Impact of Spacer Nature and Counter Ions on Rheological Behavior of Novel Polymer-Cationic Gemini Surfactant Systems at High Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymer Solutions
2.3. Rheological Measurements
3. Results and Discussion
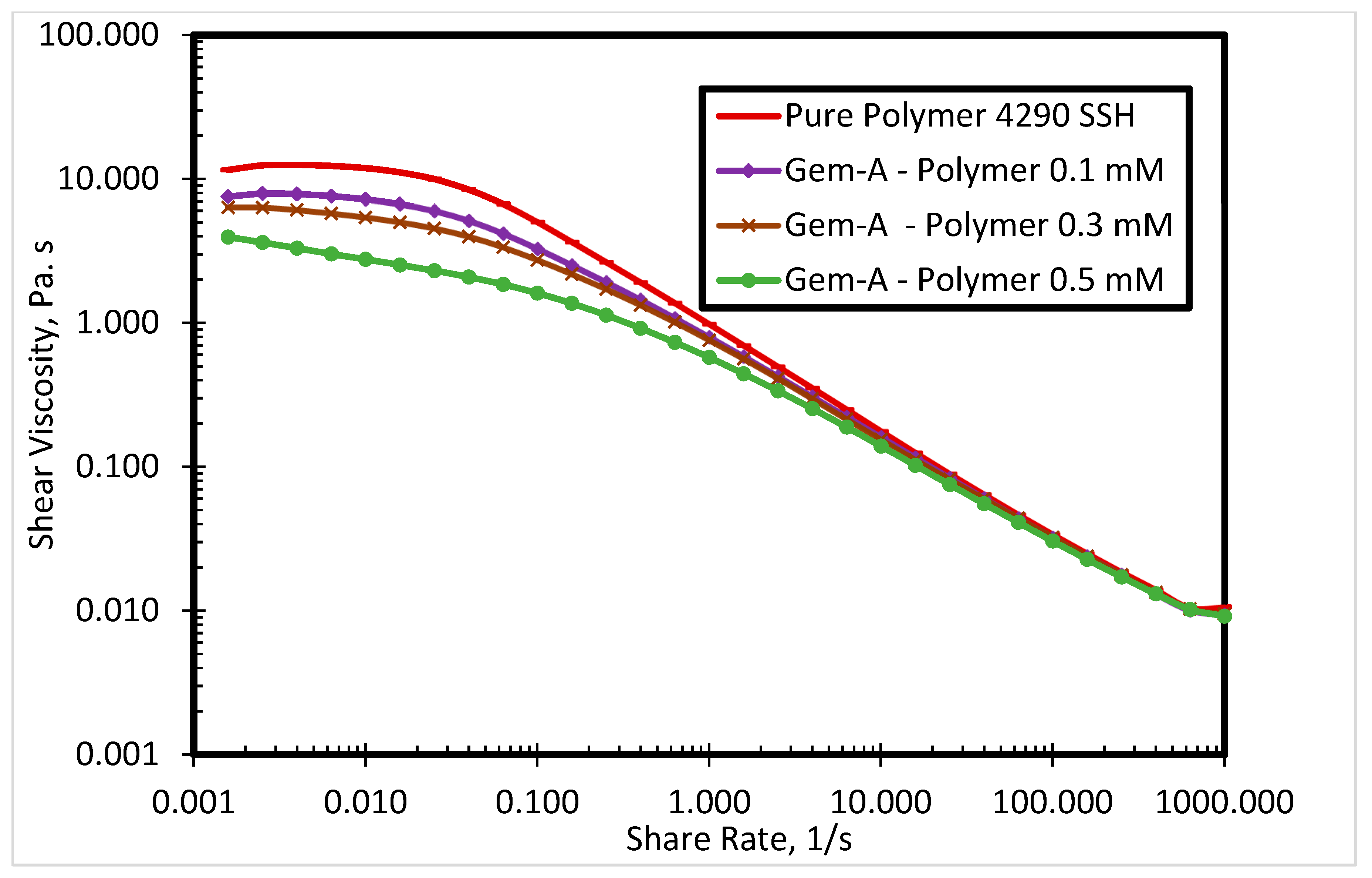
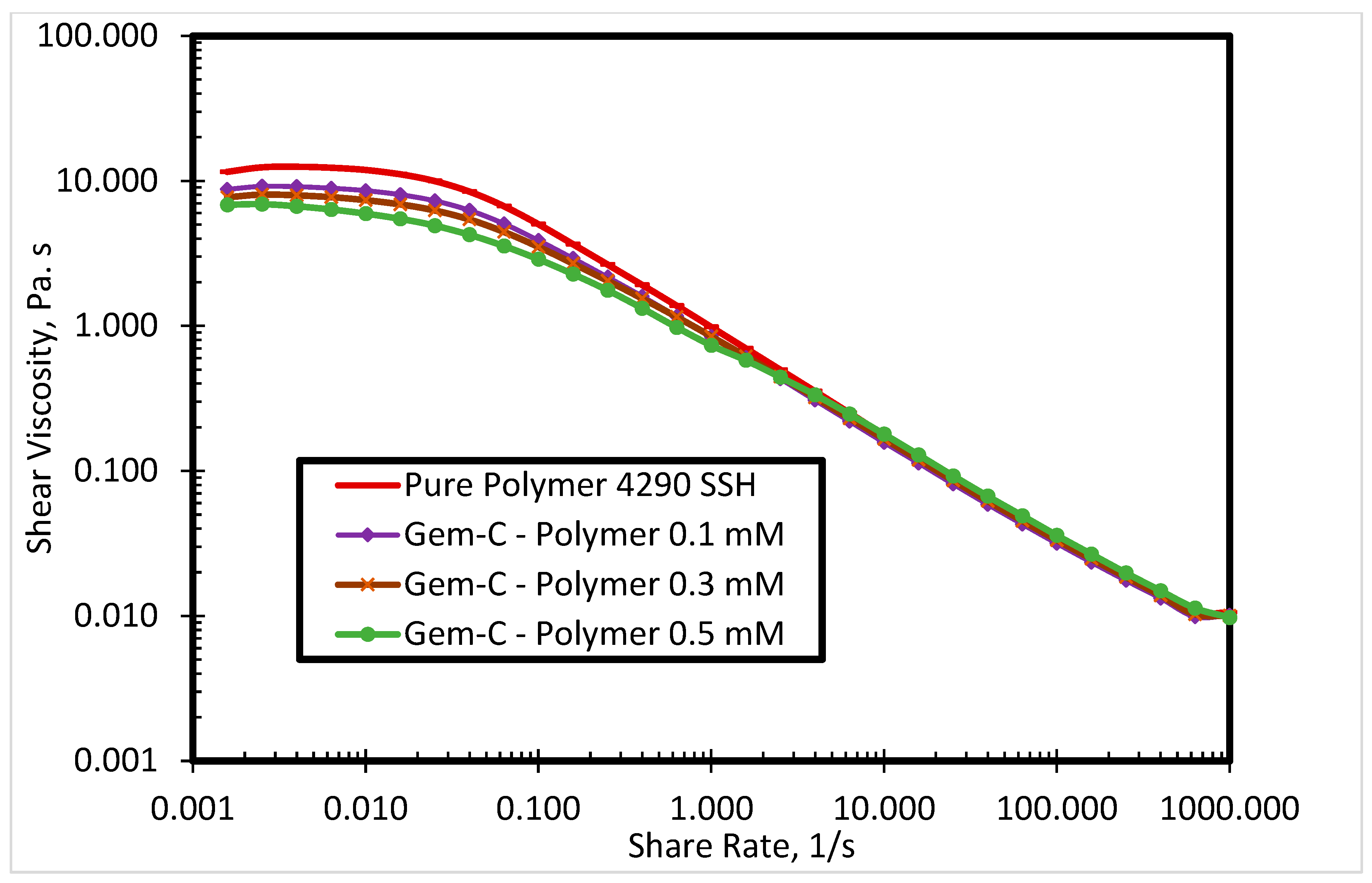
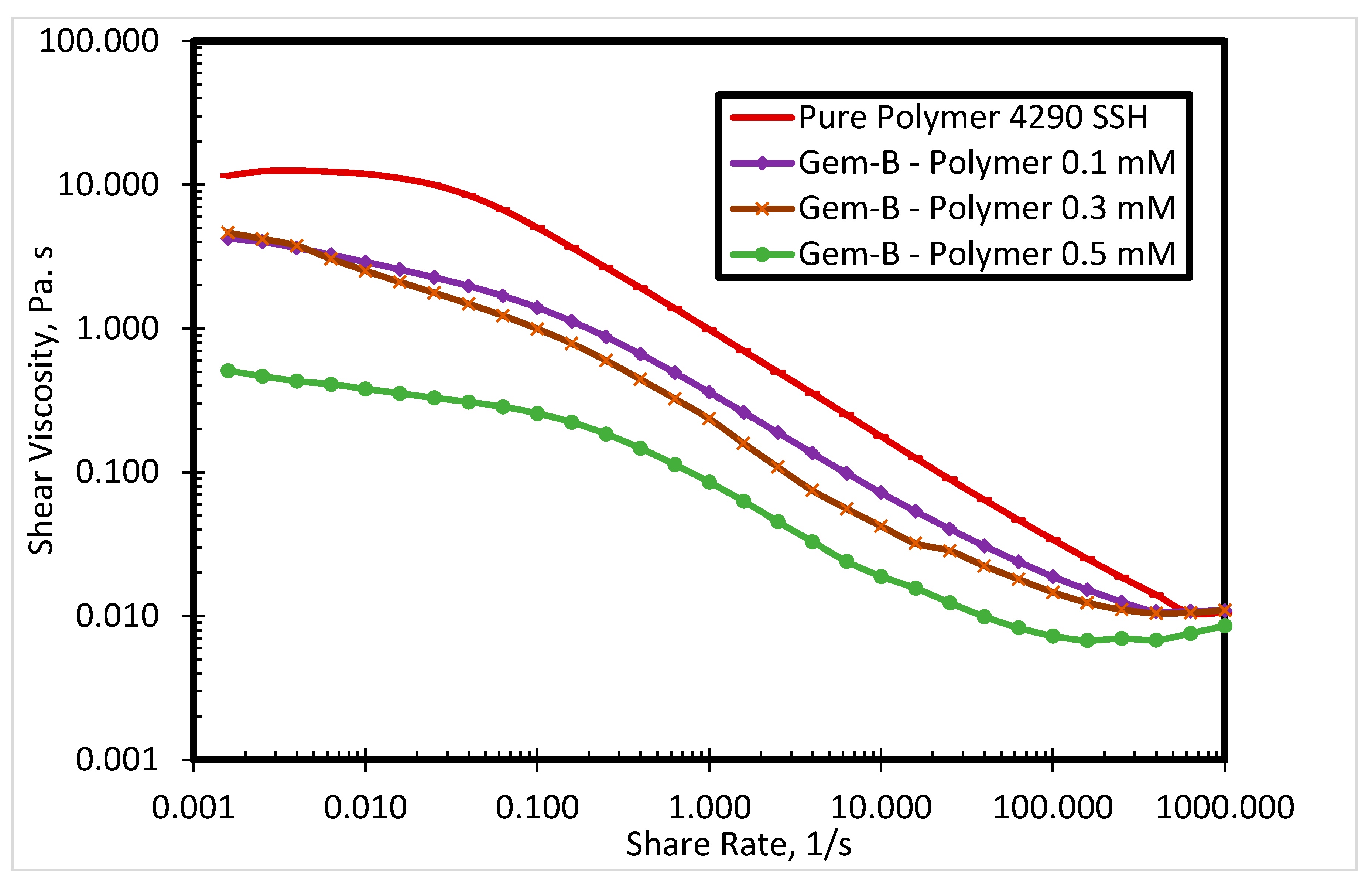
3.1. Rheological Properties of Cationic Polyacrylamide
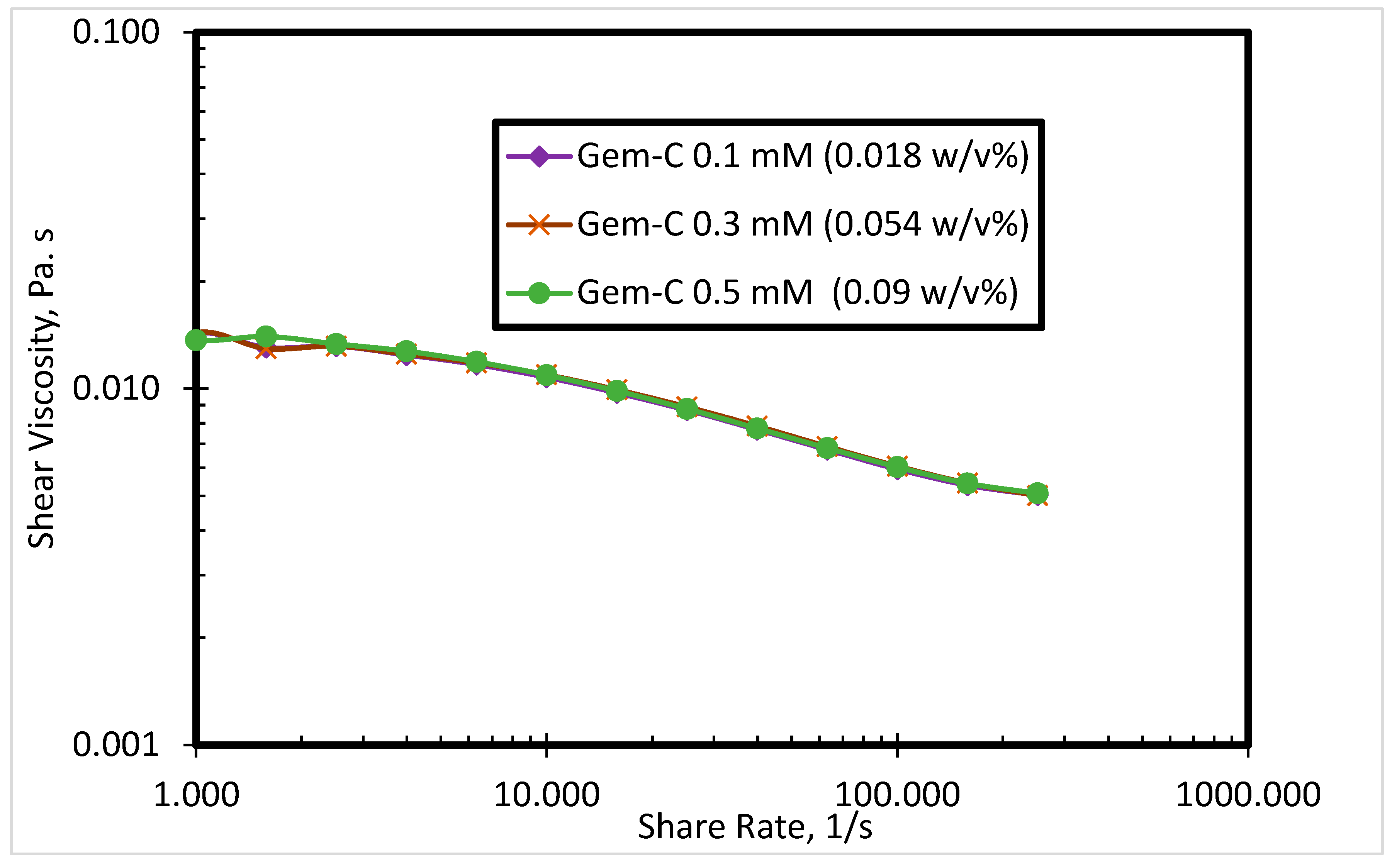
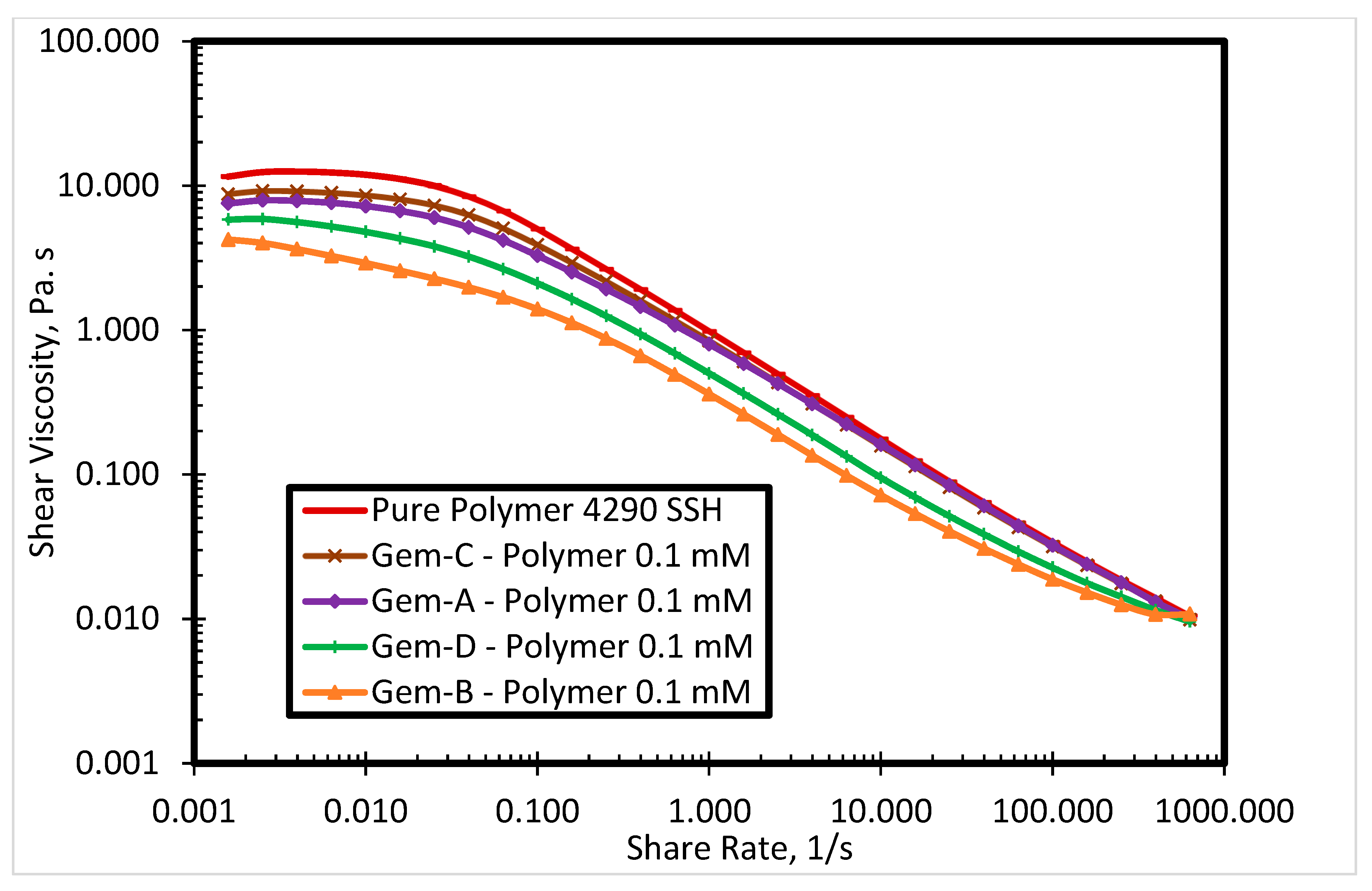
3.2. Rheological Properties of the SP System
3.2.1. Effect of Surfactant Concentration
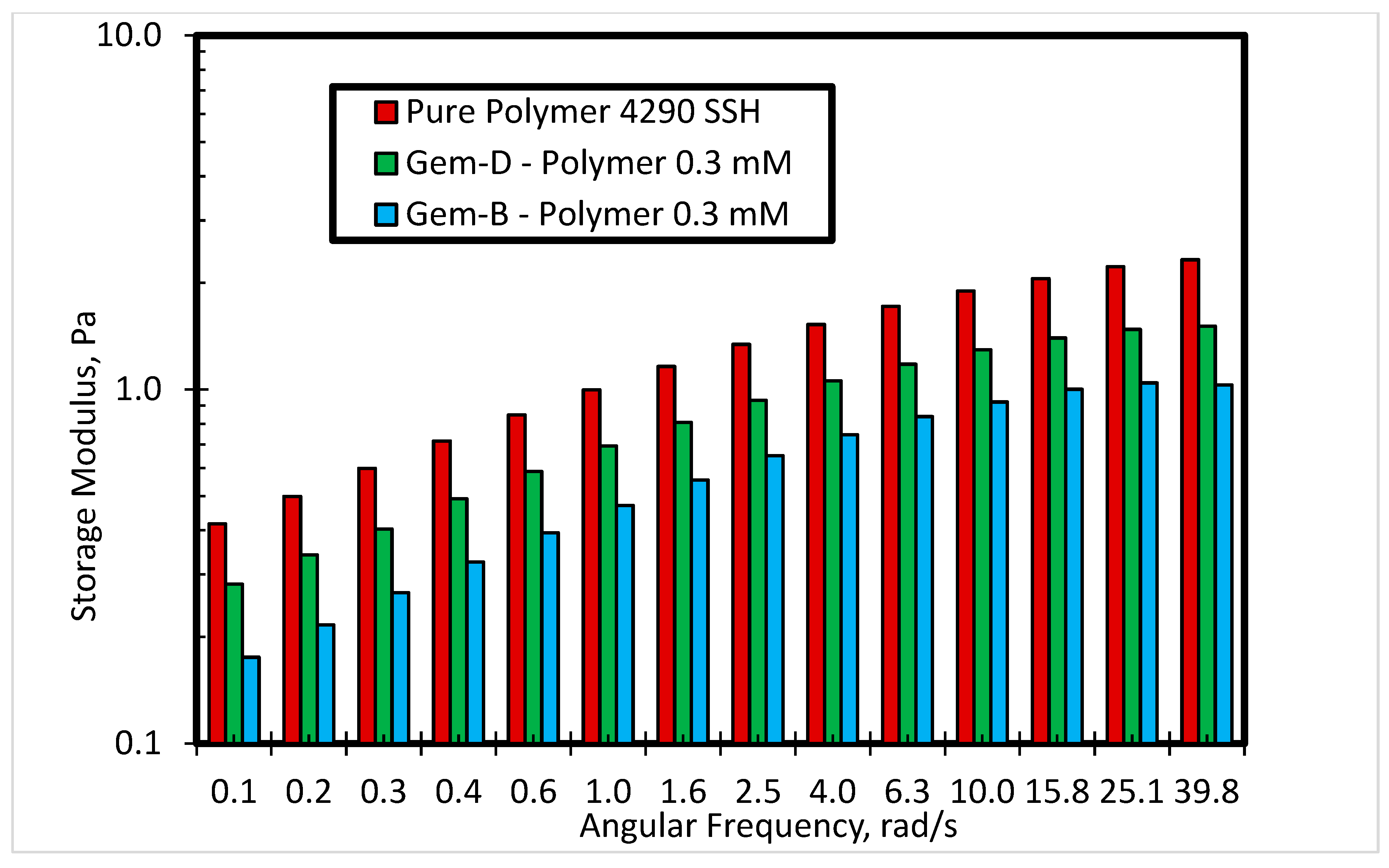
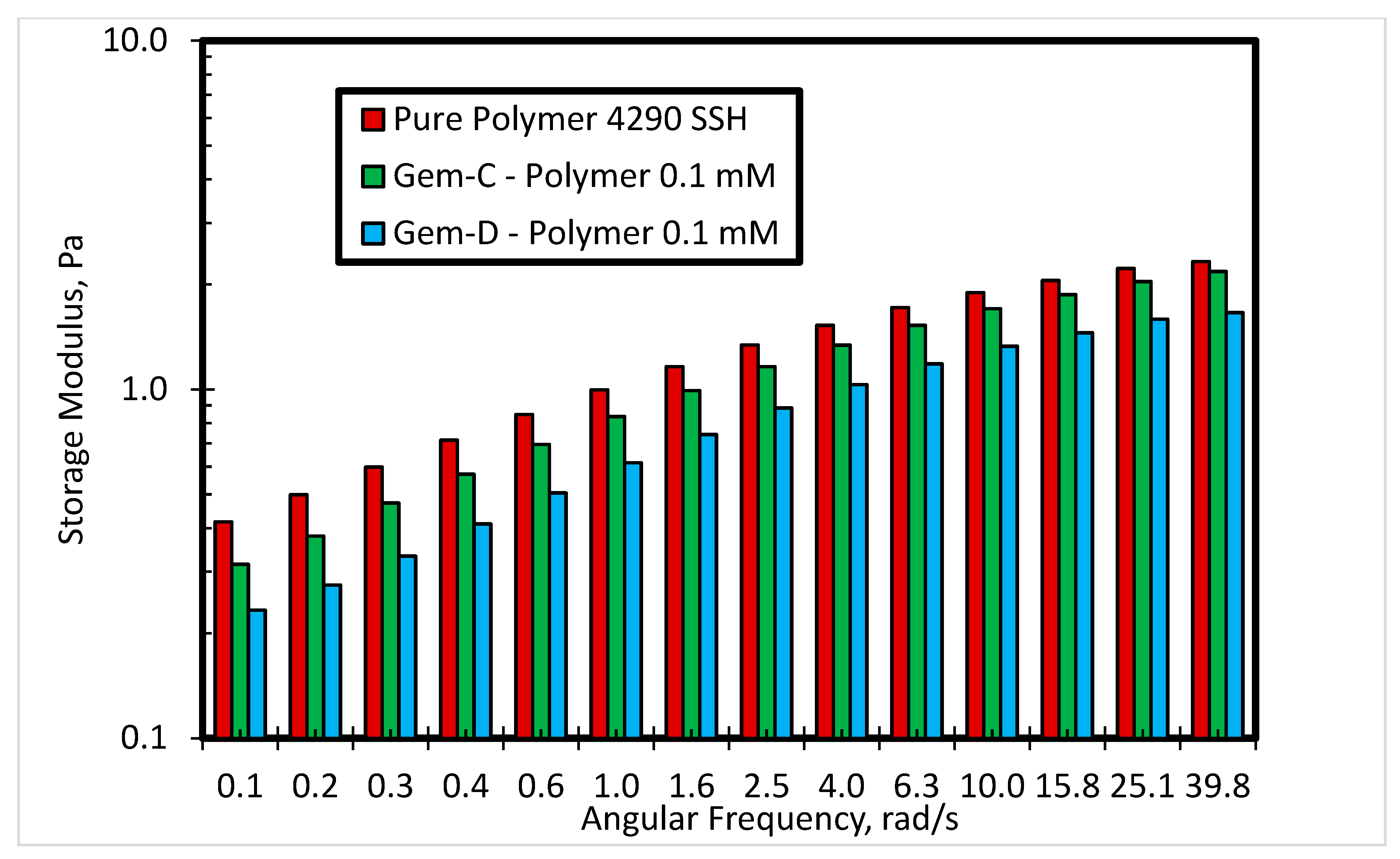
3.2.2. Effect of Spacer Nature and Counterions
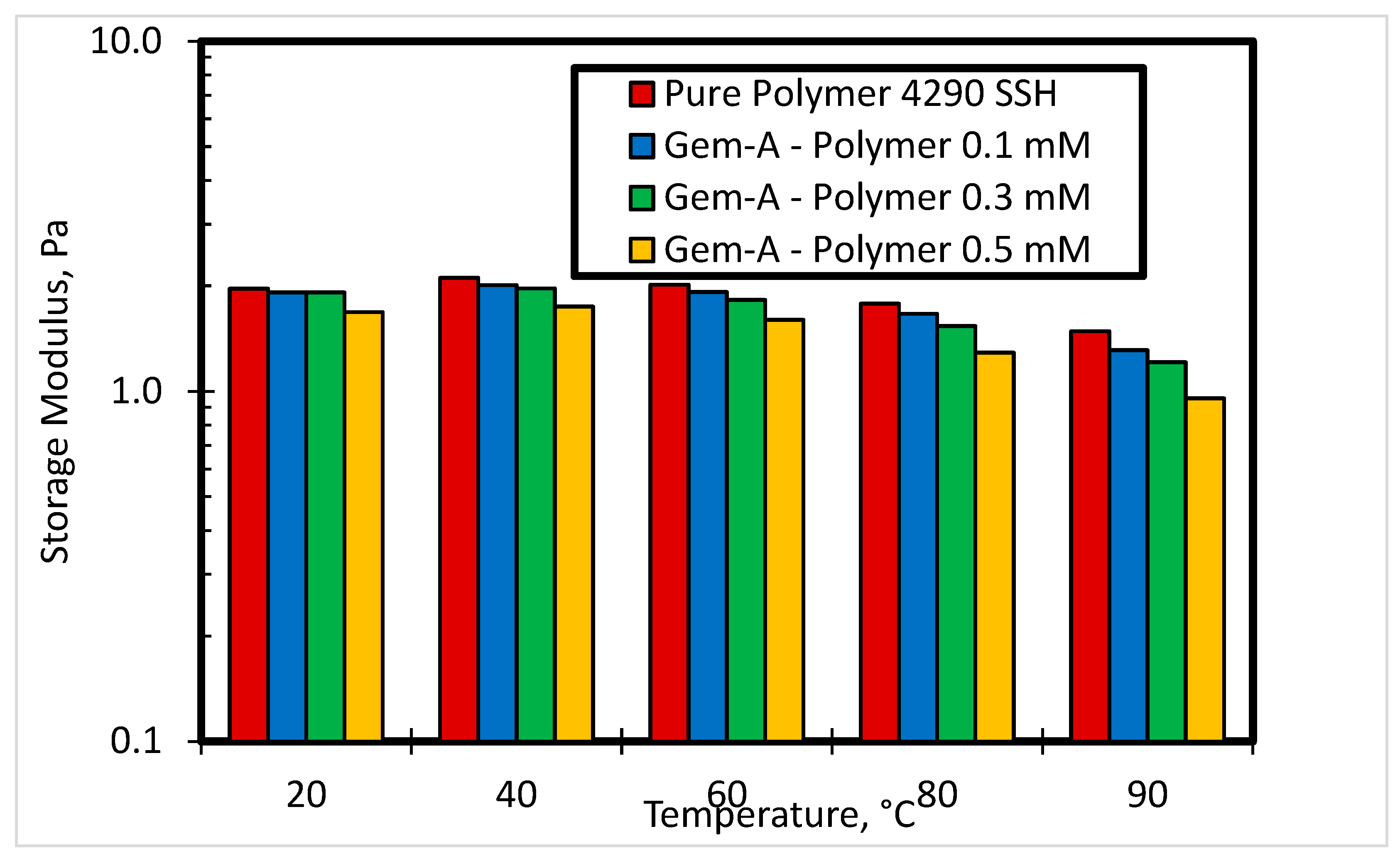
3.2.3. Effect of Temperature
4. Summary and Conclusions
- An increase in surfactant concentrations decreases the viscosity and elasticity of the SP system because of the charge screening effect.
- The inclusion of the phenyl ring in the spacer can help in improving the viscosity and elasticity of the SP system.
- The use of chloride counterions can give better rheological behavior as compared to bromide counterions.
- Finally, it was observed that counterions also influence the rheological properties significantly. The results reveal that the novel SP solution with a phenyl ring with chloride counterions performs better in comparison to a phenyl ring with bromide counterions. Moreover, the performance of the SP solution system can be further enhanced by the addition of another phenyl ring in the spacer.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yasari, E.; Pishvaie, M.R.; Khorasheh, F.; Salahshoor, K.; Kharrat, R. Application of multi-criterion robust optimization in water-flooding of oil reservoir. J. Pet. Sci. Eng. 2013, 109, 1–11. [Google Scholar] [CrossRef]
- Thomas, S. Enhanced oil recovery-an overview molecular structures of heavy oils and coal liquefaction products structure moléculaire des huiles lourdes et produits de liquéfaction du charbon. Oil Gas Sci. Technol. L’IFP 2008, 63, 9–19. [Google Scholar] [CrossRef]
- Wu, Y.; Mahmoudkhani, A.; Watson, P.; Fenderson, T.; Nair, M. Development of new polymers with better performance under conditions of high temperature and high salinity. In Proceedings of the Society of Petroleum Engineers-SPE EOR Conference at Oil and Gas West Asia 2012, Muscat, Oman, 16–18 April 2012; Volume 2, pp. 902–912. [Google Scholar]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussien, I.A.; Pabon, M. Evaluation of rheological and thermal properties of a new fluorocarbon surfactant-polymer system for EOR applications in high-temperature and high-salinity oil reservoirs. J. Surfactants Deterg. 2014, 17, 985–993. [Google Scholar] [CrossRef]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Half-century of heavy oil polymer flooding from laboratory core floods to pilot tests and field applications. In Proceedings of the Society of Petroleum Engineers-SPE Canada Heavy Oil Technical Conference 2015 (CHOC 2015), Calgary, AB, Canada, 9–11 June 2015; pp. 453–478. [Google Scholar]
- Olayiwola, S.O.; Dejam, M. A comprehensive review on interaction of nanoparticles with low salinity water and surfactant for enhanced oil recovery in sandstone and carbonate reservoirs. Fuel 2019, 241, 1045–1057. [Google Scholar] [CrossRef]
- Amirian, E.; Dejam, M.; Chen, Z. Performance forecasting for polymer flooding in heavy oil reservoirs. Fuel 2018, 216, 83–100. [Google Scholar] [CrossRef]
- Mashayekhizadeh, V.; Kord, S.; Dejam, M. EOR potential within Iran. Spec. Top. Rev. Porous Media 2014, 5, 325–354. [Google Scholar] [CrossRef]
- Sagir, M.; Talebian, S.H. Screening of CO2-philic surfactants morphology for high temperature-pressure sandstone reservoir conditions. J. Pet. Sci. Eng. 2020, 186, 106789. [Google Scholar] [CrossRef]
- Azam, M.R.; Tan, I.M.; Ismail, L.; Mushtaq, M.; Nadeem, M.; Sagir, M. Static adsorption of anionic surfactant onto crushed Berea sandstone. J. Pet. Explor. Prod. Technol. 2013, 3, 195–201. [Google Scholar] [CrossRef]
- McGuire, P.L.; Stalkup, F.I. Performance Analysis and Optimization of the Prudhoe Bay Miscible Gas Project; Society of Petroleum Engineers: Richardson, TX, USA, 1995. [Google Scholar]
- Yousef, A.A.; Al-Saleh, S.H.; Al-Kaabi, A.; Al-Jawfi, M.S. Laboratory investigation of the impact of injection-water salinity and ionic content on oil recovery from carbonate reservoirs. SPE Reserv. Eval. Eng. 2011, 14, 578–593. [Google Scholar] [CrossRef]
- Ghosh, P.; Sharma, H.; Mohanty, K.K. Development of surfactant-polymer sp processes for high temperature and high salinity carbonate reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 24–26 September 2018. [Google Scholar]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: Half-century studies. J. Pet. Sci. Eng. 2016, 142, 85–100. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Wang, D.; Zhu, X.; Zhu, Y.; Bai, X.; Yang, Q. Improvement of foaming ability of surfactant solutions by water-soluble polymers: Experiment and molecular dynamics simulation. Polymers 2020, 12, 571. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Xie, L.; Su, X.; Feng, Y. The thermoviscosifying behavior of water-soluble polymer based on graft polymerization of pluronic F127 with acrylamide and 2-acrylamido-2-methylpropane sulfonic acid sodium salt. Polymers 2019, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Novriansyah, A.; Bae, W.; Park, C.; Permadi, A.K.; Sri Riswati, S. Optimal design of alkaline-surfactant-polymer flooding under low salinity environment. Polymers 2020, 12, 626. [Google Scholar] [CrossRef]
- Deng, X.; Kamal, M.S.; Patil, S.; Hussain, S.M.S.; Zhou, X. A review on wettability alteration in carbonate rocks: Wettability modifiers. Energy Fuels 2020, 34, 31–54. [Google Scholar] [CrossRef]
- Urbissinova, T.; Trivedi, J.J.; Kuru, E. Effect of elasticity during viscoelastic polymer flooding-a possible mechanism of increasing the sweep efficiency. In Proceedings of the SPE Western Regional Meeting, Anaheim, CA, USA, 27–29 May 2010. [Google Scholar]
- Wang, D.; Cheng, J.; Xia, H.; Li, Q.; Shi, J. Viscous-elastic fluids can mobilize oil remaining after water-flood by force parallel to the oil-water interface. In Proceedings of the Society of Petroleum Engineers-SPE Asia Pacific Improved Oil Recovery Conference (APIORC 2001), Kuala Lumpur, Malaysia, 6–9 October 2001. [Google Scholar]
- Xia, H.; Ju, Y.; Kong, F.; Wu, J. Effect of elastic behavior of HPAM solutions on displacement efficiency under mixed wettability conditions. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. [Google Scholar]
- Xia, H.; Wang, D.; Wu, W.; Jiang, H. Effect of the visco-elasticity of displacing fluids on the relationship of capillary number and displacement efficiency in weak oil-wet cores. In Proceedings of the Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 30 October–1 November 2007. [Google Scholar]
- Xia, H.; Wang, D.; Wu, J.; Kong, F. Elasticity of HPAM solutions increases displacement efficiency under mixed wettability conditions. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 18–20 October 2004. [Google Scholar]
- Wang, D.; Xia, H.; Liu, Z.; Yang, Q. Study of the mechanism of polymer solution with visco-elastic behavior increasing microscopic oil displacement efficiency and the forming of steady “Oil thread” flow channels. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 April 2001. [Google Scholar]
- Urbissinova, T.S.; Trivedi, J.; Kuru, E. Effect of elasticity during viscoelastic polymer flooding: A possible mechanism of increasing the sweep efficiency. J. Can. Pet. Technol. 2010, 49, 49–56. [Google Scholar] [CrossRef]
- Liu, S.; University, R.; Leslie Zhang, D.; Westport, I.; Yan, W.; Puerto, M.; Hirasaki, G.J.; Miller, C.A. Favorable attributes of alkaline-surfactant-polymer flooding. SPE J. 2008, 13, 5–16. [Google Scholar] [CrossRef]
- Olayiwola, S.O.; Dejam, M. Mathematical modelling of surface tension of nanoparticles in electrolyte solutions. Chem. Eng. Sci. 2019, 197, 345–356. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Padmanabhan, E.; Junin, R.; Samin, A.M.; Gbadamosi, A.O.; Oguamah, I. A comprehensive review of experimental studies of nanoparticles-stabilized foam for enhanced oil recovery. J. Pet. Sci. Eng. 2018, 164, 43–74. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Yekeen, N.; Agi, A.; Oseh, J.O. Recent advances and prospects in polymeric nanofluids application for enhanced oil recovery. J. Ind. Eng. Chem. 2018, 66, 1–19. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef]
- Methemitis, C.; Morcellet, M.; Sabbadin, J.; Francois, J. Interactions between partially hydrolyzed polyacrylamide and ionic surfactants. Eur. Polym. J. 1986, 22, 619–627. [Google Scholar]
- Hussain, S.S.M.; Kamal, M.S. Effect of large spacer on surface activity, thermal, and rheological properties of novel amido-amine cationic gemini surfactants. J. Mol. Liq. 2017, 242, 1131–1137. [Google Scholar] [CrossRef]
- Hussain, S.S.M.; Kamal, M.S.; Sultan, A.S. Amido-amine-based cationic gemini surfactants: Thermal and interfacial properties and interactions with cationic polyacrylamide. J. Surfactants Deterg. 2017, 20, 47–55. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Mahboob, A.; Kamal, M.S. Poly (oxyethylene)-amidoamine based gemini cationic surfactants for oilfield applications: Effect of hydrophilicity of spacer group. Materials 2020, 13, 1046. [Google Scholar] [CrossRef]
- Hussain, S.S.M.; Kamal, M.S.; Murtaza, M. Synthesis of novel ethoxylated quaternary ammonium gemini surfactants for enhanced oil recovery application. Energies 2019, 12, 1731. [Google Scholar] [CrossRef]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S.E. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Kalam, S.; Kamal, M.S.; Patil, S.; Hussain, S.M.S. Role of counterions and nature of spacer on foaming properties of novel polyoxyethylene cationic gemini surfactants. Processes 2019, 7, 502. [Google Scholar] [CrossRef]
- Kamal, M.S.; Shakil Hussain, S.M.; Fogang, L.T. A zwitterionic surfactant bearing unsaturated tail for enhanced oil recovery in high-temperature high-salinity reservoirs. J. Surfactants Deterg. 2018, 21, 165–174. [Google Scholar] [CrossRef]
- Kamal, M.S.; Mahmoud, M.; Hanfi, M. Effects of rheological behavior of viscoelastic surfactants on formation damage in carbonate rocks. J. Surfactants Deterg. 2018, 21, 677–685. [Google Scholar] [CrossRef]
- Nasir Janjua, A.; Sultan, A.S.; Shahzad Kamal, M. Ultra-low interfacial tension, thermal stability and static adsorption of novel viscoelastic surfactant with heavy reservoir oil. In Proceedings of the Society of Petroleum Engineers-SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition 2018 (SATS 2018), Dammam, Saudi Arabia, 23–26 April 2018. [Google Scholar]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on surfactant flooding: Phase behavior, retention, IFT, and field applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Kamal, M.S.; Shakil Hussain, S.M.; Fogang, L.T.; Sultan, A.S. Impact of spacer and hydrophobic tail on interfacial and rheological properties of cationic amido-amine gemini surfactants for EOR application. Tenside Surfact. Deterg. 2018, 55, 491–497. [Google Scholar] [CrossRef]
- Riswati, S.S.; Bae, W.; Park, C.; Permadi, A.K.; Efriza, I.; Min, B. Experimental analysis to design optimum phase type and salinity gradient of alkaline surfactant polymer flooding at low saline reservoir. J. Pet. Sci. Eng. 2019, 173, 1005–1019. [Google Scholar] [CrossRef]
- Kulawardana, E.U.; Koh, H.; Kim, D.H.; Liyanage, P.J.; Upamali, K.; Huh, C.; Weerasooriya, U.; Pope, G.A. Rheology and transport of improved eor polymers under harsh reservoir conditions. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012; pp. 1–14. [Google Scholar]
- Salihu, S.M.; Abubakar, A.J.; Meisam, B.; Emmanuel, U.A.; Hassan, K.Y.; Aminu, A.Y. Effect of Temperature and salt concentration on rheological behaviour of surfactin. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 5–7 August 2019. [Google Scholar]
- Kamal, S.M.; Hussien, A.I.; Sultan, S.A.; Han, M. Rheological study on ATBS-AM copolymer-surfactant system in high-temperature and high-salinity environment. J. Chem. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, J.; Han, Y.; Wang, H.; Feng, Y. Laboratory study on the potential EOR use of HPAM/VES hybrid in high-temperature and high-salinity oil reservoirs. J. Chem. 2013, 2013, 8. [Google Scholar] [CrossRef]






| Polymer Name | Molecular Weight (million g/mol) |
|---|---|
| FO 4290 SSH | 8.3–10.8 |
| FO 4440 SSH | 6.8–9.1 |
| FO 4290 SH | 5.7–8.3 |
| FO 4800 SH | 4.7–6.7 |
| Ions | Seawater (ppm) |
|---|---|
| Sodium | 18,300 |
| Calcium | 650 |
| Magnesium | 2110 |
| Chloride | 32,200 |
| Bicarbonate | 120 |
| Sulfate | 4290 |
| TDS | 57,560 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalam, S.; Kamal, M.S.; Patil, S.; Hussain, S.M.S. Impact of Spacer Nature and Counter Ions on Rheological Behavior of Novel Polymer-Cationic Gemini Surfactant Systems at High Temperature. Polymers 2020, 12, 1027. https://doi.org/10.3390/polym12051027
Kalam S, Kamal MS, Patil S, Hussain SMS. Impact of Spacer Nature and Counter Ions on Rheological Behavior of Novel Polymer-Cationic Gemini Surfactant Systems at High Temperature. Polymers. 2020; 12(5):1027. https://doi.org/10.3390/polym12051027
Chicago/Turabian StyleKalam, Shams, Muhammad Shahzad Kamal, Shirish Patil, and Syed Muhammad Shakil Hussain. 2020. "Impact of Spacer Nature and Counter Ions on Rheological Behavior of Novel Polymer-Cationic Gemini Surfactant Systems at High Temperature" Polymers 12, no. 5: 1027. https://doi.org/10.3390/polym12051027
APA StyleKalam, S., Kamal, M. S., Patil, S., & Hussain, S. M. S. (2020). Impact of Spacer Nature and Counter Ions on Rheological Behavior of Novel Polymer-Cationic Gemini Surfactant Systems at High Temperature. Polymers, 12(5), 1027. https://doi.org/10.3390/polym12051027







