giusy Curcuruto, Daniela Zampino, Nadka Tzankova Dintcheva, Giovanni Filippone, Raniero Mendichi, and Sabrina Carola Carroccio. 2020. "Role of Organo-Modifier and Metal Impurities of Commercial Nanoclays in the Photo- and Thermo-Oxidation of Polyamide 11 Nanocomposites" Polymers 12, no. 5: 1034. https://doi.org/10.3390/polym12051034
APA StyleUssia, M., Curcuruto, G., Zampino, D., Dintcheva, N. T., Filippone, G., Mendichi, R., & Carroccio, S. C. (2020). Role of Organo-Modifier and Metal Impurities of Commercial Nanoclays in the Photo- and Thermo-Oxidation of Polyamide 11 Nanocomposites. Polymers, 12(5), 1034. https://doi.org/10.3390/polym12051034







