Synthesis and Characterization of Hollow-Sphered Poly(N-methyaniline) for Enhanced Electrical Conductivity Based on the Anionic Surfactant Templates and Doping
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PNMA Synthesis
2.3. De-Doping/Re-Doping Step
2.4. Characterization
3. Results and Discussion
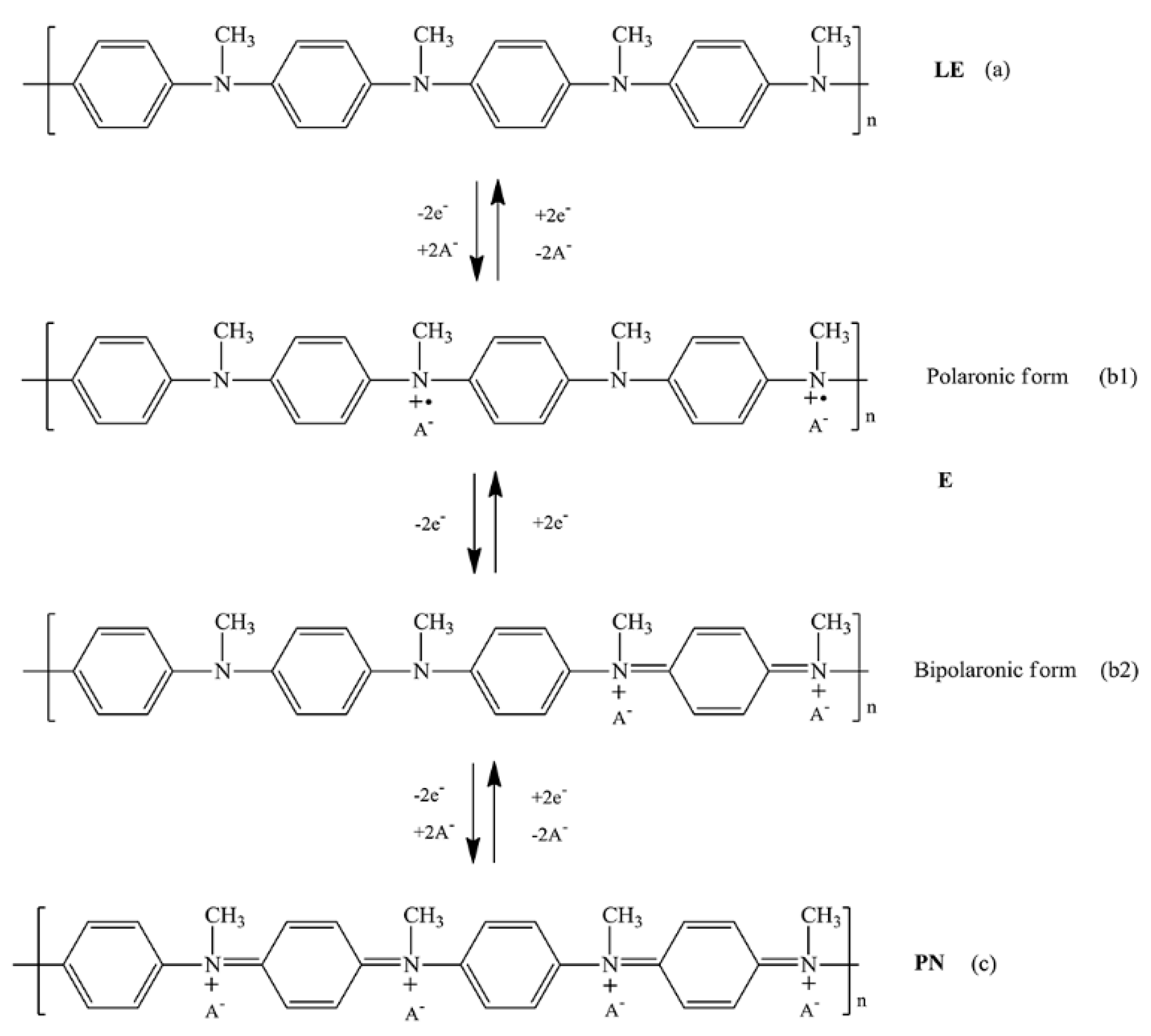
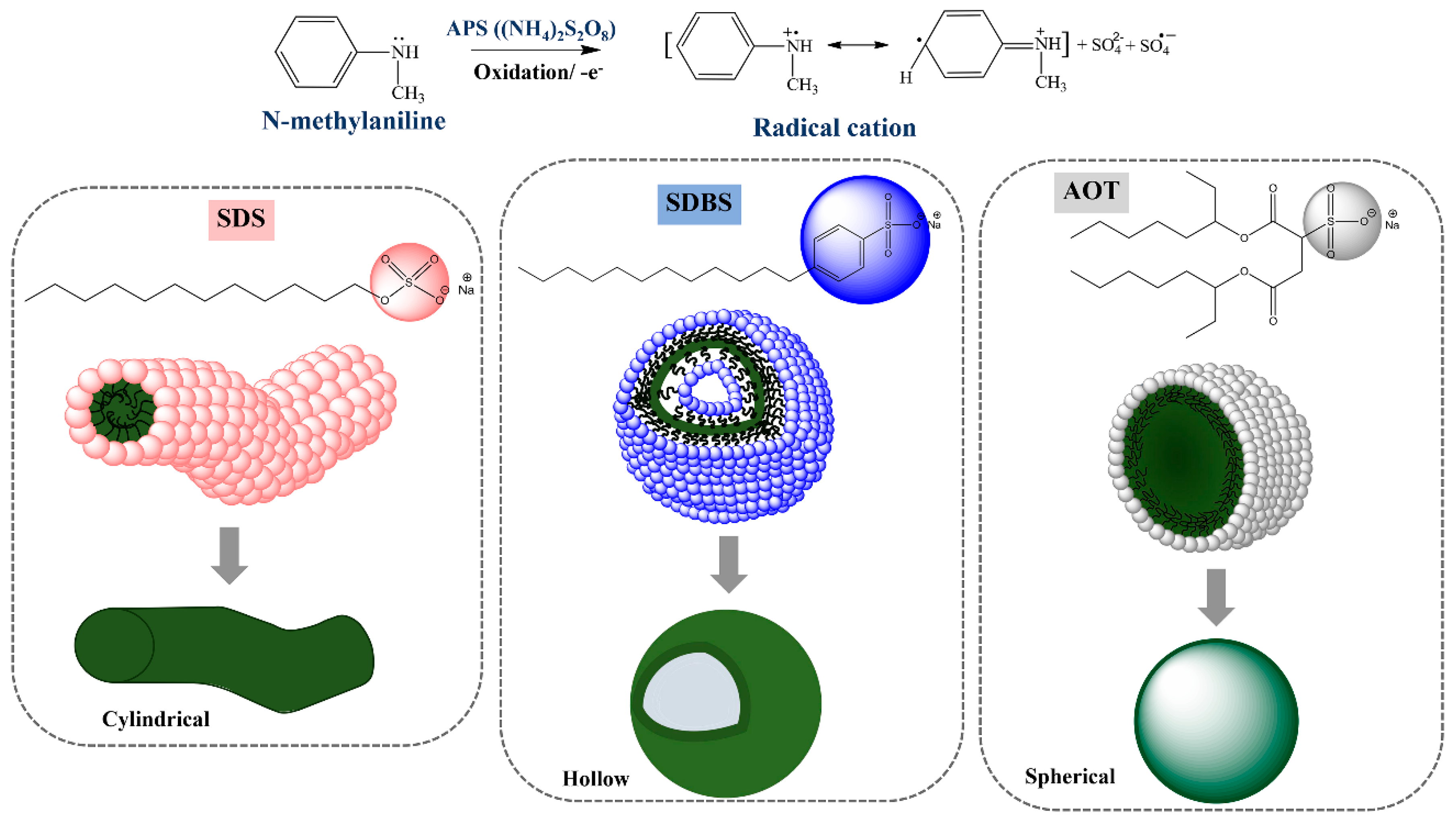
3.1. Structural Confirmation of the Synthesized PNMA
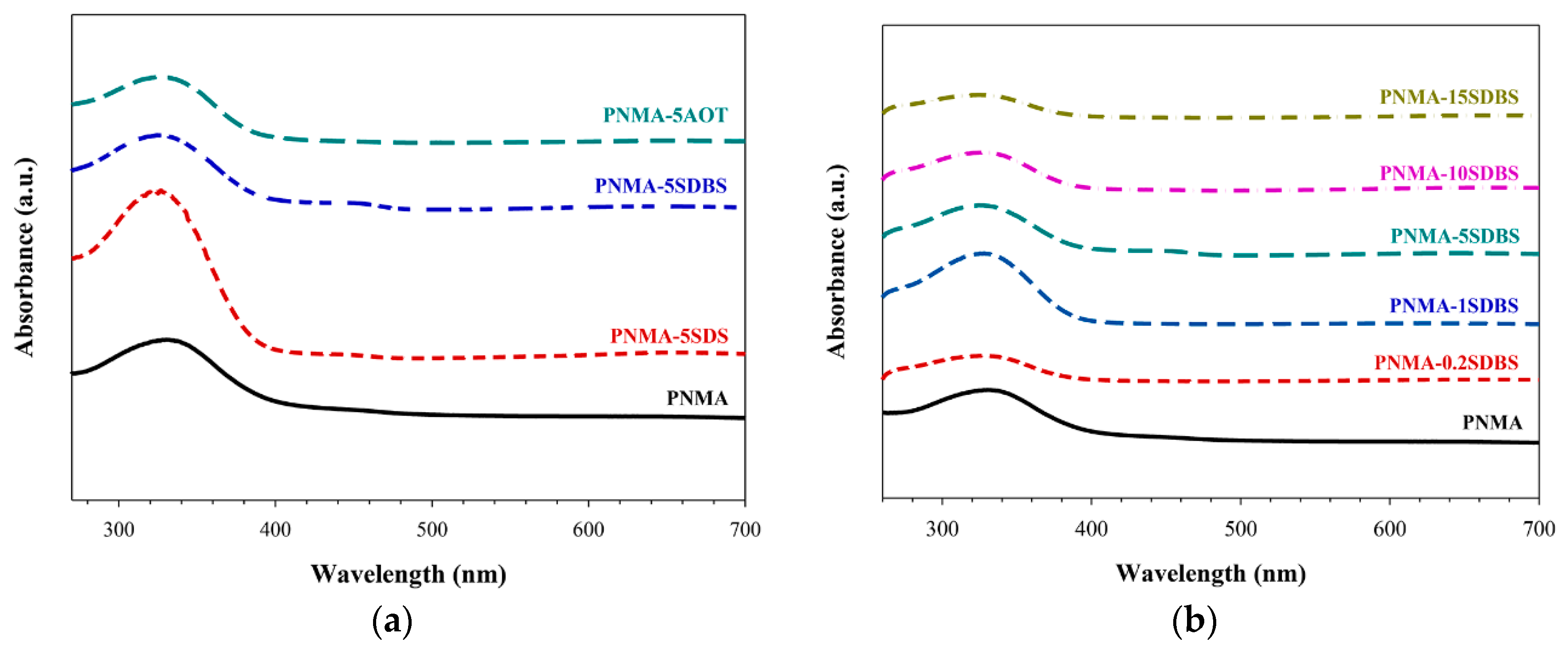
3.2. UV-Vis Analysis
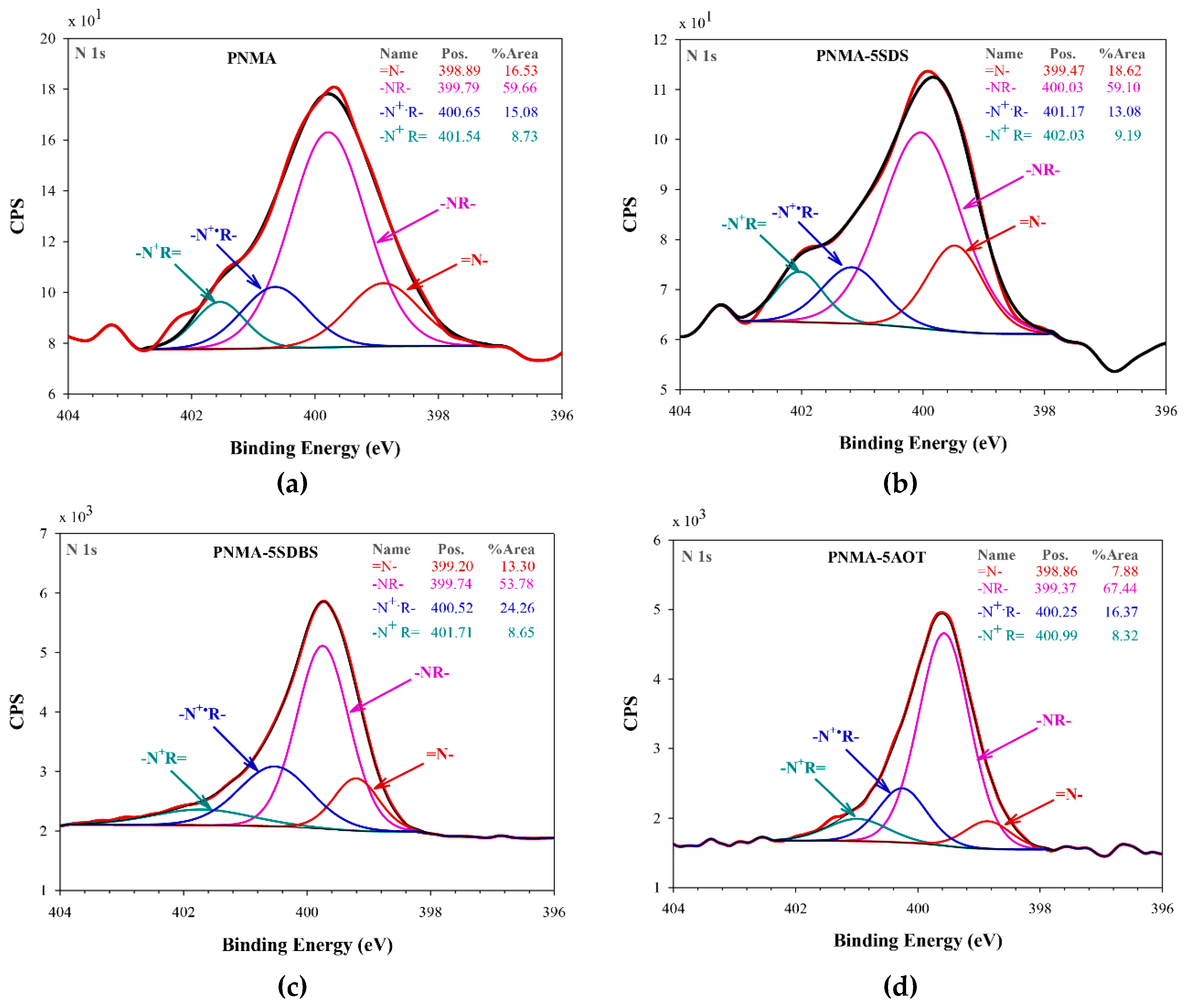
3.3. X-Ray Photoelectron Spectroscopy
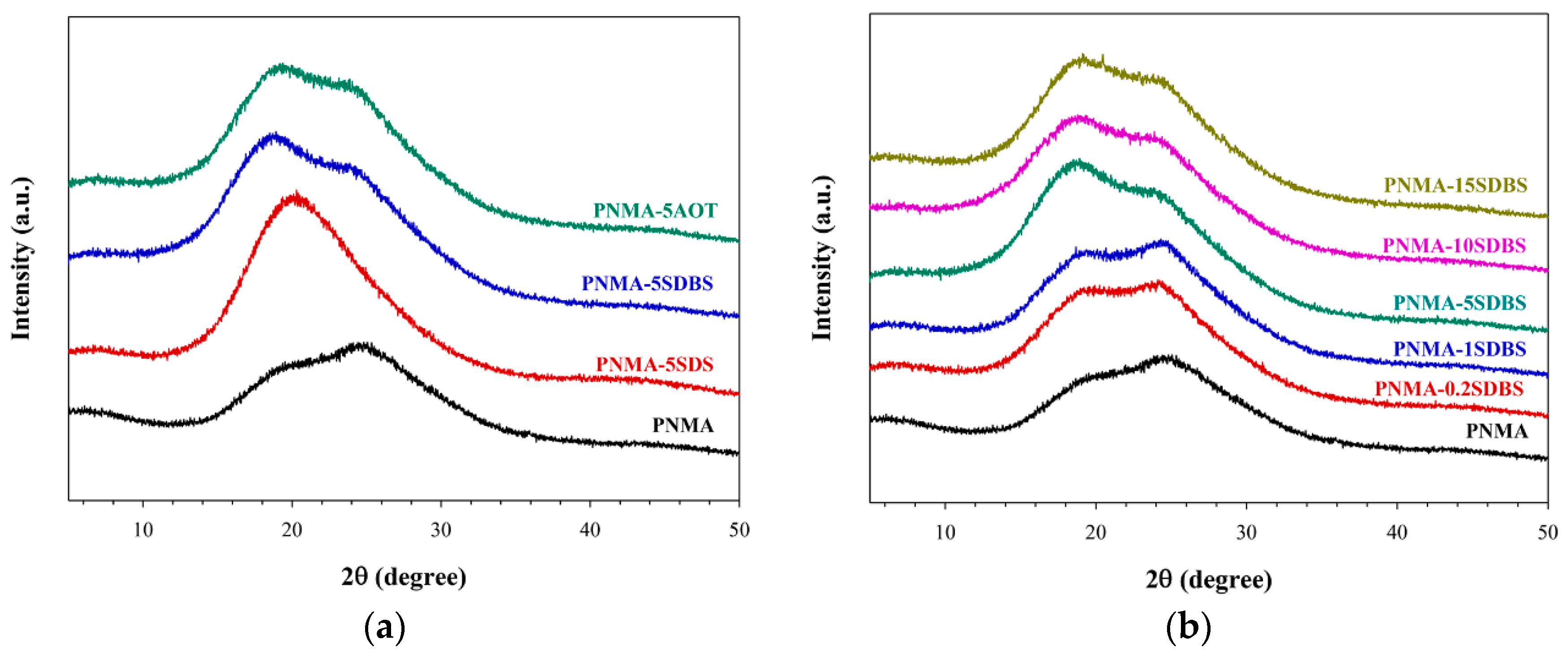
3.4. X-Ray Diffraction
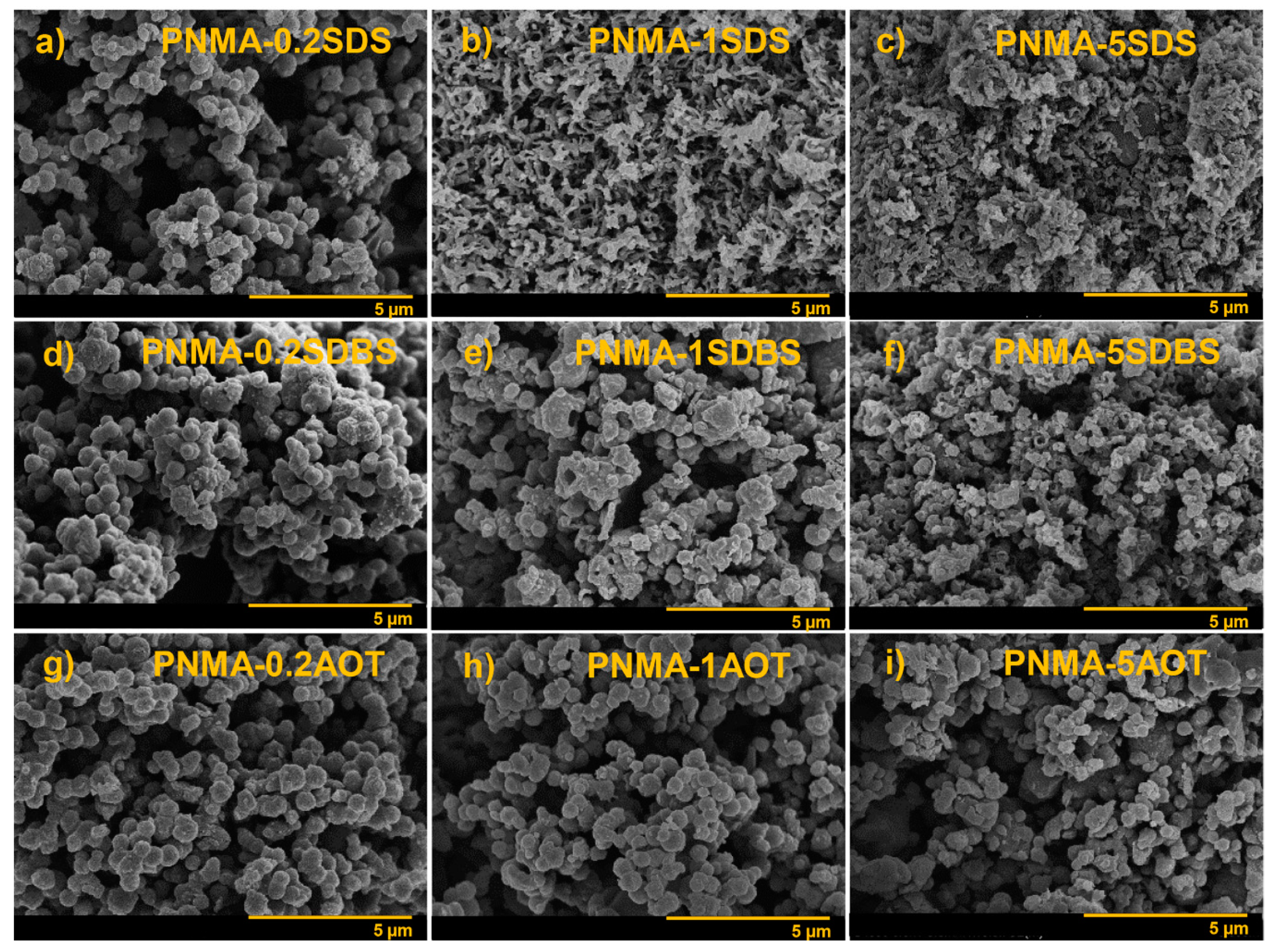
3.5. Morphology of PNMA
3.6. Thermal Stability
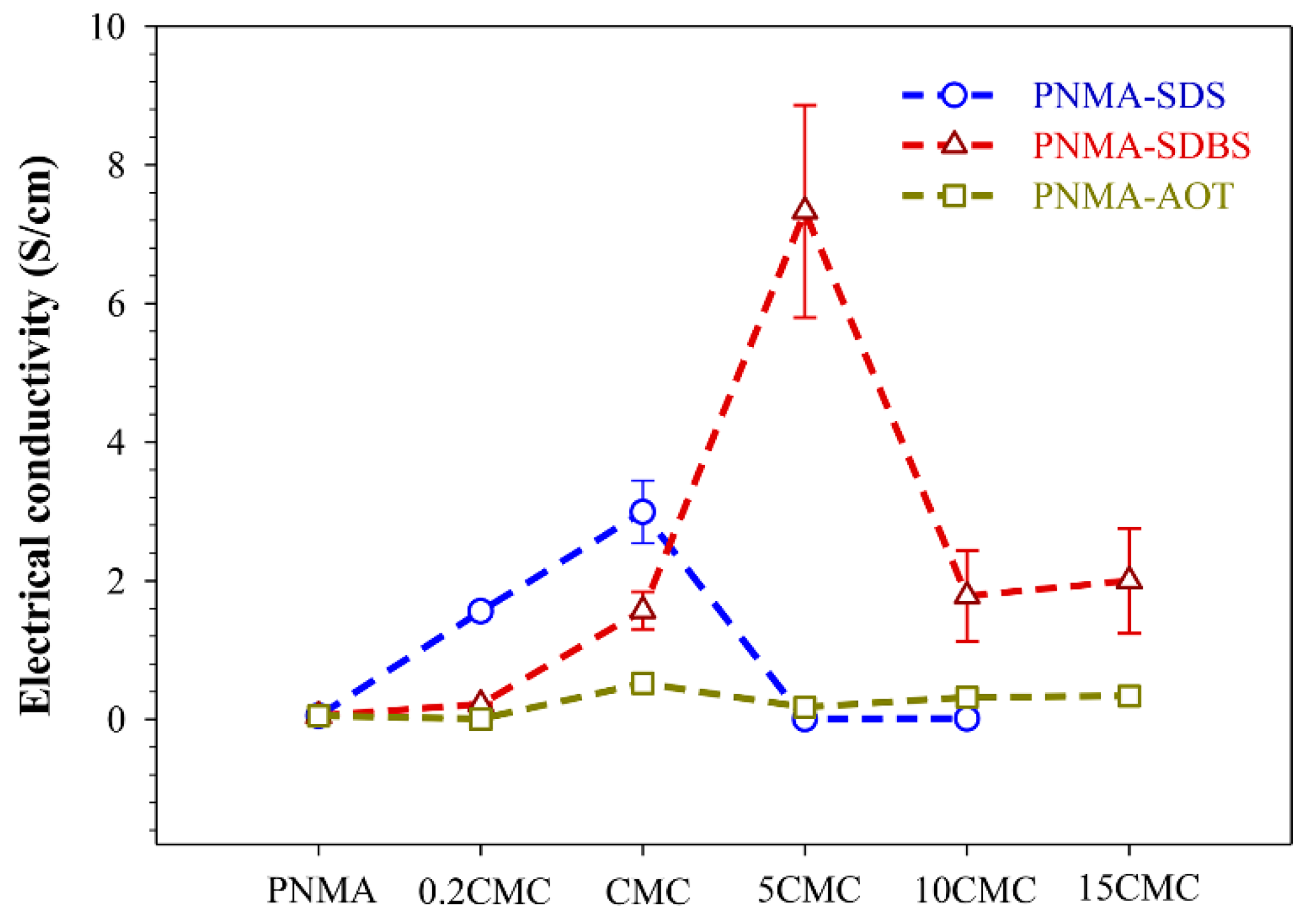
3.7. Electrical Conductivity
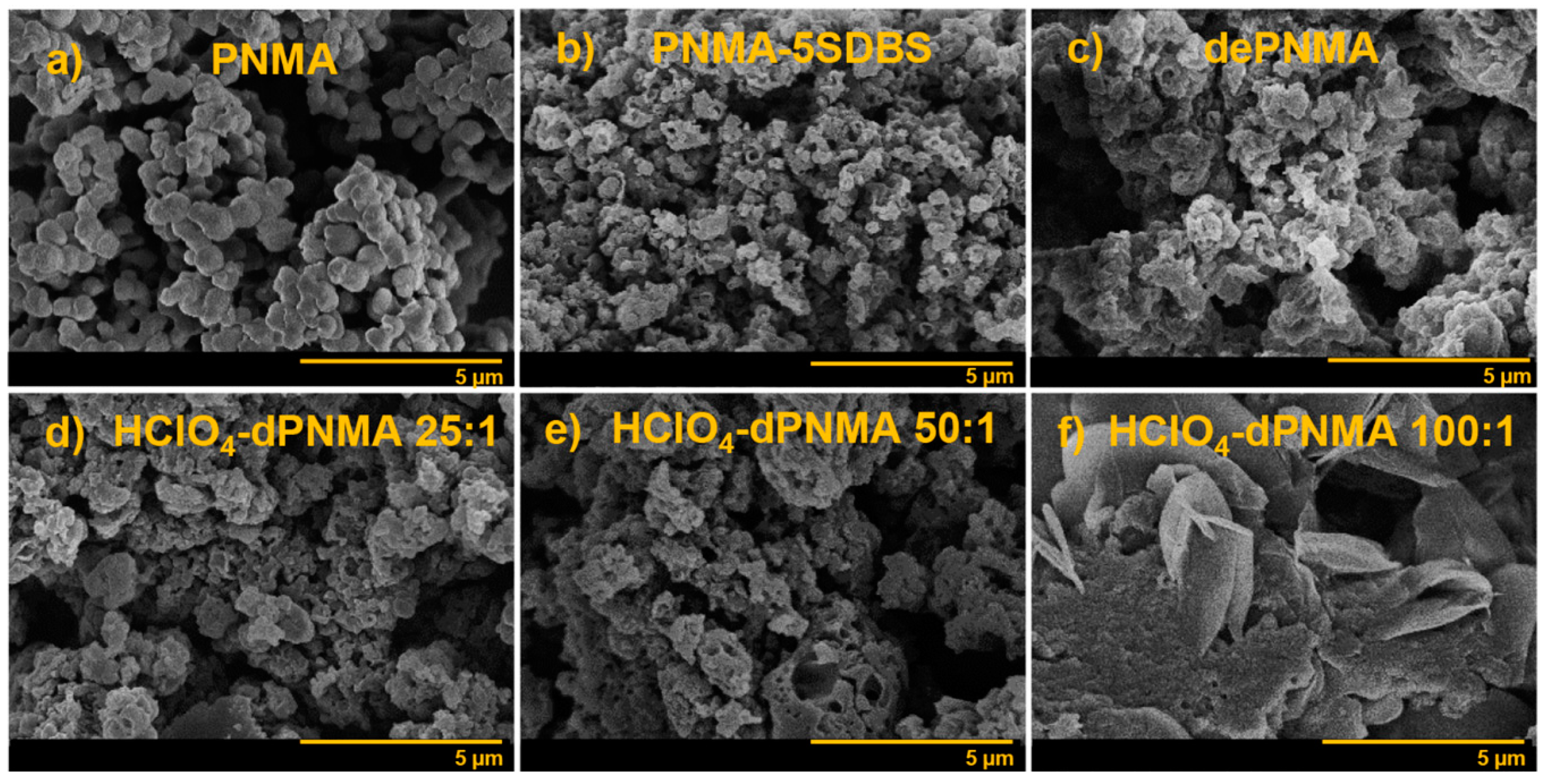
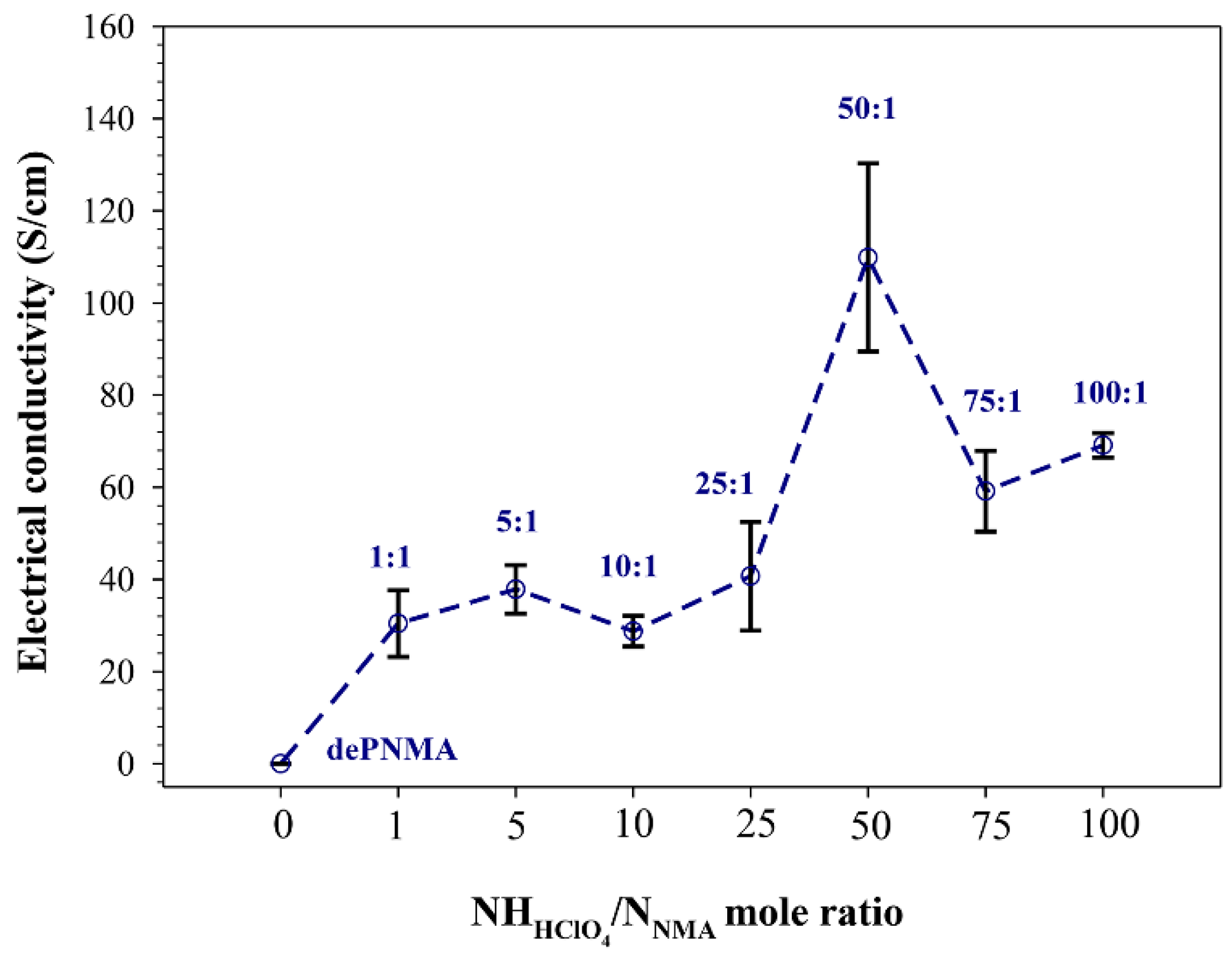
3.8. De-Doping/Re-Doping of PNMA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pan, L.; Uiu, H.; Dou, C.; Li, Y.; Pu, L.; Xu, J.; Shi, Y. Conducting polymer nanostructures: Template synthesis and applications in energy storage. Int. J. Mol. Sci. 2010, 11, 2636–2657. [Google Scholar] [CrossRef] [PubMed]
- Wan, M. A template-free method towards conducting polymer nanostructures. Adv. Mater. 2008, 20, 2926–2932. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Rajesha; Ahujab, T.; Kumar, D. Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications. Sens. Actuator B-Chem. 2009, 136, 275–286. [Google Scholar] [CrossRef]
- Ghosh, S.; Maiyalakan, T.; Basu, R.N. Nanostructured conducting polymers for energy applications: Towards a sustainable platform. Nanoscale 2016, 8, 6921–6947. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The applications of polymers in solar cells: A review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, X. Intrinsically conducting polymers for electromagnetic interference shielding. Polym. Adv. Technol. 2005, 16, 344–351. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RCS Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Tran, H.D.; Li, D.; Kaner, R.B. One-dimensional conducting polymer nanostructures: Bulk synthesis and applications. Adv. Mater. 2009, 21, 1487–1499. [Google Scholar] [CrossRef]
- Zhao, X.; Zhan, X. Electron transporting semiconducting polymers in organic electronics. Chem. Soc. Rev. 2011, 40, 3728–3743. [Google Scholar] [CrossRef]
- He, Y.; Lu, J. Synthesis of polyaniline nanostructures with controlled morphology by a two-phase strategy. React. Funct. Polym. 2007, 67, 476–480. [Google Scholar] [CrossRef]
- Petkovich, N.D.; Stein, A. Controlling macro- and mesostructures with hierarchical porosity through combined hard and soft templating. Chem. Soc. Rev. 2013, 42, 3721–3739. [Google Scholar] [CrossRef]
- Direksilp, C.; Sirivat, A. Tunable size and shape of conductive poly(N-methylaniline) based on surfactant template and doping. Polym. Int. 2019, 68, 1042–1053. [Google Scholar] [CrossRef]
- Kapil, A.; Taunk, M.; Chand, S. Preparation and characterization of chemically synthesized poly(N-methylaniline). Synth. Met. 2009, 159, 1267–1271. [Google Scholar] [CrossRef]
- Yakhmi, J.V.; Saxena, V.; Aswal, D.K. 2-Conducting polymer sensors, actuators and field-effect transistors. In Functional Materials: Preparation, Processing and Applications; Banerjee, S., Tyagi, A.K., Eds.; Elsevier: Waltham, MA, USA, 2012; pp. 61–110. [Google Scholar]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.P.; Kaner, R.B. Conducting Polymers. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–8. [Google Scholar]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef]
- Antony, M.J.; Jayakannan, M. Self-assembled anionic micellar template for polypyrrole, polyaniline, and their random copolymer nanomaterials. J. Polym. Sci. Part B-Polym. Phys. 2009, 47, 830–846. [Google Scholar] [CrossRef]
- Hoshina, Y.; Zaragoza-Contreras, E.A.; Farnood, R.; Kobayashi, T. Nanosized polypyrrole affected by surfactant agitation for emulsion polymerization. Polym. Bull. 2012, 68, 1689–1705. [Google Scholar] [CrossRef]
- Uygun, A.; Aslan, E. Comparative study of conducting polyaniline/copper and polyaniline/nickel composites in the presence of surfactants. Polym. Int. 2010, 59, 1162–1167. [Google Scholar] [CrossRef]
- García-Fernández, M.J.; Sancho-Querol, S.; Pastor-Blas, M.M.; Escribano, A.S. Surfactant-assisted synthesis of conducting polymers: Application to the removal of nitrates from water. J. Colloid Interface Sci. 2017, 494, 98–106. [Google Scholar] [CrossRef][Green Version]
- Yano, J.; Sanada, K.; Patil, R.; Ooyama, Y.; Komaguchi, K.; Harima, Y. Poly(N-methylaniline) microsphere formation and control of the average diameter by simple chemical polymerization. Mater. Chem. Phys. 2007, 106, 279–285. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Khanna, P.K. Synthesis and humidity sensing properties of conducting poly(N-methyl aniline) doped with different acids. Sens. Actuator B-Chem. 2006, 115, 140–149. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Khanna, P.K. Synthesis and characterization of poly(N-Methyl Aniline) doped with sulphonic acids: Their application as humidity sensors. J. Appl. Polym. Sci. 2006, 99, 812–820. [Google Scholar] [CrossRef]
- Chabukaswar, V.; Dhomase, N.; Bhavsar, S.; Horne, A.; Mohite, K.; Gaikwad, V. Studies on morphology and conductivity of poly(N-methyl aniline) nanoparticles prepared in nonstirred reaction medium. Macromol. Symp. 2010, 298, 43–50. [Google Scholar] [CrossRef]
- Palazzesi, F.; Calvaresi, M.; Zerbetto, F. A molecular dynamics investigation of structure and dynamics of SDS and SDBS micelles. Soft Matter 2011, 7, 9148–9156. [Google Scholar] [CrossRef]
- Permpool, T.; Sirivat, A.; Aussawasathien, D. Synthesis of polydiphenylamine with tunable size and shape via emulsion polymerization. Polym. Int. 2014, 63, 2076–2083. [Google Scholar] [CrossRef]
- Neoh, K.G.; Kang, E.T.; Tan, K.L. Limitations of the X-ray photoelectron spectroscopy technique in the study of electroactive polymers. J. Phys. Chem. B 1997, 101, 726–731. [Google Scholar] [CrossRef]
- Bavastrello, V.; Correia Terencio, T.B.; Nicolini, C. Synthesis and characterization of polyaniline derivatives and related carbon nanotubes nanocomposites—Study of optical properties and band gap calculation. Polymer 2011, 52, 46–54. [Google Scholar] [CrossRef]
- Changqing, Y.; Gao, L.; Zhou, F.; Duan, G. Facile synthesis of polyaniline nanotubes using self-assembly method based on the hydrogen bonding: Mechanism and application in gas sensing. Polymers 2017, 9, 544. [Google Scholar]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef]
- Zeybeka, B.; Pekmez, N.Ö.; Kiliç, E. Electrochemical synthesis of bilayer coatings of poly(N-methylaniline) and polypyrrole on mild steel and their corrosion protection performances. Electrochim. Acta 2011, 56, 9277–9286. [Google Scholar] [CrossRef]
- Sahin, M.; Görçay, H.; Kir, E.; Sahin, Ÿ. Removal of calcium and magnesium using polyaniline and derivatives modified PVDF cation-exchange membranes by Donnan dialysis. React. Funct. Polym. 2009, 69, 673–680. [Google Scholar] [CrossRef]
- Manohar, S.K.; Macdiarmid, A.G.; Kromack, K.R.; Jinder, J.M.; Epstein, E.J. N-substituted derivatives of polyaniline. Synth. Met. 1989, 29, 349–356. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yildiz, A. Poly(N-methylaniline) coatings on stainless steelby electropolymerization. Corros. Sci. 2007, 49, 2905–2919. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, J.; Chao, D.; Chen, J.; Zhang, W.; Wei, Y. Poly (N-methylaniline)/multi-walled carbon nanotube composites-synthesis, characterization, and electrical properties. J. Appl. Polym. Sci. 2006, 100, 2356–2361. [Google Scholar] [CrossRef]
- Li, C.-Y.; Chiu, W.-Y.; Don, T.-M. Polyurethane/polyaniline and polyurethane-poly(methyl methacrylate)/polyaniline conductive core-shell particles: Preparation, morphology, and conductivity. J. Polym. Sci. Polym. Chem. 2007, 45, 3902–3911. [Google Scholar] [CrossRef]
- Ibrahim, K.A. Synthesis and characterization of polyaniline and poly(aniline-co-o-nitroaniline) using vibrational spectroscopy. Arab. J. Chem. 2017, 10, S2668–S2674. [Google Scholar] [CrossRef]
- Castillo-Reyes, B.E.; Ovando-Medina, V.M.; González-Ortega, O.; Alonso-Dávila, P.A.; Juárez-Ramírez, I.; Martínez-Gutiérrez, H.; Márquez-Herrera, A. TiO2/polypyrrole nanocomposites photoactive under visible light synthesized by heterophase polymerization in the presence of different surfactants. Res. Chem. Intermed. 2015, 41, 8211–8231. [Google Scholar] [CrossRef]
- Hassan, P.A.; Sawant, S.N.; Bagkar, N.C.; Yakhmi, J.V. Polyaniline nanoparticles prepared in rodlike micelles. Langmuir 2004, 20, 4874–4880. [Google Scholar] [CrossRef]
- Lehr, I.L.; Saidman, S.B. Electrodeposition of polypyrrole on aluminium in the presence of sodium bis(2-ethylhexyl) sulfosuccinate. Mater. Chem. Phys. 2006, 100, 262–267. [Google Scholar] [CrossRef]
- Sakmeche, N.; Aeiyach, S.; Aaron, J.-J.; Jouini, M.; Lacroix, J.C.; Lacaze, P.-C. Improvement of the electrosynthesis and physicochemical properties of poly(3,4-ethylenedioxythiophene) using a sodium dodecyl sulfate micellar aqueous medium. Langmuir 1999, 15, 2566–2574. [Google Scholar] [CrossRef]
- Golczak, S.; Kanciurzewska, A.; Fahlman, M.; Langer, K.; Langer, J.J. Comparative XPS surface study of polyaniline thin films. Solid State Ion. 2008, 179, 2234–2239. [Google Scholar] [CrossRef]
- Lopes, E.S.; Domingos, E.; Neves, R.S.; Romão, W.; de Souza, K.R.; Valaski, R.; Archanjo, B.S.; Souza, F.G., Jr.; Silva, A.M.; Kuznetsov, A.; et al. The role of intermolecular interactions in polyaniline/polyamide-6,6 pressure-sensitive blends studied by DFT and 1H NMR. Eur. Polym. J. 2016, 85, 588–604. [Google Scholar] [CrossRef]
- Kim, B.-J.; Oh, S.-G.; Han, M.-G.; Im, S.-S. Preparation of polyaniline nanoparticles in micellar solutions as polymerization medium. Langmuir 2000, 16, 5841–5845. [Google Scholar] [CrossRef]
- Arteshi, Y.; Aghanekad, A.; Davaran, S.; Omidi, Y. Biocompatible and electroconductive polyaniline-based biomaterials for electrical stimulation. Eur. Polym. J. 2018, 108, 150–170. [Google Scholar] [CrossRef]
- Li, L.; Qiu, H.; Qian, H.; Hao, B.; Liang, X. Controlled synthesis of the poly(N-methylaniline)/Zn0.6Mn0.2Ni0.2Fe2O4 composites and its electrical-magnetic property. J. Phys. Chem. C 2010, 114, 6712–6717. [Google Scholar] [CrossRef]
- Chaudhari, H.K.; Kelkar, D.S. X-ray diffraction study of doped polyaniline. J. Appl. Polym. Sci. 1996, 62, 15–18. [Google Scholar] [CrossRef]
- Chin, S.Y.; Abdullah, T.K.; Mariatti, M. One-step synthesis of conductive graphene/polyaniline nanocomposites using sodium dodecylbenzenesulfonate: Preparation and properties. J. Mater. Sci.-Mater. Electron. 2017, 28, 18418–18428. [Google Scholar] [CrossRef]
- Khafagy, R.M. Synthesis, characterization, magnetic and electrical properties of the novel conductive and magnetic Polyaniline/MgFe2O4 nanocomposite having the core–shell structure. J. Alloy Compd. 2011, 509, 9849–9857. [Google Scholar] [CrossRef]
- Palsaniya, S.; Nemade, H.B.; Dasmahapatra, A.K. Heterostructured layer growth of polyaniline by vacuum thermal evaporation and fabrication of thin-film capacitors. J. Phys. Chem. C 2019, 123, 27959–27968. [Google Scholar] [CrossRef]
- Lee, J.; Kim, E. Effect of structural and morphological changes on the conductivity of stretched PANI-DBSA/HIPS film. Bull. Korean Chem. Soc. 2011, 32, 2661–2665. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Synthesis of nano-sized polyindole via emulsion polymerization and doping. Synth. Met. 2016, 219, 142–153. [Google Scholar] [CrossRef]
- Mohanty, A.; Dey, J. Effect of the headgroup structure on the aggregation behavior and stability of self-assemblies of sodium N-[4-(n-Dodecyloxy)benzoyl]-L-aminoacidates in water. Langmuir 2007, 23, 1033–1040. [Google Scholar] [CrossRef]
- Ardyani, T.; Mohamed, A.; Bakar, S.A.; Sagisaka, M.; Umetsu, Y.; Mamat, M.H.; Ahmad, M.K.; Abdul Khalil, H.P.S.; King, S.; Rogers, S.E.; et al. Surfactants with aromatic headgroups for optimizing properties of graphene/natural rubber latex composites (NRL): Surfactants with aromatic amine polar heads. J. Colloid Interface Sci. 2019, 545, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.-J.; Lee, Y.; Choi, H.; Kim, M.-S.; Im, K.; Noh, S.; Yoon, H. Surfactant-templated synthesis of polypyrrole nanocages as redox mediators for efficient energy storage. Sci. Rep. 2015, 5, 14097. [Google Scholar] [CrossRef]
- Jang, J.; Ha, J.; Kim, S. Fabrication of polyaniline nanoparticles using microemulsion polymerization. Macromol. Res. 2007, 15, 154–159. [Google Scholar] [CrossRef]
- Perrin, F.X.; Phan, T.A.; Nguyen, D.L. Preparation and characterization of polyaniline in reversed micelles of decylphosphonic acid for active corrosion protection coatings. Eur. Polym. J. 2015, 66, 253–265. [Google Scholar] [CrossRef]
- Trchová, M.; Šedĕnková, I.; Tobolková, E.; Stejskal, J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degrad. Stabil. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Tucceri, R.; Arnal, P.M.; Scian, A.N. Spectroscopic characterization of poly(ortho-Aminophenol) film electrodes: A review article. J. Spectrosc. 2012, 2013, 951604. [Google Scholar] [CrossRef]
- Nalayama, M.; Saeki, S.; Ogura, K. In sito observation of electrochemical formation and degradation processes of polyaniline by Fourier-Transform Infrared spectroscopy. Anal. Sci. 1999, 15, 259–263. [Google Scholar]
- Zhang, S.; Shao, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene-polypyrrole nanocomposite as a highly efficient and low cost electrically switched ion exchanger for removing ClO4- from Wastewater. ACS Appl. Mater. Interfaces 2011, 3, 3633–3637. [Google Scholar] [CrossRef] [PubMed]
- Choeichom, P.; Sirivat, A. Tuning poly(p-phenylene) nano-size for enhancing electrical conductivity based on surfactant templates and doping. Curr. Appl. Phys. 2018, 18, 686–697. [Google Scholar] [CrossRef]
- Jiang, J.; Yan, C.-S.; Liu, W. Facile template-free route to poly(N-methylaniline) microspheres in aqueous solution. Mater. Lett. 2009, 63, 2188–2190. [Google Scholar] [CrossRef]
- Patil, R.; Sanada, K.; Jiang, X.; Harima, Y.; Masaoka, K.; Yamasaki, S.; Yano, J. Microspheres of conducting poly(N-methylaniline). Polym. J. 2004, 36, 549–555. [Google Scholar] [CrossRef]
| Synthesis Condition | Designation | Shape | Particle Size a | Electrical Conductivity | Doping Level b |
|---|---|---|---|---|---|
| (nm) | (S cm−1) | ||||
| PNMA without surfactant | PNMA | Spherical | 581 ± 84 | (5.15 ± 1.16) × 10−2 | 23.81 |
| Effects of surfactant types and concentrations | |||||
| PNMA- SDS at 0.2CMC | PNMA-0.2SDS | spherical | 530 ± 78 | 1.56 ± 0.02 | 27.2 |
| PNMA- SDS at CMC | PNMA-1SDS | spherical mixed rod-like | 555 ± 98 L/D 300 ± 100 | 2.99 ± 0.45 | 29.27 |
| PNMA- SDS at 5CMC | PNMA-5SDS | irregular | 963 ± 542 | (2.15 ± 0.17) × 10−3 | 22.27 |
| PNMA- SDS at 10CMC | PNMA-10SDS | N/A | N/A | (9.20 ± 2.65) × 10−3 | 22.99 |
| PNMA-SDBS at 0.2CMC | PNMA-0.2SDBS | Spherical | 467 ± 60 | (2.16 ± 0.25) × 10−1 | 24.4 |
| PNMA-SDBS at CMC | PNMA-1SDBS | Spherical mixed hollow | 437 ± 81 t* = 54 ± 17 | 1.57 ± 0.27 | 27.52 |
| PNMA-SDBS at 5CMC | PNMA-5SDBS | Hollow | 301 ± 58 t* = 36 ± 13 | 7.33 ± 1.53 | 32.91 |
| PNMA-SDBS at 10CMC | PNMA-10SDBS | Hollow | 290 ± 53 t* = 27 ± 7 | 1.78 ± 0.66 | 28.49 |
| PNMA-SDBS at 15CMC | PNMA-15SDBS | Hollow | 304 ± 32 t* = 33 ± 6 | 2.00 ± 0.76 | 28.67 |
| PNMA- AOT at 0.2CMC | PNMA-0.2AOT | Spherical | 522 ± 80 | (2.49 ± 0.86) × 10−3 | 22.69 |
| PNMA- AOT at CMC | PNMA-1AOT | Spherical | 479 ± 80 | (5.20 ± 0.20) × 10−1 | 25.48 |
| PNMA- AOT at 5CMC | PNMA-5AOT | Spherical | 407 ± 68 | (1.74 ± 0.12) × 10−1 | 24.69 |
| PNMA- AOT at 10CMC | PNMA-10AOT | Spherical | 459 ± 40 | (3.21 ± 0.74) × 10−1 | 25.02 |
| PNMA- AOT at 15CMC | PNMA-15AOT | Spherical | 467 ± 50 | (3.35 ± 1.40) × 10−1 | 25.17 |
| Effect of dopant mole ratio | |||||
| de-doped PNMA-5SDBS | dePNMA | Irregular | N/A | (4.47 ± 0.92) × 10−6 | 14.93 |
| /NNMA mole ratio of 1:1 | dPNMA 1:1 | Irregular | 1238 ± 400 | 30.41 ± 7.23 | 37.24 |
| /NNMA mole ratio of 5:1 | dPNMA 5:1 | Irregular | 1891 ± 761 | 37.85 ± 5.26 | 38.83 |
| /NNMA mole ratio of 10:1 | dPNMA 10:1 | Irregular | 2406 ± 1343 | 28.76 ± 3.32 | 36.37 |
| /NNMA mole ratio of 25:1 | dPNMA 25:1 | Irregular | 1718 ± 532 | 40.70 ± 11.80 | 41.09 |
| /NNMA mole ratio of 50:1 | dPNMA 50:1 | Irregular | N/A | 109.84 ± 20.44 | 52.45 |
| /NNMA mole ratio of 75:1 | dPNMA 75:1 | N/A | N/A | 59.15 ± 8.74 | 43.31 |
| /NNMA mole ratio of 100:1 | dPNMA 100:1 | N/A | N/A | 69.11 ± 2.64 | 44.14 |
| Synthesis Condition | Shape | Particle Size a (nm) | Electrical Conductivity (S cm−1) | Ref. |
|---|---|---|---|---|
| PNMA-APS-SDBS at 5CMC in water via chemical polymerization re-doped by HClO4 | Hollow | 551 ± 193 t* = 48 ± 18 | 15.53 ± 2.59 | [13] |
| PNMA-APS-p-TSA via chemical polymerization | Fibrillar | 700 | 1.49 × 10−3 | [25] |
| PNMA-APS-DL-Tartaric acid using acrylic acid as a template via chemical polymerization | Coral-like | N/A | 3.4 × 10−2 | [26] |
| PNMA-APS in water via chemical polymerization | granular particle | 1200–1800 | N/A | [65] |
| PNMA in HClO4 solution via electrochemical polymerization | Spherical | 1900 | N/A | [66] |
| PNMA-APS-HCl via chemical polymerization | N/A | N/A | 5.4 × 10−1 | [24] |
| PNMA-APS-SDBS at 5CMC in HCl media via chemical polymerization re-doped by HClO4 | Irregular | N/A | 109.84 ± 20.44 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Direksilp, C.; Sirivat, A. Synthesis and Characterization of Hollow-Sphered Poly(N-methyaniline) for Enhanced Electrical Conductivity Based on the Anionic Surfactant Templates and Doping. Polymers 2020, 12, 1023. https://doi.org/10.3390/polym12051023
Direksilp C, Sirivat A. Synthesis and Characterization of Hollow-Sphered Poly(N-methyaniline) for Enhanced Electrical Conductivity Based on the Anionic Surfactant Templates and Doping. Polymers. 2020; 12(5):1023. https://doi.org/10.3390/polym12051023
Chicago/Turabian StyleDireksilp, Chatrawee, and Anuvat Sirivat. 2020. "Synthesis and Characterization of Hollow-Sphered Poly(N-methyaniline) for Enhanced Electrical Conductivity Based on the Anionic Surfactant Templates and Doping" Polymers 12, no. 5: 1023. https://doi.org/10.3390/polym12051023
APA StyleDireksilp, C., & Sirivat, A. (2020). Synthesis and Characterization of Hollow-Sphered Poly(N-methyaniline) for Enhanced Electrical Conductivity Based on the Anionic Surfactant Templates and Doping. Polymers, 12(5), 1023. https://doi.org/10.3390/polym12051023





