Large, Rapid Swelling of High-cis Polydicyclopentadiene Aerogels Suitable for Solvent-Responsive Actuators
Abstract
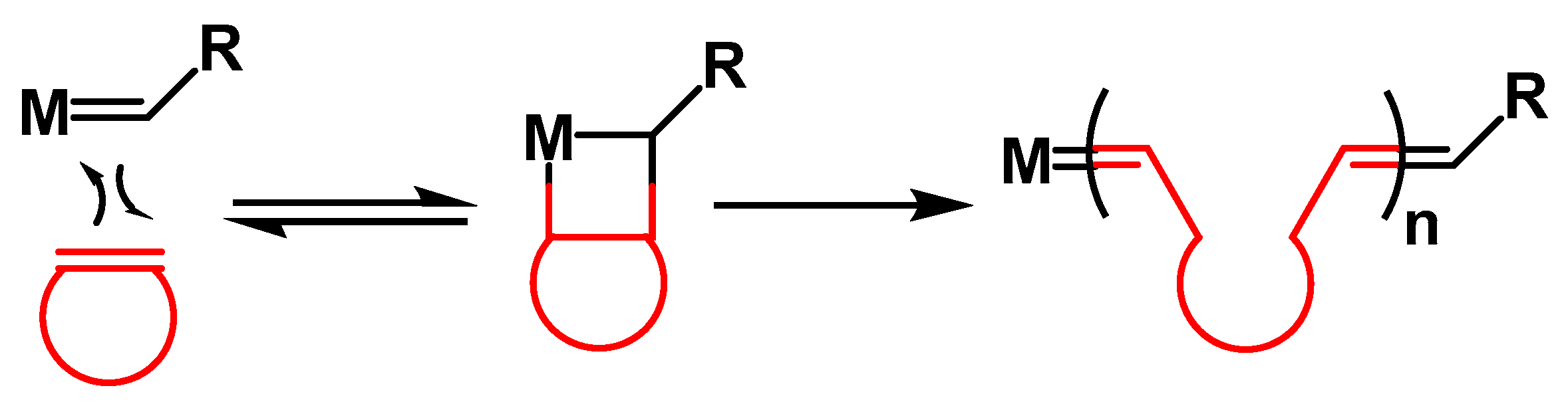
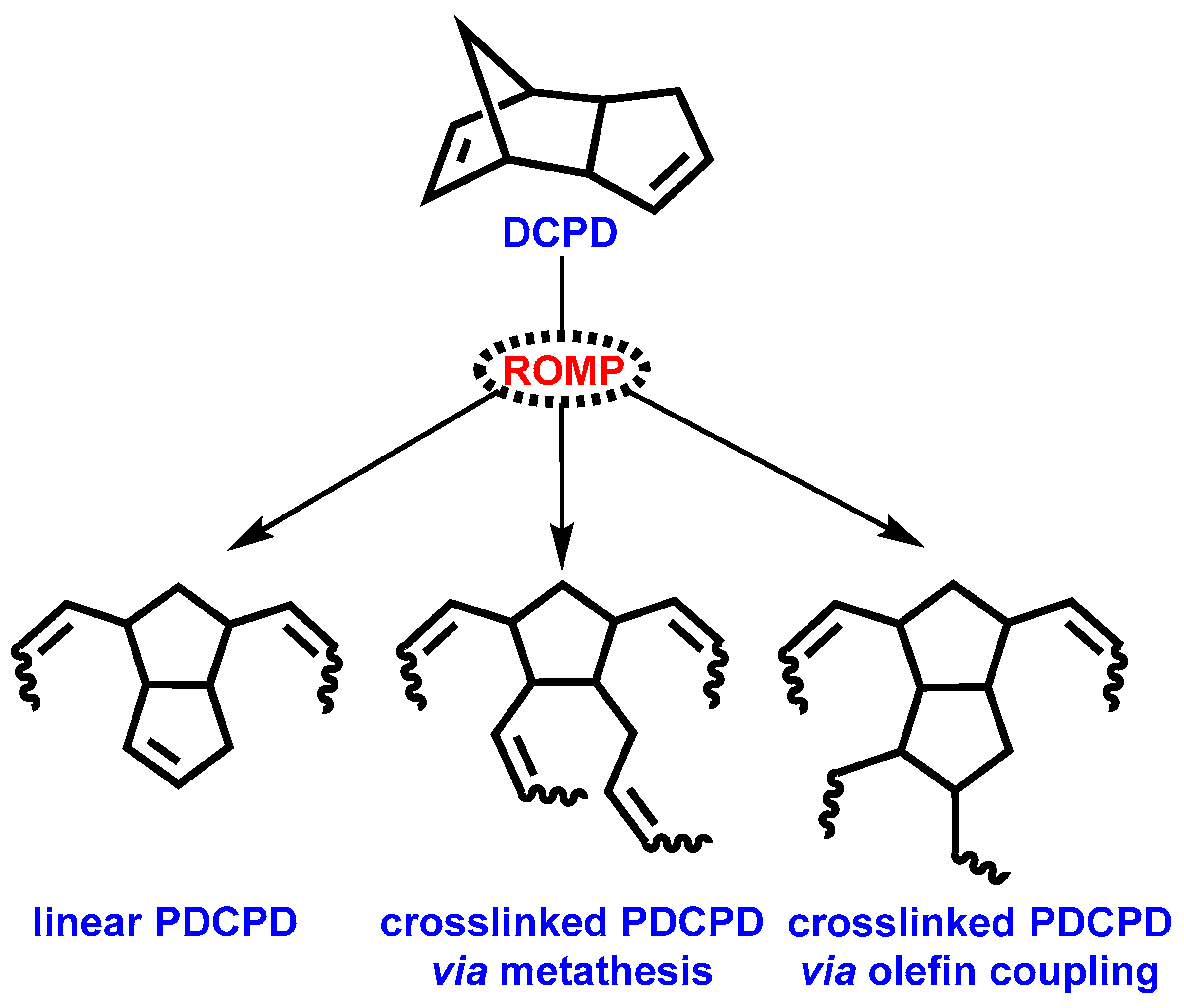
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
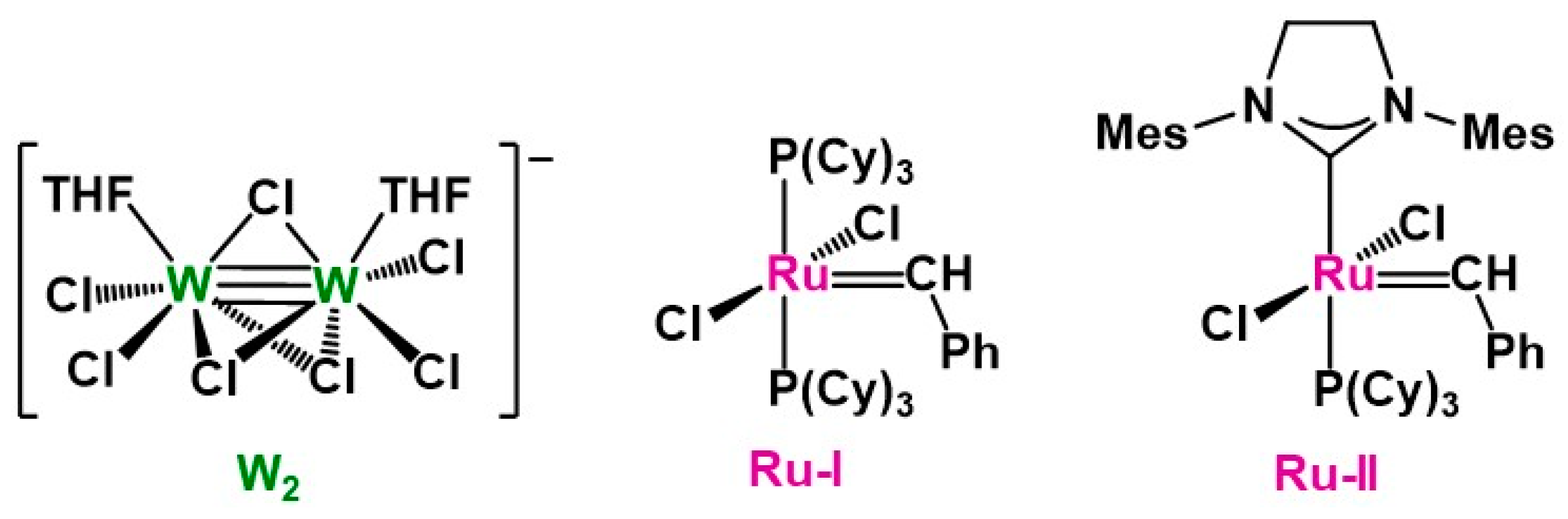
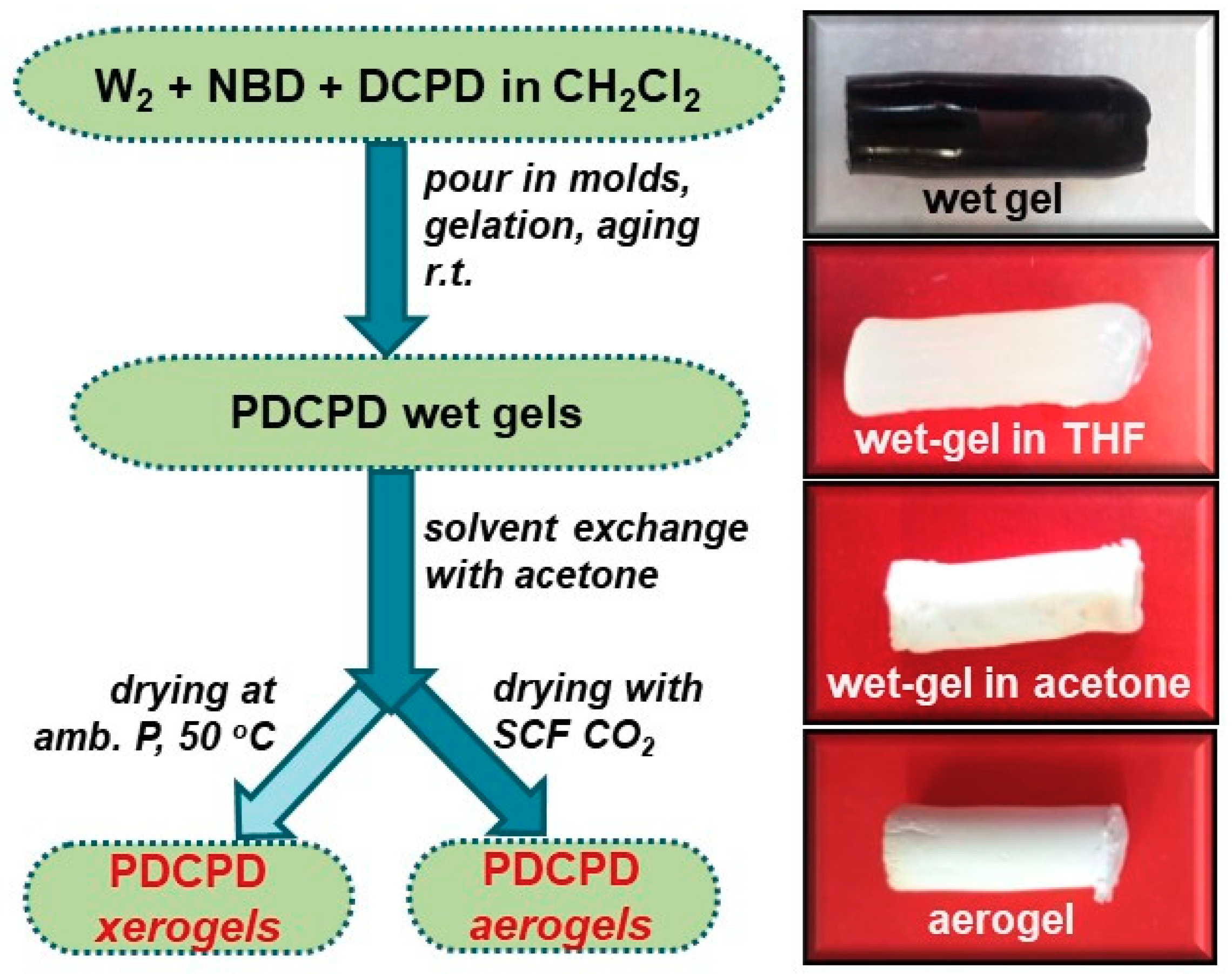
2.2. Synthesis of PDCPD Xerogels and Aerogels Using the Catalytic System W2/NBD
2.3. Study of the Swelling Behavior of PDCPD Aerogels
3. Results and Discussion
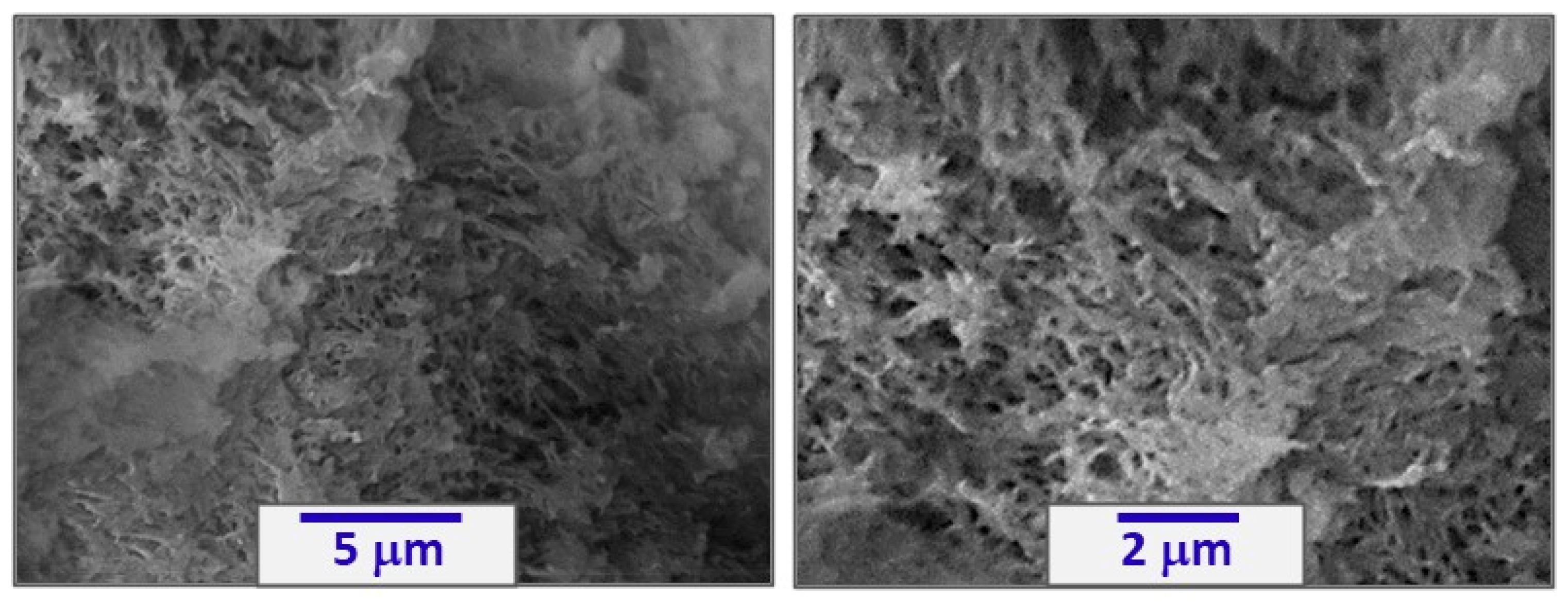
3.1. Preparation of PDCPD Xerogels and Aerogels Using the Catalytic System W2/NBD
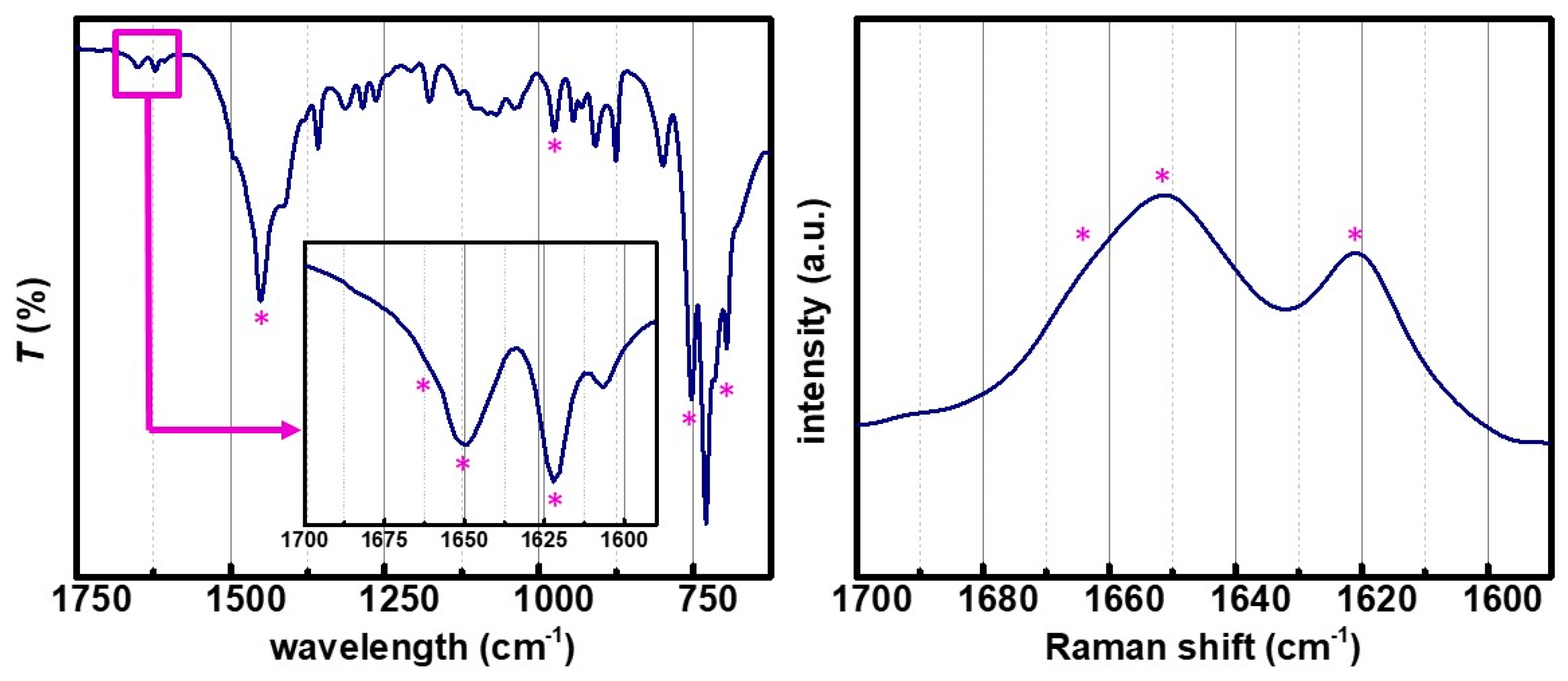
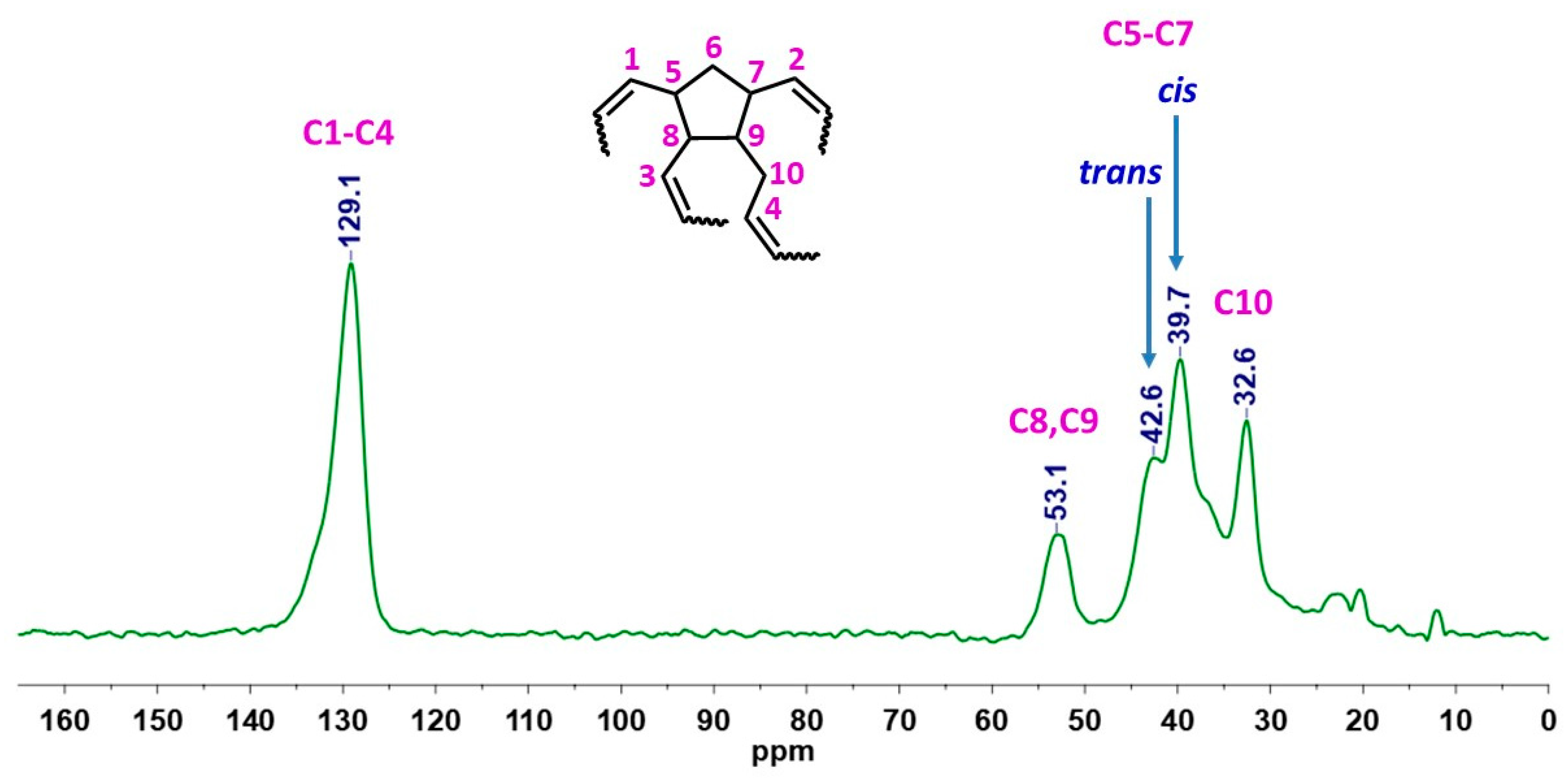
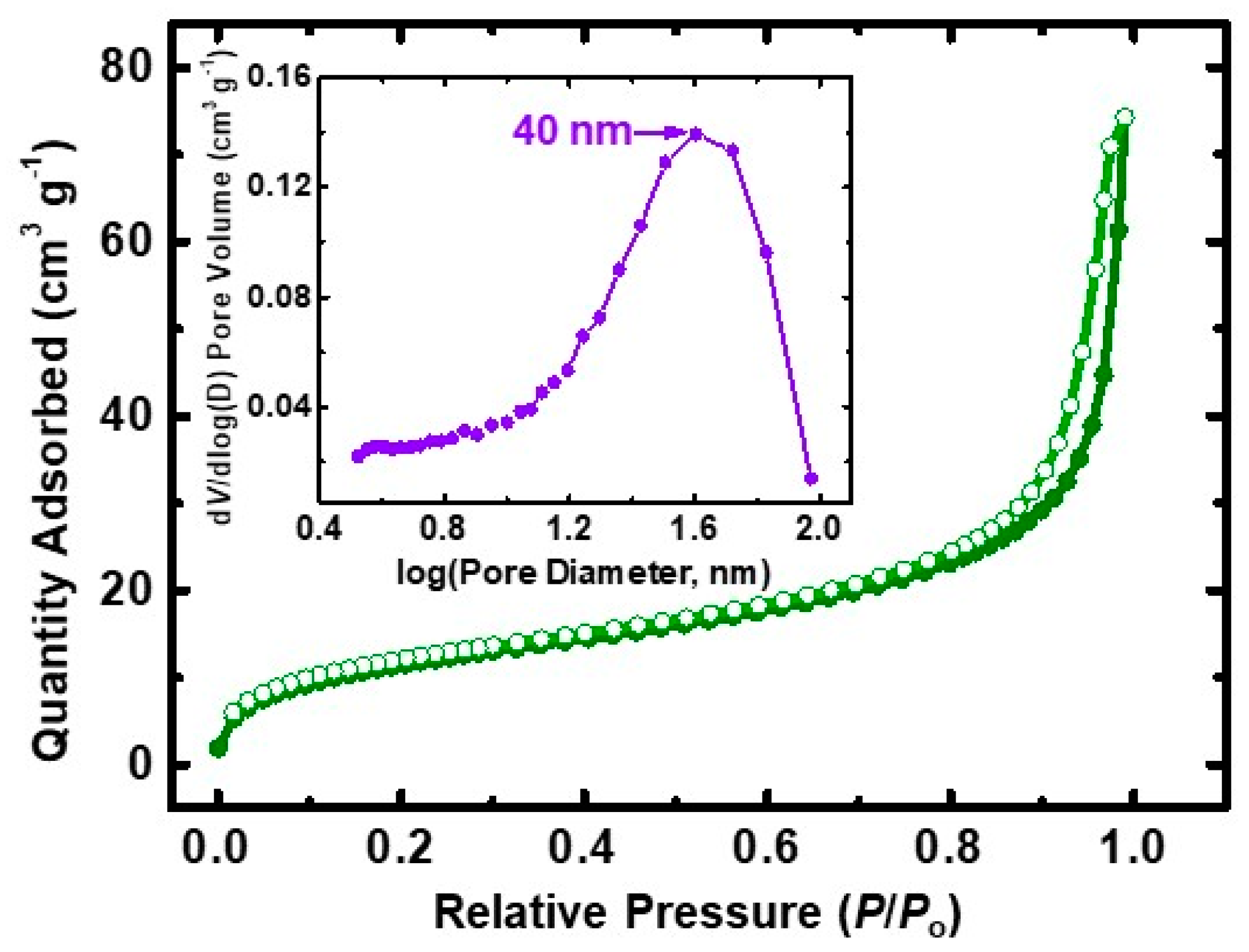
3.2. Physicochemical Characterization of PDCPD Xerogels and Aerogels
3.3. Swelling Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reim, M.; Körner, W.; Manara, J.; Korder, S.; Arduini-Schuster, M.; Ebert, H.-P.; Fricke, J. Silica aerogel granulate material for thermal insulation and daylighting. Sol. Energy 2005, 79, 131–139. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Stepanian, C.J.; Gould, G.L.; Begag, R. Aerogel Composite with Fibrous Batting. U.S. Patent 7078359B2, 18 July 2006. [Google Scholar]
- Malakooti, S.; Churu, H.G.; Lee, A.; Xu, T.; Luo, H.; Xiang, N.; Sotiriou-Leventis, C.; Leventis, N.; Lu, H. Sound insulation properties in low-density, mechanically strong and ductile nanoporous polyurea aerogels. J. Non-Cryst. Solids 2017, 476, 36–45. [Google Scholar] [CrossRef]
- Malakooti, S.; Churu, H.G.; Lee, A.; Rostami, S.; May, S.J.; Ghidei, S.; Wang, F.; Lu, Q.; Luo, H.; Xiang, N.; et al. Sound Transmission Loss Enhancement in an Inorganic-Organic Laminated Wall Panel Using Multifunctional Low-Density Nanoporous Polyurea Aerogels: Experiment and Modeling. Adv. Eng. Mater. 2018, 20, 1700937. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Hall, P.J. Preparation of controlled porosity carbon aerogels for energy storage in rechargeable lithium oxygen batteries. Electrochim. Acta 2009, 54, 7444–7451. [Google Scholar] [CrossRef] [Green Version]
- Biener, J.; Stadermann, M.; Suss, M.; Worsley, M.A.; Biener, M.M.; Rose, K.A.; Baumann, T.F. Advanced carbon aerogels for energy applications. Energy Environ. Sci. 2011, 4, 656–667. [Google Scholar] [CrossRef]
- Kabbour, H.; Baumann, T.F.; Satcher, J.H.; Saulnier, A.; Ahn, C.C. Toward New Candidates for Hydrogen Storage: High-Surface-Area Carbon Aerogels. Chem. Mater. 2006, 18, 6085–6087. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- García-González, C.A.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef] [Green Version]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, M.P.; Gonçalves, V.S.S.; Gaspar, F.B.; Nogueira, I.D.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.A.; Ikkala, O. Hydrophobic Nanocellulose Aerogels as Floating, Sustainable, Reusable, and Recyclable Oil Absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef]
- Amonette, J.E.; Matyáš, J. Functionalized silica aerogels for gas-phase purification, sensing, and catalysis: A review. Microporous Mesoporous Mater. 2017, 250, 100–119. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D Nitrogen-Doped Graphene Aerogel-Supported Fe3O4 Nanoparticles as Efficient Electrocatalysts for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl. Catal. B Environ. 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Saeed, A.M.; Wisner, C.A.; Donthula, S.; Majedi Far, H.; Sotiriou-Leventis, C.; Leventis, N. Reuseable Monolithic Nanoporous Graphite-Supported Nanocatalysts (Fe, Au, Pt, Pd, Ni, and Rh) from Pyrolysis and Galvanic Transmetalation of Ferrocene-Based Polyamide Aerogels. Chem. Mater. 2016, 28, 4867–4877. [Google Scholar] [CrossRef]
- Cai, B.; Eychmüller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2019, 31, 1804881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.-K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef] [Green Version]
- Rewatkar, P.M.; Taghvaee, T.; Saeed, A.M.; Donthula, S.; Mandal, C.; Chandrasekaran, N.; Leventis, T.; Shruthi, T.K.; Sotiriou-Leventis, C.; Leventis, N. Sturdy, Monolithic SiC and Si3N4 Aerogels from Compressed Polymer-Cross-Linked Silica Xerogel Powders. Chem. Mater. 2018, 30, 1635–1647. [Google Scholar] [CrossRef]
- Feinle, A.; Hüsing, N. Mixed metal oxide aerogels from tailor-made precursors. J. Supercrit. Fluids 2015, 106, 2–8. [Google Scholar] [CrossRef]
- Gurikov, P.; Raman, S.P.; Weinrich, D.; Fricke, M.; Smirnova, I. A novel approach to alginate aerogels: Carbon dioxide induced gelation. RSC Adv. 2015, 5, 7812–7818. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Smirnova, I.; Athamneh, T.; Papastergiou, M.; Chriti, D.; Mali, G.; Čendak, T.; Chatzichristidi, M.; Raptopoulos, G.; Gurikov, P. Mechanically-Strong Polyurea/Polyurethane-Crosslinked Alginate Aerogels. ACS Appl. Polym. Mater. 2020, 2, 1974–1988. [Google Scholar] [CrossRef]
- Baudron, V.; Taboada, M.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Production of starch aerogel in form of monoliths and microparticles. Colloid Polym. Sci. 2020, 298, 477–494. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-B.; Schiraldi, D.A. Flammability of Polymer/Clay Aerogel Composites: An Overview. Polym. Rev. 2018, 59, 1–24. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Gurikov, P.; Raptopoulos, G.; Chriti, D.; Papastergiou, M.; Kypritidou, Z.; Skounakis, V.; Argyraki, A. Strategies toward catalytic biopolymers: Incorporation of tungsten in alginate aerogels. Polyhedron 2018, 154, 209–216. [Google Scholar] [CrossRef]
- Mandal, C.; Donthula, S.; Far, H.M.; Saeed, A.M.; Sotiriou-Leventis, C.; Leventis, N. Transparent, mechanically strong, thermally insulating cross-linked silica aerogels for energy-efficient windows. J. Sol-Gel Sci. Technol. 2019, 92, 84–100. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Mulik, S.; Sotiriou-Leventis, C. The effect of compactness on the carbothermal conversion of interpenetrating metal oxide/resorcinol-formaldehyde nanoparticle networks to porous metals and carbides. J. Mater. Chem. 2010, 20, 7456–7471. [Google Scholar] [CrossRef]
- Rewatkar, P.M.; Soni, R.U.; Sotiriou-Leventis, C.; Leventis, N. A Cobalt Sunrise: Thermites Based on LiClO4-Filled Co(0) Aerogels Prepared from Polymer-Cross-Linked Cobaltia Xerogel Powders. ACS Appl. Mater. Interfaces 2019, 11, 22668–22676. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Gao, J.; Yao, J.; Zhang, Q. Recent progress in metal-organic frameworks-based hydrogels and aerogels and their applications. Coord. Chem. Rev. 2019, 398, 213016. [Google Scholar] [CrossRef]
- Alshrah, M.; Tran, M.-P.; Gong, P.; Naguib, H.E.; Park, C.B. Development of high-porosity resorcinol formaldehyde aerogels with enhanced mechanical properties through improved particle necking under CO2 supercritical conditions. J. Colloid Interface Sci. 2017, 485, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Time-Efficient Acid-Catalyzed Synthesis of Resorcinol−Formaldehyde Aerogels. Chem. Mater. 2007, 19, 6138–6144. [Google Scholar] [CrossRef]
- Mulik, S.; Sotiriou-Leventis, C.; Leventis, N. Macroporous Electrically Conducting Carbon Networks by Pyrolysis of Isocyanate-Cross-Linked Resorcinol-Formaldehyde Aerogels. Chem. Mater. 2008, 20, 6985–6997. [Google Scholar] [CrossRef]
- Williams, J.C.; Nguyen, B.N.; McCorkle, L.; Scheiman, D.; Griffin, J.S.; Steiner, S.A.; Meador, M.A.B. Highly Porous, Rigid-Rod Polyamide Aerogels with Superior Mechanical Properties and Unusually High Thermal Conductivity. ACS Appl. Mater. Interfaces 2017, 9, 1801–1809. [Google Scholar] [CrossRef]
- Leventis, N.; Chidambareswarapattar, C.; Mohite, D.P.; Larimore, Z.J.; Lu, H.; Sotiriou-Leventis, C. Multifunctional porous aramids (aerogels) by efficient reaction of carboxylic acids and isocyanates. J. Mater. Chem. 2011, 21, 11981–11986. [Google Scholar] [CrossRef]
- Williams, J.C.; Meador, M.A.B.; McCorkle, L.; Mueller, C.; Wilmoth, N. Synthesis and Properties of Step-Growth Polyamide Aerogels Cross-linked with Triacid Chlorides. Chem. Mater. 2014, 26, 4163–4171. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; Larimore, Z.; Sotiriou-Leventis, C.; Mang, J.T.; Leventis, N. One-step room-temperature synthesis of fibrous polyimide aerogels from anhydrides and isocyanates and conversion to isomorphic carbons. J. Mater. Chem. 2010, 20, 9666–9678. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Mohite, D.P.; Larimore, Z.J.; Mang, J.T.; Churu, G.; Lu, H. Polyimide Aerogels by Ring-Opening Metathesis Polymerization (ROMP). Chem. Mater. 2011, 23, 2250–2261. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; Xu, L.; Sotiriou-Leventis, C.; Leventis, N. Robust monolithic multiscale nanoporous polyimides and conversion to isomorphic carbons. RSC Adv. 2013, 3, 26459–26469. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Agnello, M.; McCorkle, L.; Vivod, S.L.; Wilmoth, N. Moisture-Resistant Polyimide Aerogels Containing Propylene Oxide Links in the Backbone. ACS Appl. Mater. Interfaces 2016, 8, 29073–29079. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Alemán, C.R.; Hanson, K.; Ramirez, N.; Vivod, S.L.; Wilmoth, N.; McCorkle, L. Polyimide Aerogels with Amide Cross-Links: A Low Cost Alternative for Mechanically Strong Polymer Aerogels. ACS Appl. Mater. Interfaces 2015, 7, 1240–1249. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Meador, M.A.B.; McCorkle, L.S.; Scheiman, D.A.; McCrone, J.D.; Wilkewitz, B. Poly(maleic anhydride) cross-linked polyimide aerogels: Synthesis and properties. RSC Adv. 2016, 6, 26055–26065. [Google Scholar] [CrossRef]
- Vivod, S.L.; Meador, M.A.B.; Pugh, C.; Wilkosz, M.; Calomino, K.; McCorkle, L. Toward Improved Optical Transparency of Polyimide Aerogels. ACS Appl. Mater. Interfaces 2020, 12, 8622–8633. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; McCarver, P.M.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Fractal Multiscale Nanoporous Polyurethanes: Flexible to Extremely Rigid Aerogels from Multifunctional Small Molecules. Chem. Mater. 2013, 25, 3205–3224. [Google Scholar] [CrossRef]
- Kanellou, A.; Anyfantis, G.C.; Chriti, D.; Raptopoulos, G.; Pitsikalis, M.; Paraskevopoulou, P. Poly(urethane-norbornene) Aerogels via Ring Opening Metathesis Polymerization of Dendritic Urethane-Norbornene Monomers: Structure-Property Relationships as a Function of an Aliphatic Versus an Aromatic Core and the Number of Peripheral Norbornene Moieties. Molecules 2018, 23, 1007. [Google Scholar] [CrossRef] [Green Version]
- Leventis, N.; Sotiriou-Leventis, C.; Chidambareswarapattar, C. Porous Polyurethane Networks and Methods of Preparation. U.S. Patent 20180251623A1, 6 September 2018. [Google Scholar]
- Papastergiou, M.; Kanellou, A.; Chriti, D.; Raptopoulos, G.; Paraskevopoulou, P. Poly(Urethane-Acrylate) Aerogels via Radical Polymerization of Dendritic Urethane-Acrylate Monomers. Materials 2018, 11, 2249. [Google Scholar] [CrossRef] [Green Version]
- Papastergiou, M.; Chriti, D.; Damalas, D.E.; Raptopoulos, G.; Paraskevopoulou, P. Poly(urethane-acrylate) aerogels from the isocyanurate trimer of isophorone diisocyanate. J. Supercrit. Fluids 2019, 148, 42–54. [Google Scholar] [CrossRef]
- Bang, A.; Buback, C.; Sotiriou-Leventis, C.; Leventis, N. Flexible Aerogels from Hyperbranched Polyurethanes: Probing the Role of Molecular Rigidity with Poly(Urethane Acrylates) Versus Poly(Urethane Norbornenes). Chem. Mater. 2014, 26, 6979–6993. [Google Scholar] [CrossRef]
- Taghvaee, T.; Donthula, S.; Rewatkar, P.M.; Majedi Far, H.; Sotiriou-Leventis, C.; Leventis, N. K-Index: A Descriptor, Predictor, and Correlator of Complex Nanomorphology to Other Material Properties. ACS Nano 2019, 13, 3677–3690. [Google Scholar] [CrossRef]
- Chriti, D.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P. Millimeter-Size Spherical Polyurea Aerogel Beads with Narrow Size Distribution. Gels 2018, 4, 66. [Google Scholar] [CrossRef] [Green Version]
- Leventis, N.; Sotiriou-Leventis, C.; Chandrasekaran, N.; Mulik, S.; Larimore, Z.J.; Lu, H.; Churu, G.; Mang, J.T. Multifunctional Polyurea Aerogels from Isocyanates and Water. A Structure−Property Case Study. Chem. Mater. 2010, 22, 6692–6710. [Google Scholar] [CrossRef]
- Paraskevopoulou, P.; Chriti, D.; Raptopoulos, G.; Anyfantis, G.C. Synthetic Polymer Aerogels in Particulate Form. Materials 2019, 12, 1543. [Google Scholar] [CrossRef] [Green Version]
- Leventis, N.; Sotiriou-Leventis, C.; Saeed, A.M.; Donthula, S.; Majedi Far, H.; Rewatkar, P.M.; Kaiser, H.; Robertson, J.D.; Lu, H.; Churu, G. Nanoporous Polyurea from a Triisocyanate and Boric Acid: A Paradigm of a General Reaction Pathway for Isocyanates and Mineral Acids. Chem. Mater. 2016, 28, 67–78. [Google Scholar] [CrossRef]
- Ho Kim, S.; Worsley, M.A.; Valdez, C.A.; Shin, S.J.; Dawedeit, C.; Braun, T.; Baumann, T.F.; Letts, S.A.; Kucheyev, S.O.; Jen, J.; et al. Exploration of the versatility of ring opening metathesis polymerization: An approach for gaining access to low density polymeric aerogels. RSC Adv. 2012, 2, 8672–8680. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Gould, G.L. Polydicyclopentadiene based aerogel: A new insulation material. J. Sol-Gel Sci. Technol. 2007, 44, 29–40. [Google Scholar] [CrossRef]
- Mohite, D.P.; Mahadik-Khanolkar, S.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Polydicyclopentadiene aerogels grafted with PMMA: I. Molecular and interparticle crosslinking. Soft Matter 2013, 9, 1516–1530. [Google Scholar] [CrossRef]
- Mohite, D.P.; Mahadik-Khanolkar, S.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Polydicyclopentadiene aerogels grafted with PMMA: II. Nanoscopic characterization and origin of macroscopic deformation. Soft Matter 2013, 9, 1531–1539. [Google Scholar] [CrossRef]
- Bang, A.; Mohite, D.; Saeed, A.M.; Leventis, N.; Sotiriou-Leventis, C. Polydicyclopentadiene aerogels from first- versus second-generation Grubbs’ catalysts: A molecular versus a nanoscopic perspective. J. Sol-Gel Sci. Technol. 2015, 75, 460–474. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, S.J.; Lenhardt, J.M.; Braun, T.; Sain, J.D.; Valdez, C.A.; Leif, R.N.; Kucheyev, S.O.; Wu, K.J.J.; Biener, J.; et al. Deterministic Control over High-Z Doping of Polydicyclopentadiene-Based Aerogel Coatings. ACS Appl. Mater. Interfaces 2013, 5, 8111–8119. [Google Scholar] [CrossRef]
- Lee, J.K.; Gould, G.L. Microporous Polyolefin-Based Aerogels. U.S. Patent 9469739B2, 18 October 2016. [Google Scholar]
- Sutthasupa, S.; Shiotsuki, M.; Sanda, F. Recent advances in ring-opening metathesis polymerization, and application to synthesis of functional materials. Polym. J. 2010, 42, 905–915. [Google Scholar] [CrossRef]
- Leitgeb, A.; Wappel, J.; Slugovc, C. The ROMP toolbox upgraded. Polymer 2010, 51, 2927–2946. [Google Scholar] [CrossRef]
- Nomura, K.; Abdellatif, M.M. Precise synthesis of polymers containing functional end groups by living ring-opening metathesis polymerization (ROMP): Efficient tools for synthesis of block/graft copolymers. Polymer 2010, 51, 1861–1881. [Google Scholar] [CrossRef] [Green Version]
- Slugovc, C. Industrial Applications of Olefin Metathesis Polymerization. In Olefin Metathesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 329–333. ISBN 978-1-118-71161-3. [Google Scholar]
- Robertson, I.D.; Yourdkhani, M.; Centellas, P.J.; Aw, J.E.; Ivanoff, D.G.; Goli, E.; Lloyd, E.M.; Dean, L.M.; Sottos, N.R.; Geubelle, P.H.; et al. Rapid energy-efficient manufacturing of polymers and composites via frontal polymerization. Nature 2018, 557, 223–227. [Google Scholar] [CrossRef]
- Davidson, T.A.; Wagener, K.B.; Priddy, D.B. Polymerization of Dicyclopentadiene: A Tale of Two Mechanisms. Macromolecules 1996, 29, 786–788. [Google Scholar] [CrossRef]
- Della Martina, A.; Hilborn, J.G.; Mühlebach, A. Macroporous Cross-Linked Poly(dicyclopentadiene). Macromolecules 2000, 33, 2916–2921. [Google Scholar] [CrossRef]
- Rule, J.D.; Moore, J.S. ROMP Reactivity of endo- and exo-Dicyclopentadiene. Macromolecules 2002, 35, 7878–7882. [Google Scholar] [CrossRef]
- Autenrieth, B.; Jeong, H.; Forrest, W.P.; Axtell, J.C.; Ota, A.; Lehr, T.; Buchmeiser, M.R.; Schrock, R.R. Stereospecific Ring-Opening Metathesis Polymerization (ROMP) of endo-Dicyclopentadiene by Molybdenum and Tungsten Catalysts. Macromolecules 2015, 48, 2480–2492. [Google Scholar] [CrossRef]
- Raptopoulos, G.; Anyfantis, G.C.; Chriti, D.; Paraskevopoulou, P. Synthesis and structural characterization of poly(dicyclopentadiene) gels obtained with a novel ditungsten versus conventional W and Ru mononuclear catalysts. Inorg. Chim. Acta 2017, 460, 69–76. [Google Scholar] [CrossRef]
- Floros, G.; Saragas, N.; Paraskevopoulou, P.; Psaroudakis, N.; Koinis, S.; Pitsikalis, M.; Hadjichristidis, N.; Mertis, K. Ring Opening Metathesis Polymerization of Norbornene and Derivatives by the Triply Bonded Ditungsten Complex Na[W2(µ-Cl)3Cl4(THF)2]·(THF)3. Polymers 2012, 4, 1657–1673. [Google Scholar] [CrossRef] [Green Version]
- Floros, G.; Agrafioti, F.; Grigoropoulos, A.; Paraskevopoulou, P.; Mertis, K.; Tseklima, M.; Veli, M.; Pitsikalis, M. Statistical copolymers of norbornene and 5-vinyl-2-norbornene by a ditungsten complex mediated ring-opening metathesis Polymerization: Synthesis, thermal properties, and kinetics of thermal decomposition. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4835–4844. [Google Scholar] [CrossRef]
- Saragas, N.; Floros, G.; Raptopoulos, G.; Pitsikalis, M.; Paraskevopoulou, P.; Mertis, K. Exploring the Reactivity of Na[W2(μ-Cl)3Cl4(THF)2]∙(THF)3 towards the Polymerization of Selected Cycloolefins. Molecules 2015, 20, 21896–21908. [Google Scholar] [CrossRef] [Green Version]
- Raptopoulos, G.; Kyriakou, K.; Mali, G.; Scarpellini, A.; Anyfantis, G.C.; Mavromoustakos, T.; Pitsikalis, M.; Paraskevopoulou, P. Copolymerization of Norbornene and Norbornadiene Using a cis-Selective Bimetallic W-Based Catalytic System. Polymers 2017, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Chriti, D.; Grigoropoulos, A.; Raptopoulos, G.; Charalambidis, G.; Nikolaou, V.; Coutsolelos, A.G.; Pitsikalis, M.; Mertis, K.; Paraskevopoulou, P. Metathesis Polymerization Reactions Induced by the Bimetallic Complex (Ph4P)2[W2(μ-Br)3Br6]. Polymers 2015, 7, 2611–2624. [Google Scholar] [CrossRef] [Green Version]
- Chriti, D.; Raptopoulos, G.; Anyfantis, G.C.; Paraskevopoulou, P. An Extreme Case of Swelling of Mostly cis-Polydicyclopentadiene by Selective Solvent Absorption—Application in Decontamination and Environmental Remediation. ACS Appl. Polym. Mater. 2019, 1, 1648–1659. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Corten, C.; Kuckling, D.; Muller, M.; Shi, Z.; Sorber, J.; Arndt, K.-F. Application of Polyelectrolytic Temperature-Responsive Hydrogels in Chemical Sensors. Macromol. Symp. 2007, 254, 314–321. [Google Scholar] [CrossRef]
- Zhao, Q.; Dunlop, J.W.C.; Qiu, X.; Huang, F.; Zhang, Z.; Heyda, J.; Dzubiella, J.; Antonietti, M.; Yuan, J. An instant multi-responsive porous polymer actuator driven by solvent molecule sorption. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Schute, K.; Jansen, F.; Rose, M. Solvent-Responsive and Switchable Nanofiltration Membranes based on Hypercrosslinked Polymers with Permanent Porosity. ChemNanoMat 2018, 4, 562–567. [Google Scholar] [CrossRef]
- Shiraki, T.; Dawn, A.; Tsuchiya, Y.; Shinkai, S. Thermo- and Solvent-Responsive Polymer Complex Created from Supramolecular Complexation between a Helix-Forming Polysaccharide and a Cationic Polythiophene. J. Am. Chem. Soc. 2010, 132, 13928–13935. [Google Scholar] [CrossRef]
- Ehrenhofer, A.; Elstner, M.; Wallmersperger, T. Normalization of hydrogel swelling behavior for sensoric and actuatoric applications. Sens. Actuators B Chem. 2018, 255, 1343–1353. [Google Scholar] [CrossRef]
- Gerlach, G.; Arndt, K.-F. (Eds.) Hydrogel Sensors and Actuators: Engineering and Technology; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-540-75644-6. [Google Scholar]
- Chisholm, M.H.; Eichhorn, B.W.; Folting, K.; Huffman, J.C.; Ontiveros, C.D.; Streib, W.E.; Van der Sluys, W.G. Preparation and characterization of NaW2Cl7(THF)5. A synthetically useful precursor for X3W≡WX3 compounds where X = CH2-t-Bu, NMe2 and O-t-Bu. Inorg. Chem. 1987, 26, 3182–3186. [Google Scholar] [CrossRef]
- Abadie, M.J.; Dimonie, M.; Couve, C.; Dragutan, V. New catalysts for linear polydicyclopentadiene synthesis. Eur. Polym. J. 2000, 36, 1213–1219. [Google Scholar] [CrossRef]
- Saragas, N. Selective Polymerization of Alkynes and Cycloolefins with Bimetallic Complexes of the Transition Metals. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2009. [Google Scholar]
- Barnes, S.E.; Brown, E.C.; Corrigan, N.; Coates, P.D.; Harkin-Jones, E.; Edwards, H.G.M. Raman spectroscopic studies of the cure of dicyclopentadiene (DCPD). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2946–2952. [Google Scholar] [CrossRef]
- Schaubroeck, D.; Brughmans, S.; Vercaemst, C.; Schaubroeck, J.; Verpoort, F. Qualitative FT-Raman investigation of the ring opening metathesis polymerization of dicyclopentadiene. J. Mol. Catal. A Chem. 2006, 254, 180–185. [Google Scholar] [CrossRef]
- Carvalho, V.P., Jr.; Ferraz, C.P.; Lima-Neto, B.S. Tailored norbornene-based copolymer with systematic variation of norbornadiene as a crosslinker obtained via ROMP with alternative amine Ru catalysts. Eur. Polym. J. 2012, 48, 341–349. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Fonseca, L.R.; Tomazetti, V.; Silva, F.C.S.; Lima-Neto, B.S.; Carvalho, V.P. Copolymers from norbornene and norbornadiene with organized morphologies and high Tg values obtained via ROMP with a highly reactive [RuCl3(PCy3)2] complex. New J. Chem. 2016, 40, 9424–9431. [Google Scholar] [CrossRef]
- Chaves, H.K.; Ferraz, C.P.; Carvalho, V.P., Jr.; Lima-Neto, B.S. Tuning the activity of alternative Ru-based initiators for ring-opening metathesis polymerization of norbornene and norbornadiene by the substituent in 4-CH2R-piperidine. J. Mol. Catal. A Chem. 2014, 385, 46–53. [Google Scholar] [CrossRef]
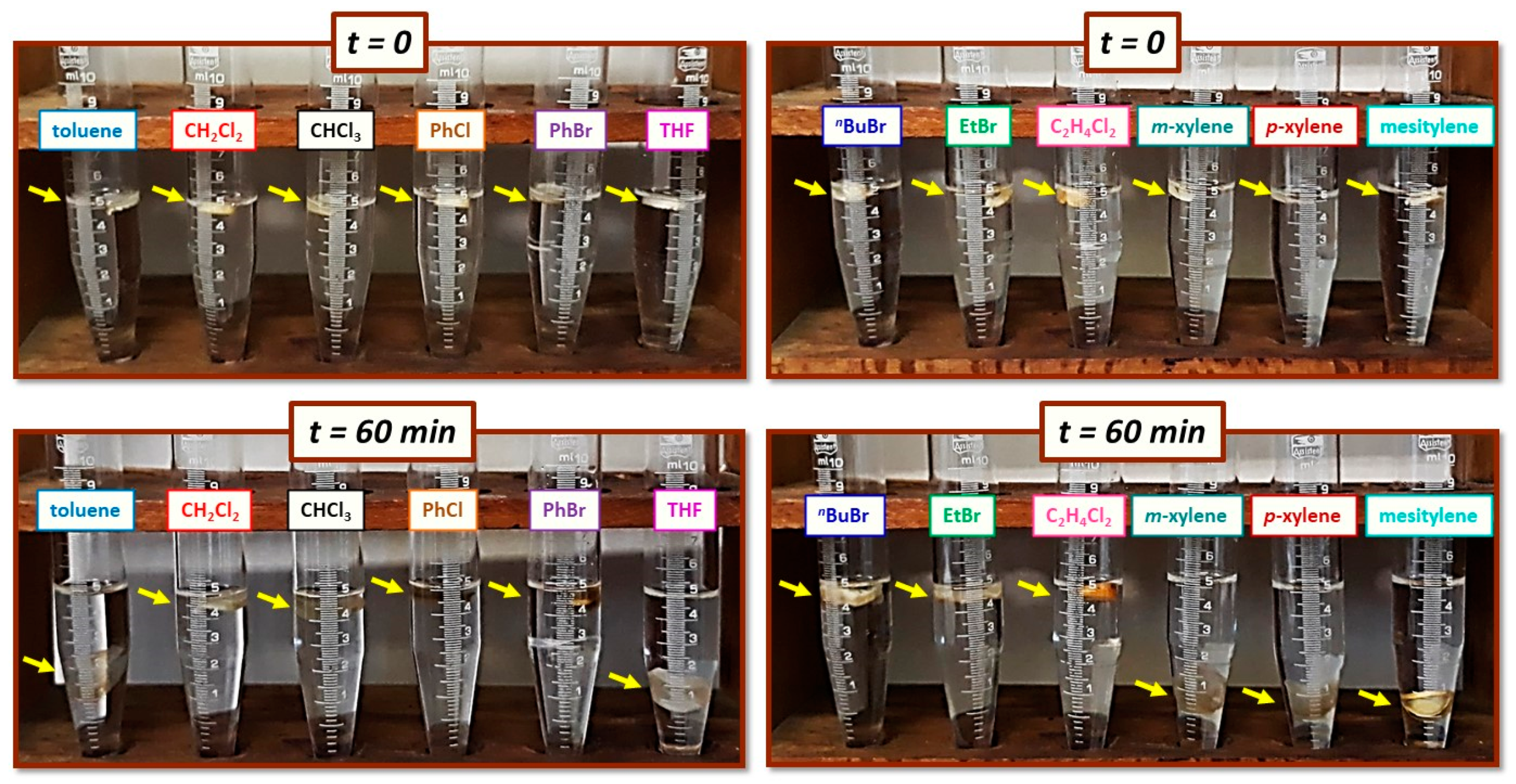
| Catalytic System (Molar Ratio) | Linear Shrinkage 1 (%) | Bulk Density ρb (g cm−3) | Skeletal Density ρs (g cm−3) | Porosity 2 Π (% v/v) | BET Surf. Area σ (m2g−1) | VTotal3 (V1.7–300nm) 4 (cm3 g−1) | Av. Pore Diameter 5 (nm) | Particle Radius 6 (nm) |
|---|---|---|---|---|---|---|---|---|
| W2/NBD 1/10 | 15 | 0.135 ± 0.009 | 1.128 ± 0.005 | 88 | 42 | 7.0 (0.1) | 11 (667) | 63 |
| W2/NBD 1/20 | 26 | 0.52 ± 0.08 | 1.348 ± 0.006 | 61 | <10 | 1.6 (0.009) | 6.6 (914) | - |
| Ru-I [63] | 12 | 0.28 ± 0.07 | 1.136 ± 0.003 | 75 | 186 | 2.7 (NA) | 21 (57) | 14.2 |
| Ru-II [63] | NA 7 | NA | 1.055 ± 0.004 | - | 38 | NA (NA) | 32 (NA) | 75 |
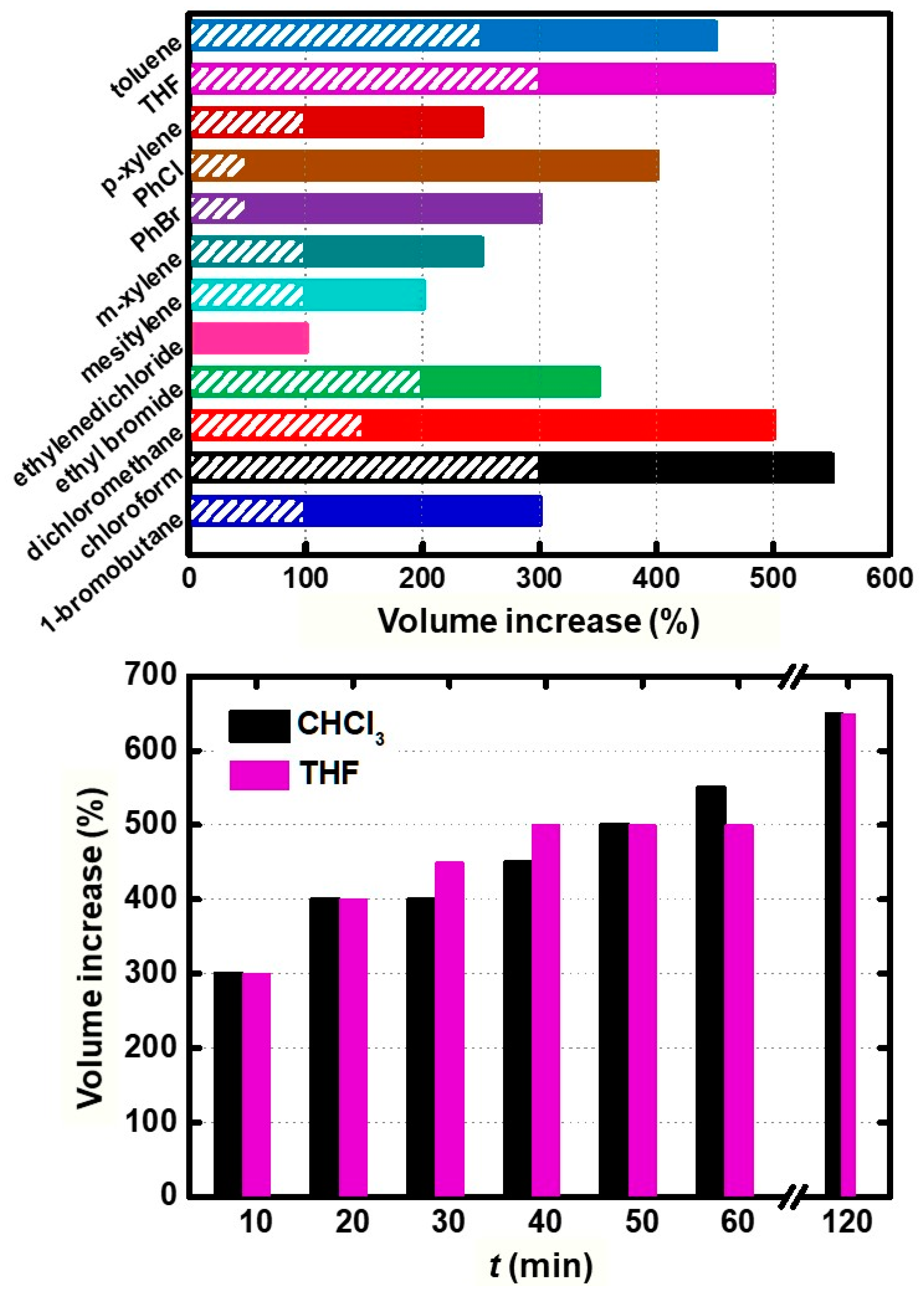
| Solvent | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Toluene | CH2Cl2 | CHCl3 | PhCl | PhBr | THF | 1-Bromo Butane | Ethyl Bromide | Ethylene Dichloride | Meta- Xylene | Para- Xylene | Mesity- lene |
| Volume Increase (ΔV, mL) | ||||||||||||
| 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | 0.25 | 0.15 | 0.30 | 0.05 | 0.05 | 0.30 | 0.10 | 0.20 | 0.00 | 0.10 | 0.10 | 0.10 |
| 20 | 0.30 | 0.35 | 0.40 | 0.20 | 0.20 | 0.40 | 0.20 | 0.20 | 0.00 | 0.10 | 0.15 | 0.10 |
| 30 | 0.30 | 0.40 | 0.40 | 0.30 | 0.20 | 0.45 | 0.20 | 0.25 | 0.10 | 0.10 | 0.25 | 0.15 |
| 40 | 0.30 | 0.40 | 0.45 | 0.30 | 0.25 | 0.50 | 0.20 | 0.30 | 0.10 | 0.20 | 0.25 | 0.15 |
| 50 | 0.40 | 0.40 | 0.50 | 0.30 | 0.30 | 0.50 | 0.20 | 0.30 | 0.10 | 0.20 | 0.25 | 0.20 |
| 60 | 0.45 | 0.50 | 0.55 | 0.40 | 0.30 | 0.50 | 0.30 | 0.35 | 0.10 | 0.25 | 0.25 | 0.20 |
| 120 | 0.45 | 0.60 | 0.65 | 0.40 | 0.40 | 0.65 | 0.30 | 0.40 | 0.10 | 0.35 | 0.40 | 0.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chriti, D.; Raptopoulos, G.; Brandenburg, B.; Paraskevopoulou, P. Large, Rapid Swelling of High-cis Polydicyclopentadiene Aerogels Suitable for Solvent-Responsive Actuators. Polymers 2020, 12, 1033. https://doi.org/10.3390/polym12051033
Chriti D, Raptopoulos G, Brandenburg B, Paraskevopoulou P. Large, Rapid Swelling of High-cis Polydicyclopentadiene Aerogels Suitable for Solvent-Responsive Actuators. Polymers. 2020; 12(5):1033. https://doi.org/10.3390/polym12051033
Chicago/Turabian StyleChriti, Despoina, Grigorios Raptopoulos, Benjamin Brandenburg, and Patrina Paraskevopoulou. 2020. "Large, Rapid Swelling of High-cis Polydicyclopentadiene Aerogels Suitable for Solvent-Responsive Actuators" Polymers 12, no. 5: 1033. https://doi.org/10.3390/polym12051033
APA StyleChriti, D., Raptopoulos, G., Brandenburg, B., & Paraskevopoulou, P. (2020). Large, Rapid Swelling of High-cis Polydicyclopentadiene Aerogels Suitable for Solvent-Responsive Actuators. Polymers, 12(5), 1033. https://doi.org/10.3390/polym12051033







