Synthesis of Phosphorus-Containing Polyanilines by Electrochemical Copolymerization
Abstract
1. Introduction
2. Experiments
3. Results and Discussion
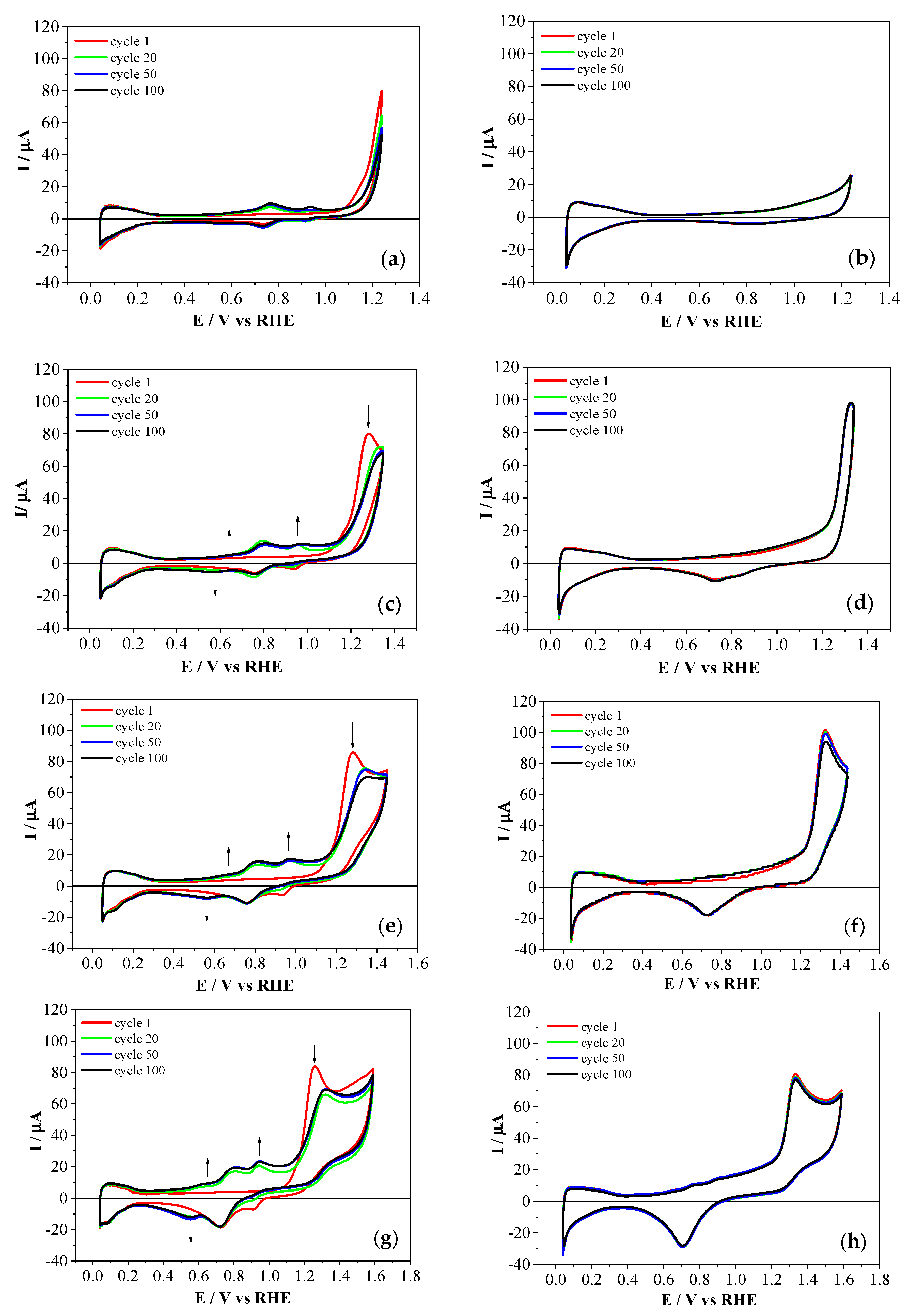
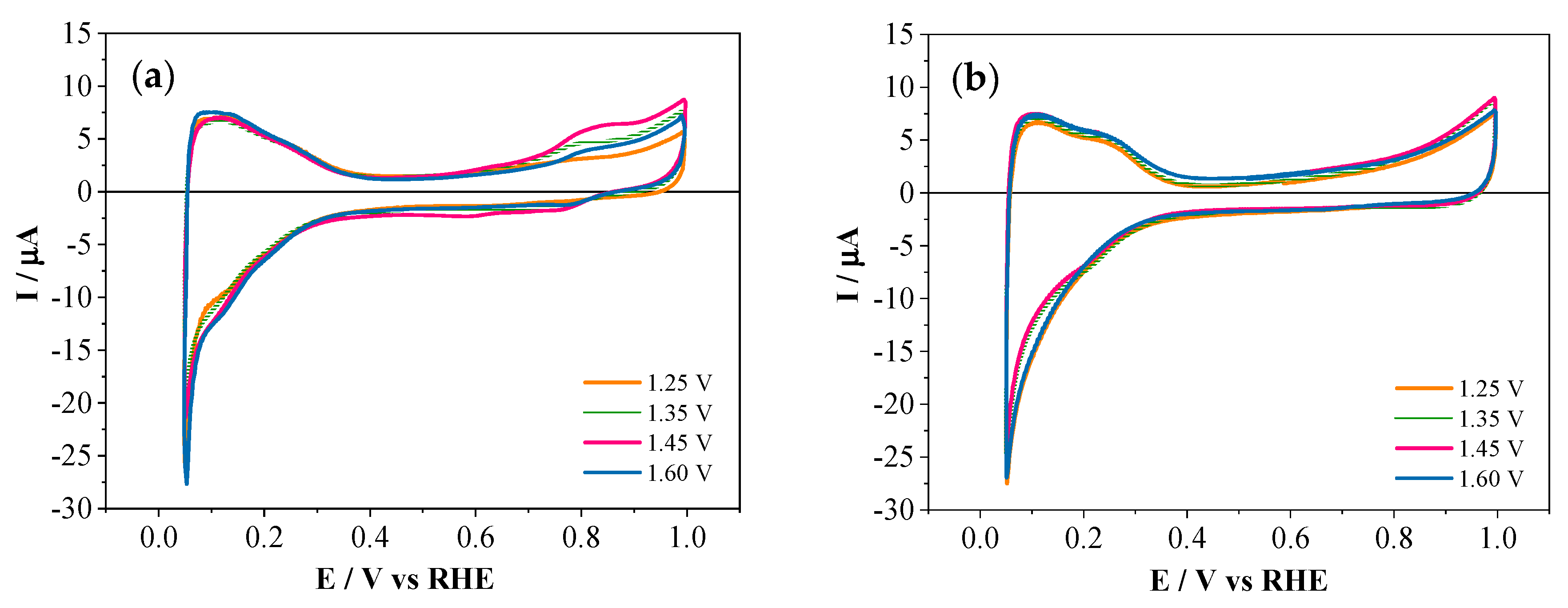
3.1. Electrochemical Homopolymerization of 2-APPA and 4-APPA
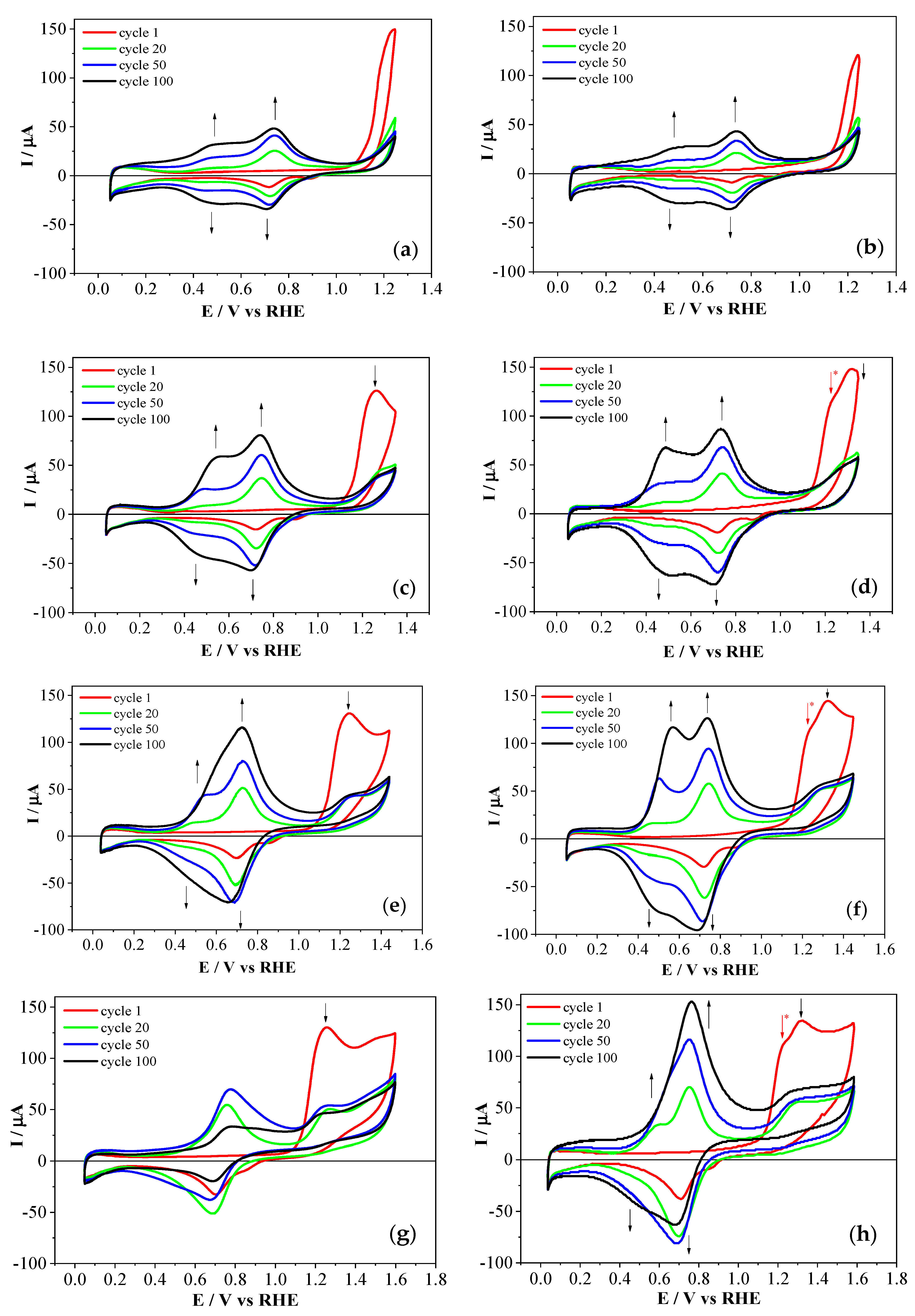
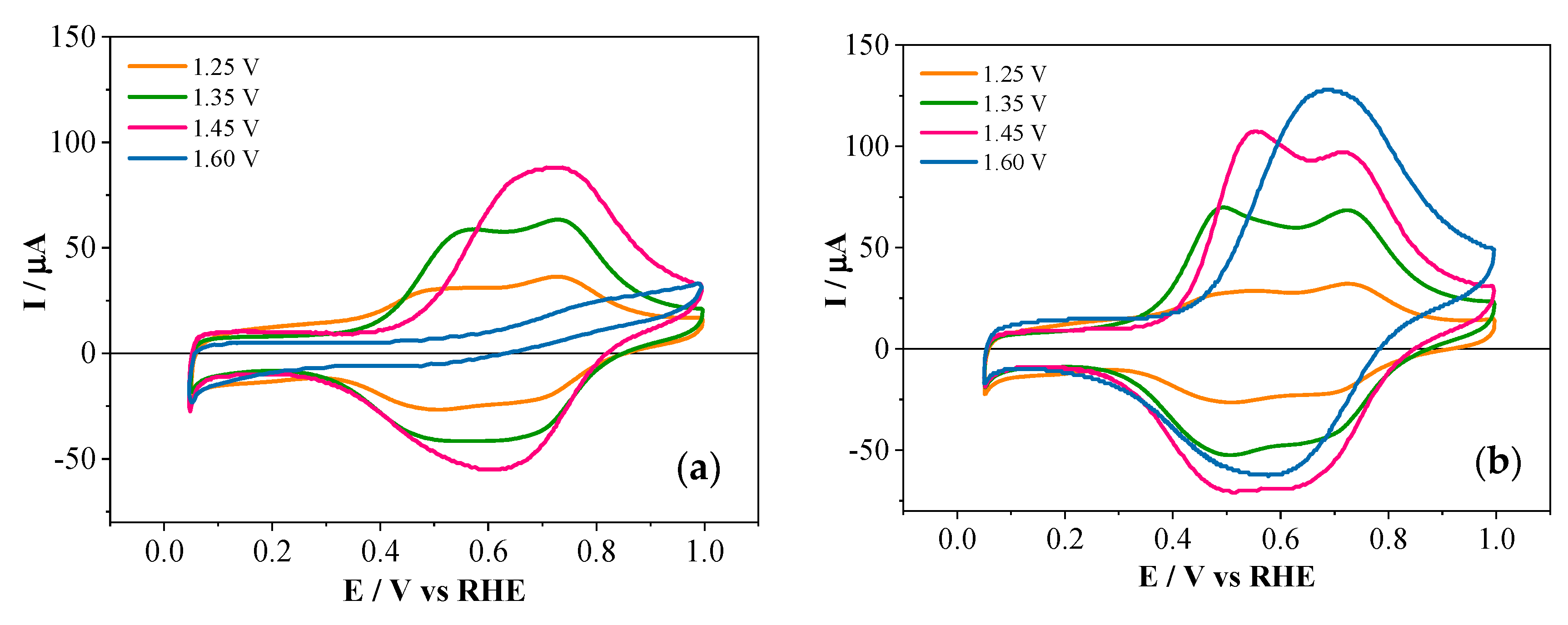
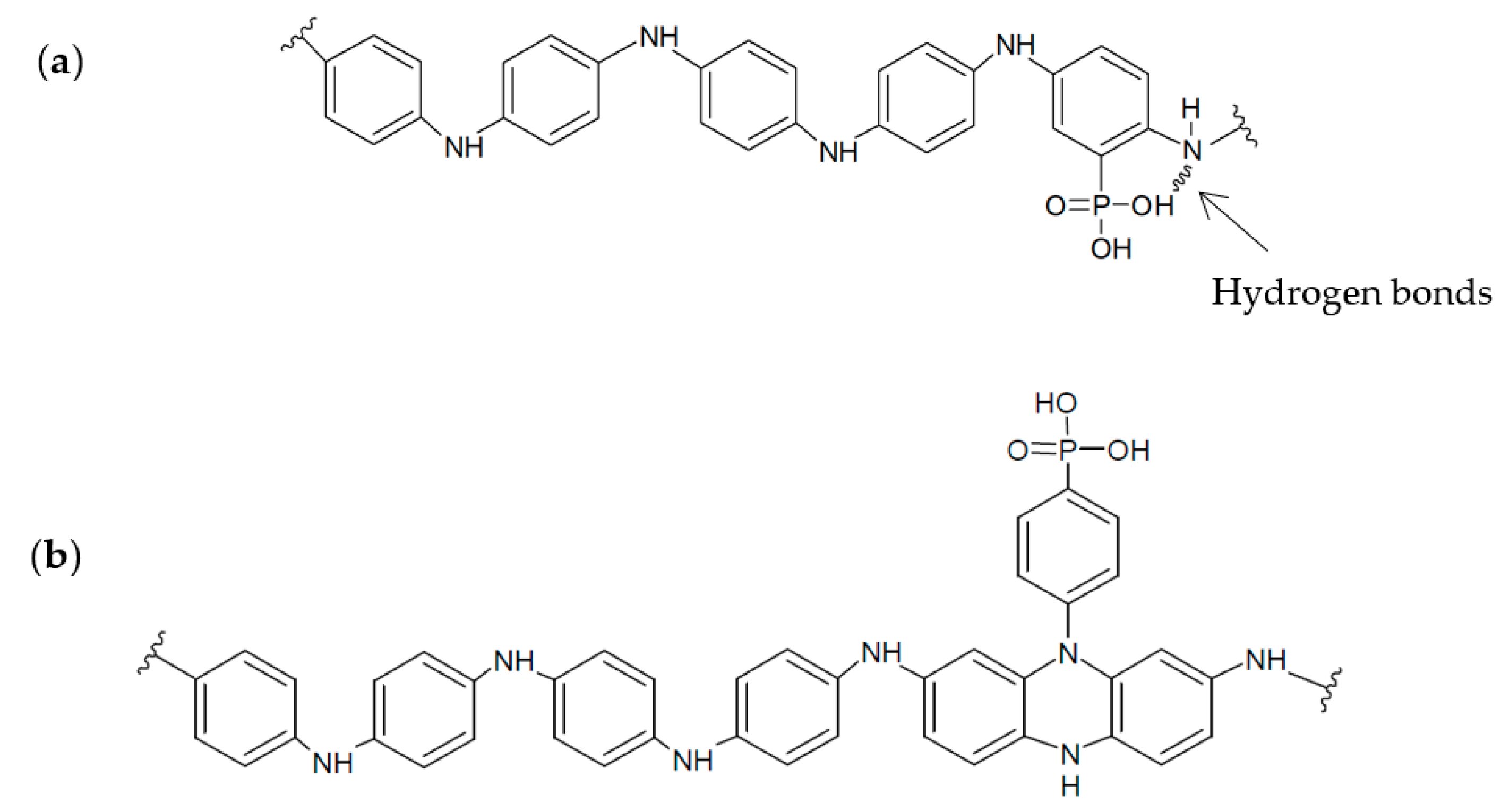
3.2. Electrochemical Copolymerization of 2-APPA and 4-APPA with Aniline
3.3. Spectro-Electrochemical Characterization
3.4. X-ray Photoelectron Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- John, R.; Reynolds, T.A.; Skotheim, B.C.T. Conjugated Polymers: Properties, Processing, and Applications, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Magu, T.O.; Agobi, A.U.; Hitler, L.; Dass, P.M. A Review on Conducting Polymers-Based Composites for Energy Storage Application. J. Chem. Rev. 2019, 1, 19–34. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Jaymand, M. Recent progress in chemical modification of polyaniline. Prog. Polym. Sci. 2013, 38, 1287–1306. [Google Scholar] [CrossRef]
- Pandey, R.K.; Lakshminarayanan, V. Electro-oxidation of formic acid, methanol, and ethanol on electrodeposited Pd-polyaniline nanofiber films in acidic and alkaline medium. J. Phys. Chem. C 2009, 113, 21596–21603. [Google Scholar] [CrossRef]
- Wei, H.; Yan, X.; Wu, S.; Luo, Z.; Wei, S.; Guo, Z. Electropolymerized Polyaniline Stabilized Tungsten Oxide Nanocomposite Films: Electrochromic Behavior and Electrochemical Energy Storage. J. Phys. Chem. C 2012, 116, 25052–25064. [Google Scholar] [CrossRef]
- Gabe, A.; Mostazo-López, M.J.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D. Synthesis of conducting polymer/carbon material composites and their application in electrical energy storage. In Hybrid Polymer Composite Materials; Thakur, V., Thakur, M., Gupta, R., Eds.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2017; pp. 173–209. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly sensitive glucose sensor based on pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 2013, 7, 3540–3546. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Oxygen-reduction catalysis of N-doped carbons prepared via heat treatment of polyaniline at over 1100 °C. Chem. Commun. 2018, 54, 4441–4444. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Pašti, I.; Gavrilov, N.; Janošević, A.; Mentus, S. Carbonised polyaniline and polypyrrole: Towards advanced nitrogen-containing carbon materials. Chem. Pap. 2013, 67, 781–813. [Google Scholar] [CrossRef]
- Xu, X.; Fu, Q.; Gu, H.; Guo, Y.; Zhou, H.; Zhang, J.; Pan, D.; Wu, S.; Dong, M.; Guo, Z. Polyaniline crystalline nanostructures dependent negative permittivity metamaterials. Polymer 2020, 188, 122129. [Google Scholar] [CrossRef]
- Malinauskas, A. Self-doped polyanilines. J. Power Sources 2004, 126, 214–220. [Google Scholar] [CrossRef]
- Gu, H.; Zhang, H.; Lin, J.; Shao, Q.; Young, D.P.; Sun, L.; Shen, T.D.; Guo, Z. Large negative giant magnetoresistance at room temperature and electrical transport in cobalt ferrite-polyaniline nanocomposites. Polymer 2018, 143, 324–330. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, H.; Zhang, Y.; Yin, Z. Corrosion protection of epoxy coatings containing 2-hydroxyphosphonocarboxylic acid doped polyaniline nanofibers. Prog. Org. Coat. 2020, 139, 105470. [Google Scholar] [CrossRef]
- Shahadat, M.; Ali, S.W.; Ahammad, S.Z.; Azam, A. Chapter 12—Polyaniline/carbon nanotube-supported nanocomposite electrode for detection of organic pollutants. In Handbook of Nanomaterials for Manufacturing Applications; Hussair, C.M., Ed.; Elsevier Publisher: Chennai, India, 2020; pp. 279–296. [Google Scholar] [CrossRef]
- Benyoucef, A.; Huerta, F.; Vázquez, J.L.; Morallón, E. Synthesis and in situ FTIRS characterization of conducting polymers obtained from aminobenzoic acid isomers at platinum electrodes. Eur. Polym. J. 2005, 41, 843–852. [Google Scholar] [CrossRef]
- Dkhili, S.; López-Bernabeu, S.; Huerta, F.; Montilla, F.; Besbes-Hentati, S.; Morallón, E. A self-doped polyaniline derivative obtained by electrochemical copolymerization of aminoterephthalic acid and aniline. Synth. Met. 2018, 245, 61–66. [Google Scholar] [CrossRef]
- Sanchís, C.; Salavagione, H.J.; Arias-Pardilla, J.; Morallón, E. Tuning the electroactivity of conductive polymer at physiological pH. Electrochim. Acta 2007, 52, 2978–2986. [Google Scholar] [CrossRef]
- Grigoras, M.; Catargiu, A.M.; Tudorache, F.; Dobromir, M. Chemical synthesis and characterization of self-doped N-propanesulfonic acid polyaniline derivatives. Iran. Polym. J. 2012, 21, 131–141. [Google Scholar] [CrossRef]
- Yue, J.; Epstein, A.J. Synthesis of self-doped conducting polyaniline. J. Am. Chem. Soc. 1990, 112, 2800–2801. [Google Scholar] [CrossRef]
- Chatterjee, K.; Ganguly, S.; Kargupta, K.; Banerjee, D. Bismuth nitrate doped polyaniline—Characterization and properties for thermoelectric application. Synth. Met. 2011, 161, 275–279. [Google Scholar] [CrossRef]
- Wei, Y.; Hariharan, R.; Patel, S.A. Chemical and electrochemical copolymerization of aniline with alkyl ring-substituted anilines. Macromolecules 1990, 23, 758–764. [Google Scholar] [CrossRef]
- Probst, M.; Holze, R. A systematic spectroelectrochemical investigation of alkyl-substituted anilines and their polymers. Macromol. Chem. Phys. 1997, 198, 1499–1509. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Arias, J.; Garcés, P.; Morallón, E.; Barbero, C.; Vázquez, J.L. Spectroelectrochemical study of the oxidation of aminophenols on platinum electrode in acid medium. J. Electroanal. Chem. 2004, 565, 375–383. [Google Scholar] [CrossRef]
- Mu, S. Electrochemical copolymerization of aniline and o-aminophenol. Synth. Met. 2004, 143, 259–268. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, D.; Mu, S. A promising copolymer of aniline and m-aminophenol: Chemical preparation, novel electric properties and characterization. Polymer 2007, 48, 1269–1275. [Google Scholar] [CrossRef]
- Quintero-Jaime, A.F.; Cazorla-Amorós, D.; Morallón, E. Electrochemical functionalization of single wall carbon nanotubes with phosphorus and nitrogen species. Electrochim. Acta 2020, 340, 135935. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Chen, T.; Liu, C.; Mu, D.; Sun, S.; Wan, W.; Wang, Z.; Wei, J.; Dai, C. From bulk to porous: Structure transformation of nitrogen and phosphorous co-doped carbon material via sodium chloride assistance and its application in lithium-sulfur batteries. J. Alloys Compd. 2020, 830, 154638. [Google Scholar] [CrossRef]
- Fonsaca, J.E.S.; Domingues, S.H.; Orth, E.S.; Zarbin, A.J.G. A black phosphorus-based cathode for aqueous Na-ion batteries operating under ambient conditions. Chem. Commun. 2020, 56, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Amaya, T.; Kurata, I.; Inada, Y.; Hatai, T.; Hirao, T. Synthesis of phosphonic acid ring-substituted polyanilines: Via direct phosphonation to polymer main chains. RSC Adv. 2017, 7, 39306–39313. [Google Scholar] [CrossRef]
- Ghil, L.J.; Youn, T.Y.; Park, N.R.; Rhee, H.W. Proton conductive Nano-channel membranes based on Polyaniline with Phosphonic acid moieties for low relative humidity. J. Nanosci. Nanotechnol. 2013, 13, 7912–7915. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Ghisolfi, A.; Grau-Marín, D.; San-Fabián, E.; Morallón, E.; Cazorla-Amorós, D. Post-synthetic efficient functionalization of polyaniline with phosphorus-containing groups. Effect of phosphorus on electrochemical properties. Eur. Polym. J. 2019, 119, 272–280. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhang, Y.; Zhang, Y. Improving the flame retardancy and electrical conductivity of epoxy resin composites by multifunctional phosphorus-containing polyaniline. Mater. Lett. 2020, 261, 127092. [Google Scholar] [CrossRef]
- Amaya, T.; Abe, Y.; Inada, Y.; Hirao, T. Synthesis of self-doped conducting polyaniline bearing phosphonic acid. Tetrahedron Lett. 2014, 55, 3976–3978. [Google Scholar] [CrossRef]
- Chan, H.S.O.; Ho, P.K.H.; Ng, S.C.; Tan, B.T.G.; Tan, K.L. A New Water-Soluble, Self-Doping Conducting Polyaniline from Poly(o-aminobenzylphosphonic acid) and Its Sodium Salts: Synthesis and Characterization. J. Am. Chem. Soc. 1995, 117, 8517–8523. [Google Scholar] [CrossRef]
- Botelho do Rego, A.M.; Ferraria, A.M.; El Beghdadi, J.; Debontridder, F.; Brogueira, P.; Naaman, R.; Rei Vilar, M. Adsorption of Phenylphosphonic Acid on GaAs Surfaces. Langmuir 2005, 21, 8765–8773. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.E.R.; Grosvenor, A.P.; Cavell, R.G.; Mar, A. X-ray Photoelectron and Absorption Spectroscopy of Metal-Rich Phosphides M2P and M3P (M = Cr−Ni). Chem. Mater. 2008, 20, 7081–7088. [Google Scholar] [CrossRef]
- Yang, H.; Bard, A.J. The application of fast scan cyclic voltammetry. Mechanistic study of the initial stage of electropolymerization of aniline in aqueous solutions. J. Electroanal. Chem. 1992, 339, 423–449. [Google Scholar] [CrossRef]
- Yagyu, S.; Yoshitake, M.; Tsud, N.; Chikyow, T. Adsorption of phenylphosphonic acid on gold and platinum surfaces. Jpn. J. Appl. Phys. 2011, 50, 081606-1–081606-5. [Google Scholar] [CrossRef]
- Laska, J.; Widlarz, J. Spectroscopic and structural characterization of low molecular weight fractions of polyaniline. Polymer 2005, 46, 1485–1495. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Cotarelo, M.A.; Huerta, F.; Quijada, C.; Mallavia, R.; Vázquez, J.L. Synthesis and Characterization of Electroactive Films Deposited from Aniline Dimers. J. Electrochem. Soc. 2006, 153, D114. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Geniès, E.M.; Penneau, J.F.; Lapkowski, M.; Boyle, A. Electropolymerisation reaction mechanism of para-aminodiphenylamine. J. Electroanal. Chem. Interfacial Electrochem. 1989, 269, 63–75. [Google Scholar] [CrossRef]
- Abidi, M.; López-Bernabeu, S.; Huerta, F.; Montilla, F.; Besbes-Hentati, S.; Morallón, E. Spectroelectrochemical study on the copolymerization of o-aminophenol and aminoterephthalic acid. Eur. Polym. J. 2017, 91, 386–395. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Socha, R.P.; Gurgul, J.; Wisniewski, M. XPS and NMR studies of phosphoric acid activated carbons. Carbon 2008, 46, 2113–2123. [Google Scholar] [CrossRef]

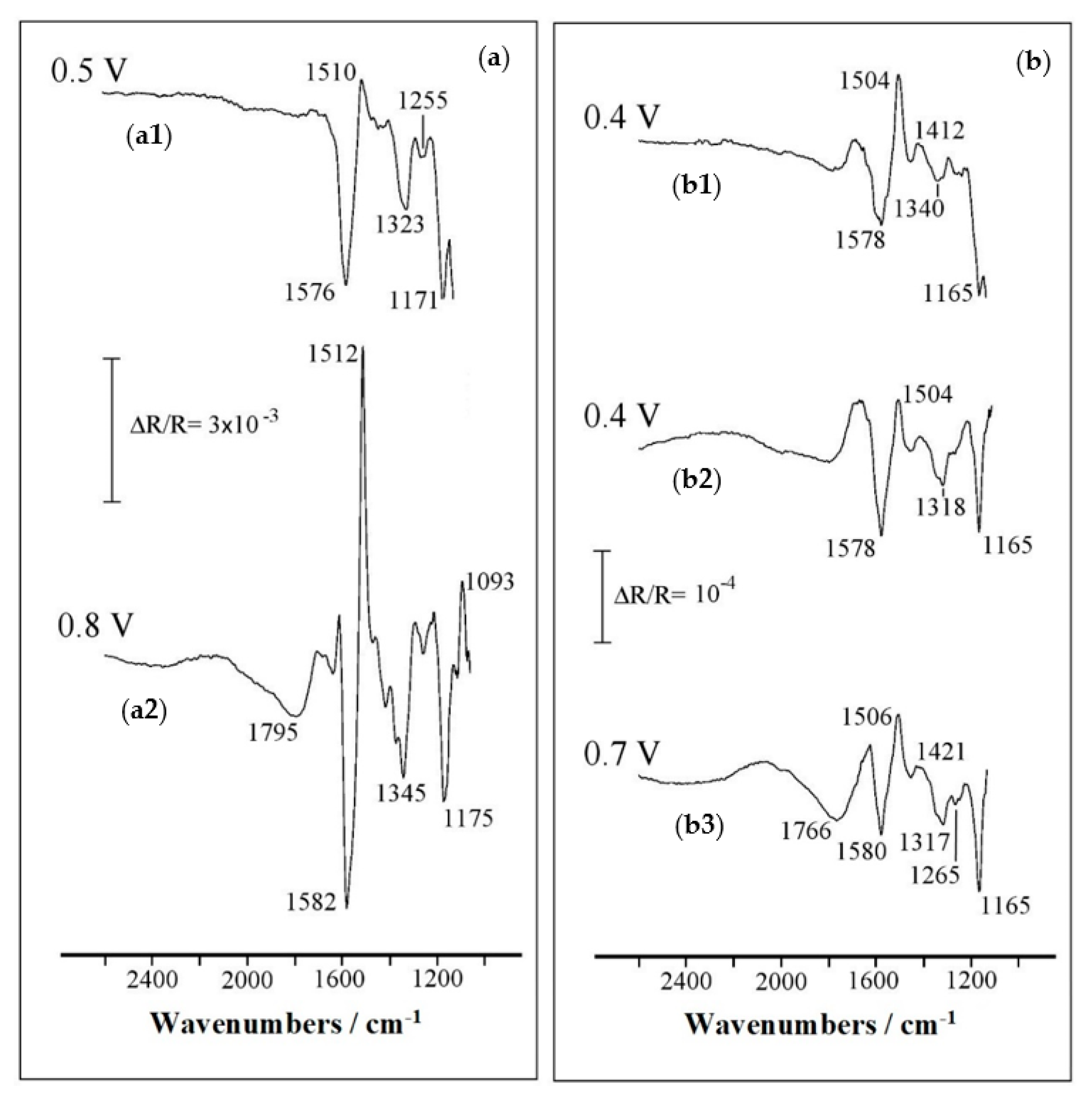

| Oxidation State | Frequency/cm−1 | Assignment | References |
|---|---|---|---|
| Reduced | 1510, 1512 | Benzenoid aromatic ring (C–C) stretching | [41,42,43] |
| 1300–1310 | Secondary aromatic amine (N–H) stretching | [42] | |
| 1220 | (P=O) stretching | [35,40] | |
| 1093 | (P–O) asymmetric stretching | [39,41] | |
| Oxidized | 1795 | (–OH) stretching in (O=P–OH) with a (–OH) single neighboring | [35,41] |
| 1576, 1582 | Quinoid ring (C–C) stretching | [42,43] | |
| 1323, 1345 | Intermediate order (C=N) stretching | [42] | |
| 1255 | (C–N•+) stretching | [42] | |
| 1171, 1175 | (C-H) bending, quinoid ring (C–N–C) stretching, aromatic ring (P–C) stretching and/or | [40,41,42,44] |
| Oxidation State | Frequency/cm−1 | Assignment | References | |
|---|---|---|---|---|
| 1.35 V | 1.45 V | |||
| Reduced | 1504 | 1504, 1506 | Benzenoid aromatic ring (C–C) stretching | [41,42,43] |
| 1300–1310 | 1300–1310 | Secondary aromatic amines (N–H) stretching | [42] | |
| 1220 | 1220 | (P=O) stretching | [35,40] | |
| Oxidized | - | 1766 | (–OH) stretching in (O=P–OH) with a (–OH) single neighboring | [35,41] |
| 1578 | 1578, 1580 | Quinoid ring (C–C) stretching | [42,43] | |
| 1340 | 1317–1318 | Intermediate order (C=N) stretching | [42] | |
| - | 1265 | (C–N•+) stretching | [42] | |
| 1165 | 1165 | (C–H) bending, quinoid ring (C–N–C) stretching, aromatic ring (P–C) stretching and/or | [41,42,43,44] | |
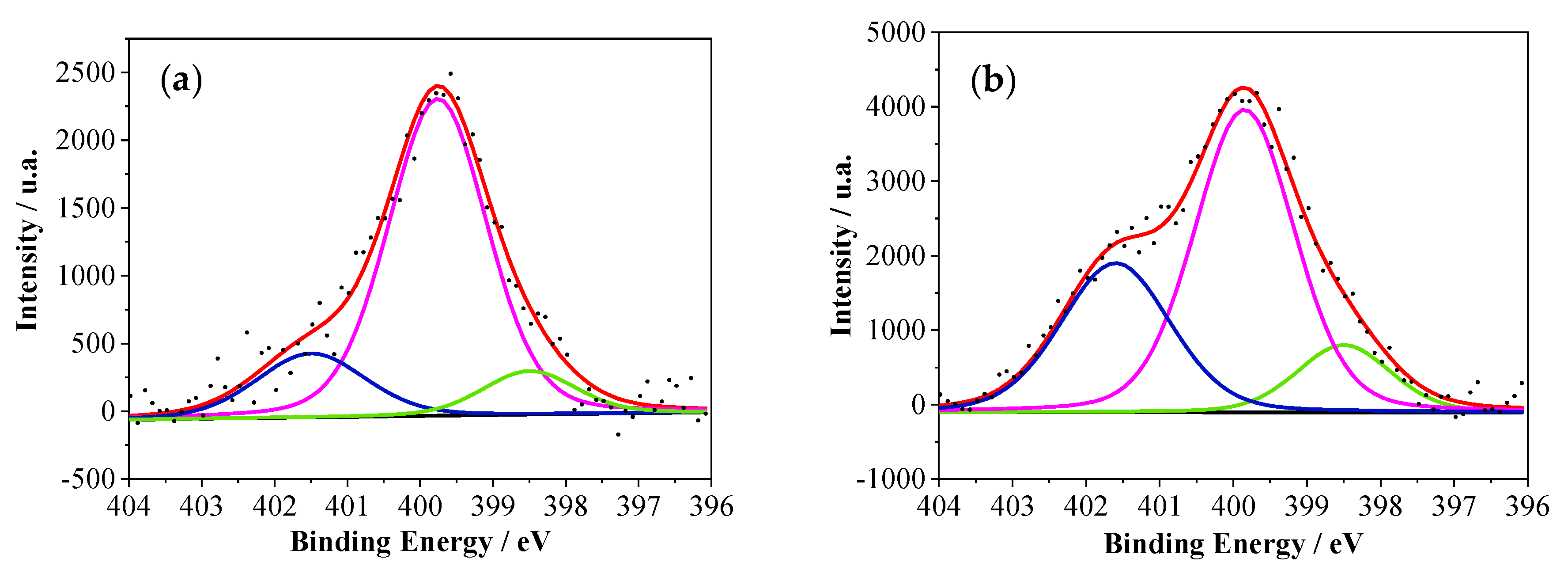
| Samples | %N1 | %N2 | %N3 | %P1 | %P2 | N/P |
|---|---|---|---|---|---|---|
| PANI | 19 | 73 | 8 | - | - | 0 |
| PANI–2APPA | 16 | 74 | 10 | 78 | 22 | 5.2 |
| PANI–4APPA | 12 | 57 | 31 | 80 | 20 | 7.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sánchez, B.; Quintero-Jaime, A.F.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Synthesis of Phosphorus-Containing Polyanilines by Electrochemical Copolymerization. Polymers 2020, 12, 1029. https://doi.org/10.3390/polym12051029
Martínez-Sánchez B, Quintero-Jaime AF, Huerta F, Cazorla-Amorós D, Morallón E. Synthesis of Phosphorus-Containing Polyanilines by Electrochemical Copolymerization. Polymers. 2020; 12(5):1029. https://doi.org/10.3390/polym12051029
Chicago/Turabian StyleMartínez-Sánchez, Beatriz, Andrés Felipe Quintero-Jaime, Francisco Huerta, Diego Cazorla-Amorós, and Emilia Morallón. 2020. "Synthesis of Phosphorus-Containing Polyanilines by Electrochemical Copolymerization" Polymers 12, no. 5: 1029. https://doi.org/10.3390/polym12051029
APA StyleMartínez-Sánchez, B., Quintero-Jaime, A. F., Huerta, F., Cazorla-Amorós, D., & Morallón, E. (2020). Synthesis of Phosphorus-Containing Polyanilines by Electrochemical Copolymerization. Polymers, 12(5), 1029. https://doi.org/10.3390/polym12051029








