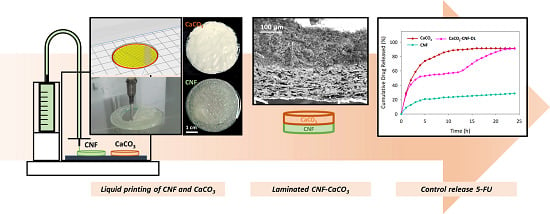
3D Printed Laminated CaCO3-Nanocellulose Films as Controlled-Release 5-Fluorouracil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNF and CaCO3–CNF Laminated Films
2.3. Liquid Deposition Modelling
2.4. Total Uptake and Release of 5-FU
2.5. Characterization
2.6. Analysis of Variance
3. Results and Discussion
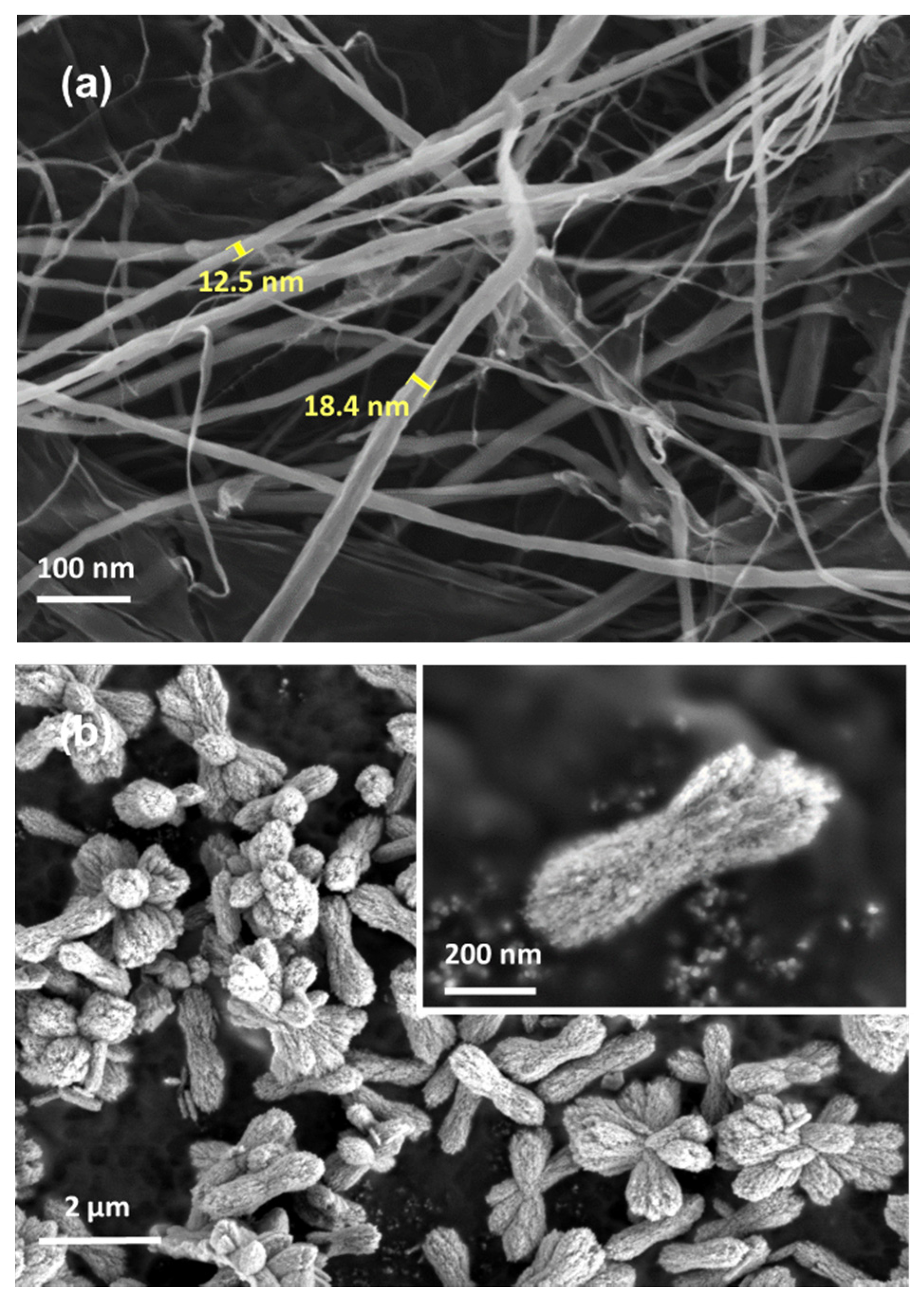
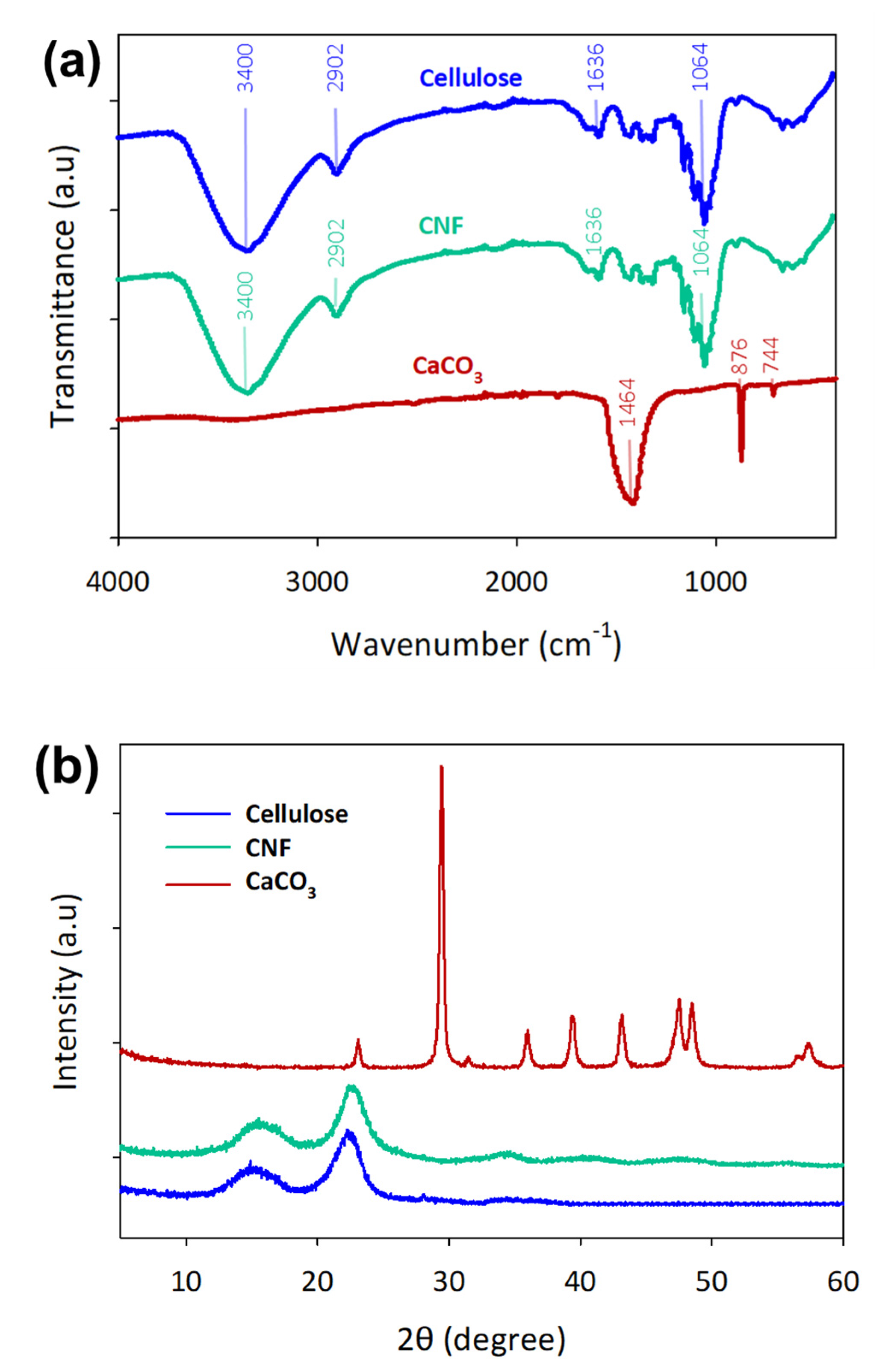
3.1. Characterization
3.2. Total Uptake and Release of 5-FU Using CNF and CaCO3 Casted Films
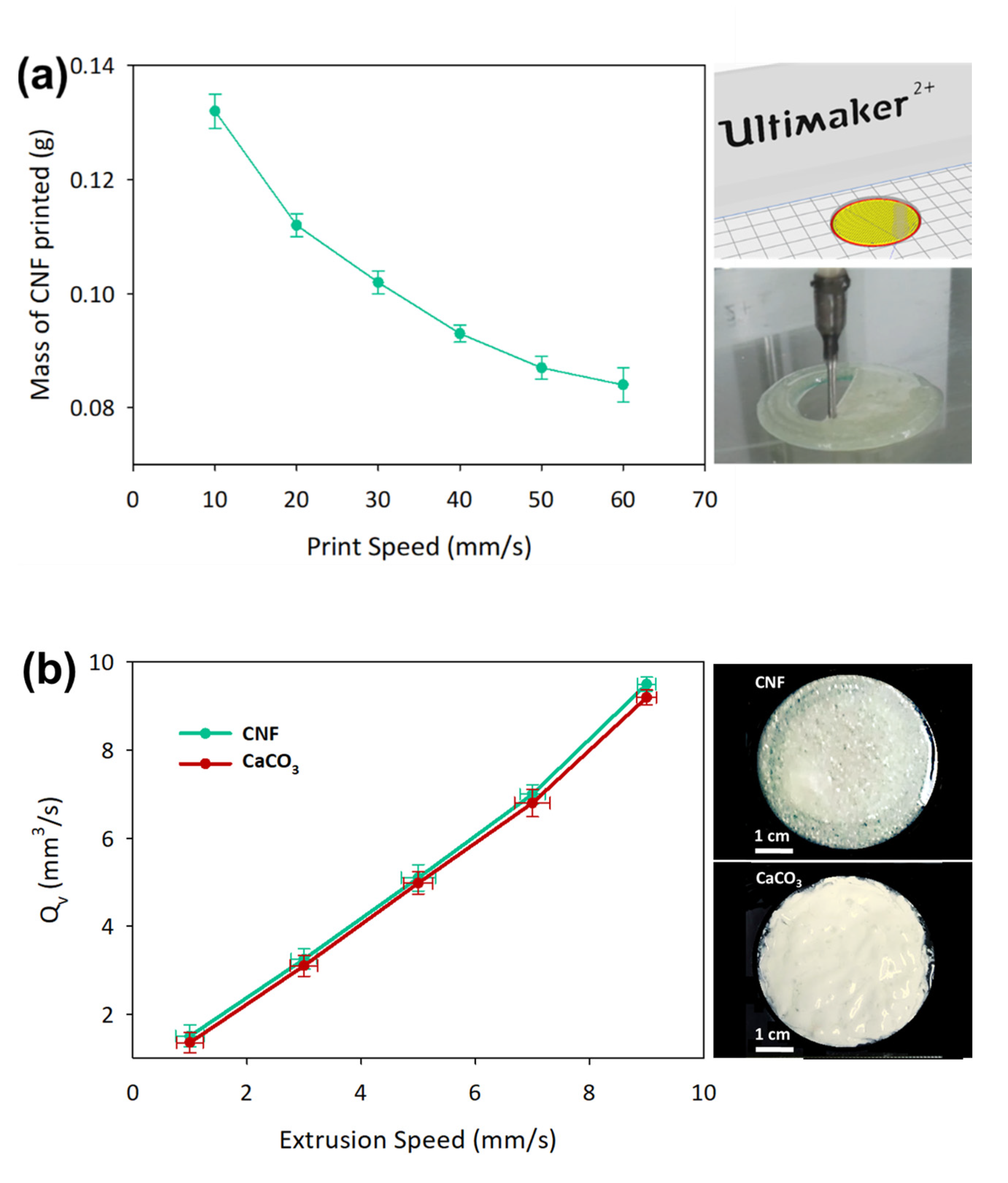
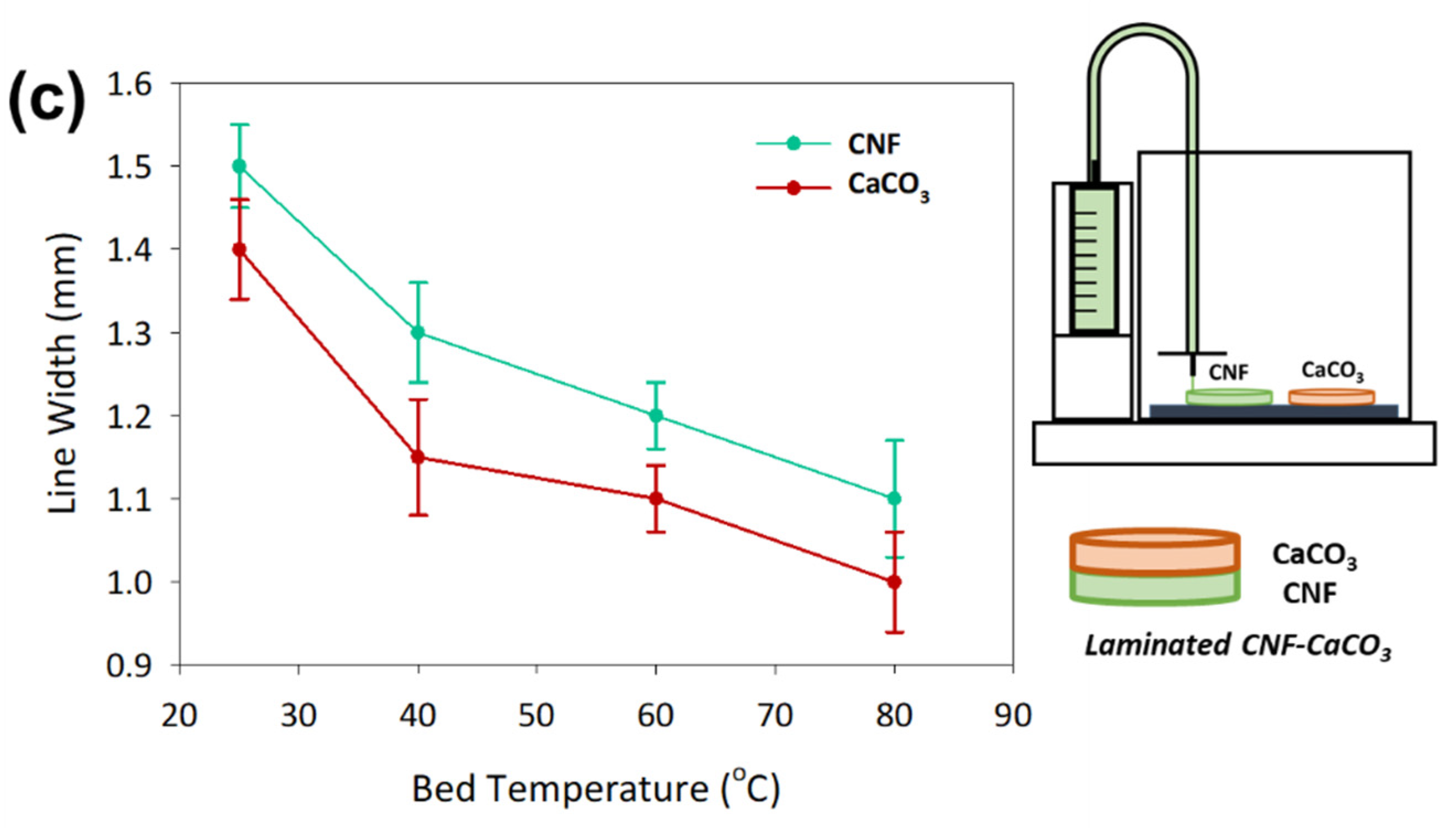
3.3. 3D Printed CaCO3-Nanocellulose Profiles
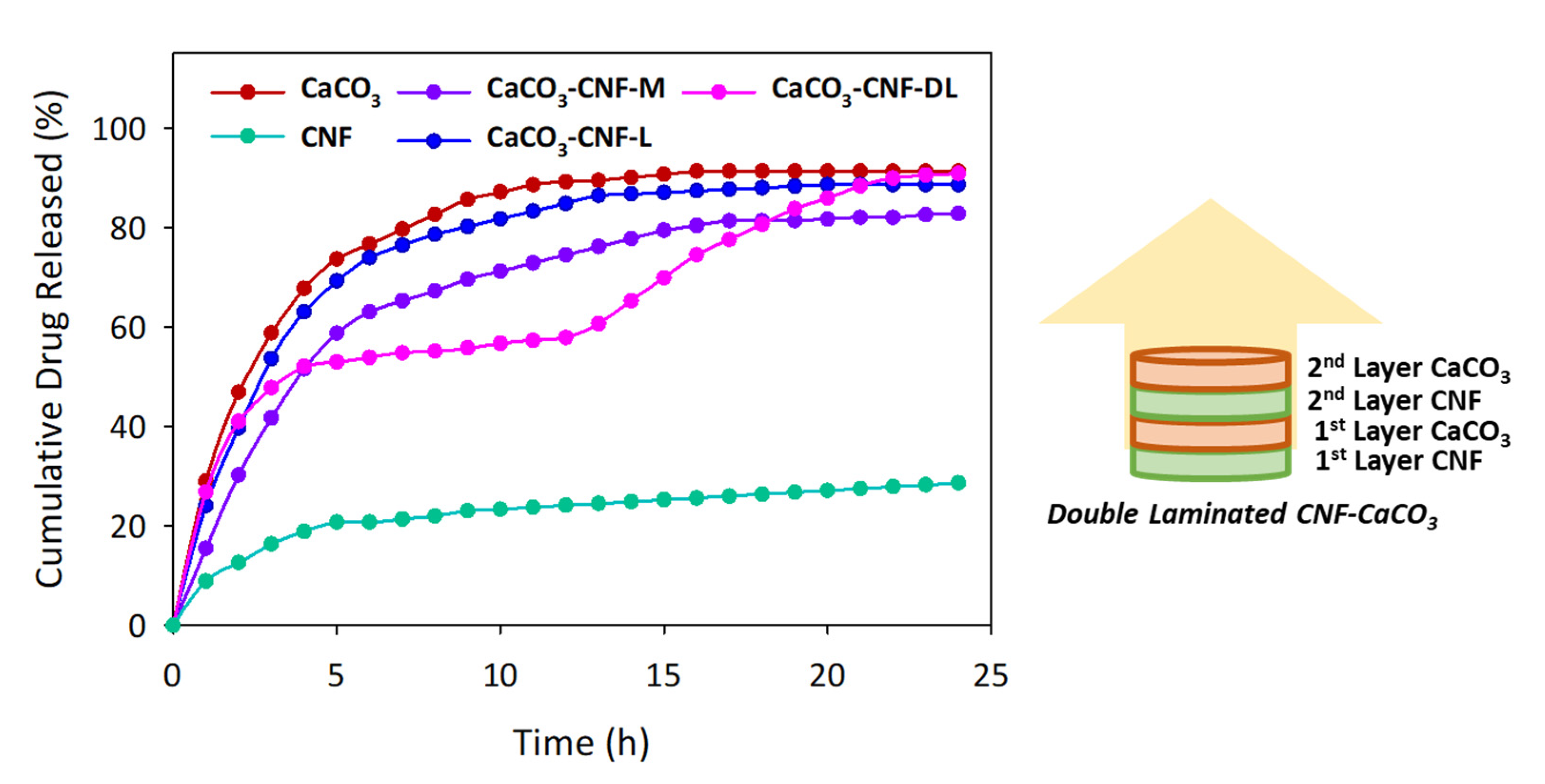
3.4. Controlled-Release 5-FU
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Holban, A.M.; Grumezescu, A.M. Nanoarchitectonics for Smart Delivery and Drug Targeting, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-323-47347-7. [Google Scholar]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target Ther. 2018, 3, 1–18. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.J.; Chen, W.S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Ortega-García, M.B.; Mesa, A.; Moya, E.L.J.; Rueda, B.; Lopez-Ordoño, G.; García, J.Á.; Conde, V.; Redondo-Cerezo, E.; Lopez-Hidalgo, J.L.; Jiménez, G.; et al. Uncovering tumour heterogeneity through PKR and nc886 analysis in metastatic colon cancer patients treated with 5-FU-based chemotherapy. Cancers 2020, 12, 379. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Tang, J.N.; Xie, H.X.; Du, Y.A.; Huang, L.; Yu, P.F.; Cheng, X.D. 5-Fluorouracil Chemotherapy of Gastric Cancer Generates Residual Cells With Properties of Cancer Stem Cells. Int. J. Biol. Sci. 2015, 11, 284–294. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Fischer, F.; Baerenfaller, K.; Jiricny, J. 5-fluorouracil is efficiently removed from DNA by the base excision and mismatch repair systems. Gastroenterology 2007, 133, 1858–1868. [Google Scholar] [CrossRef]
- Chandran, S.P.; Natarajan, S.B.; Chandraseharan, S.; Suhaini, M.; Mohd, B. Nano drug delivery strategy of 5-fluorouracil for the treatment of colorectal cancer. J. Cancer Res. Pract. 2017, 4, 45–48. [Google Scholar] [CrossRef]
- Wigmore, P.M.; Mustafa, S.; El-Beltagy, M.; Lyons, L.; Umka, J.; Bennett, G. Effects of 5-FU. Adv. Exp. Med. Biol. 2010, 678, 157–164. [Google Scholar] [PubMed]
- Thomas, S.A.; Grami, Z.; Mehta, S.; Patel, K.; North, W.; Hospital, F. Adverse Effects of 5-fluorouracil: Focus on Rare Side Effects. Cancer Cell Microenviron. 2016, 3, 1266. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.L.; Oporto-Velásquez, G.S.; Comolli, N. Evaluation of Acetaminophen Release from Biodegradable Poly (Vinyl Alcohol) (PVA) and Nanocellulose Films Using a Multiphase Release Mechanism. Nanomaterials 2020, 10, 301. [Google Scholar] [CrossRef]
- Xu, C.; Molino, B.Z.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075. [Google Scholar] [CrossRef]
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D printed of nano-cellulosic biomaterials for medical applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34. [Google Scholar] [CrossRef]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytönen, V.P. 3D-Printable Bioactivated Nanocellulose-Alginate Hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef]
- Mariani, L.M.; Turner, K.T.; Iii, W.R.J.; Considine, J.M. Printing and mechanical characterization of cellulose nanofibril materials. Cellulose 2019, 26, 2639–2651. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef]
- Viidik, L.; Seera, D.; Antikainen, O.; Kogermann, K.; Heinämäki, J.; Laidmäe, I. 3D-printability of aqueous poly(ethylene oxide) gels. Eur. Polym. J. 2019, 120, 109206. [Google Scholar] [CrossRef]
- Borujeni, S.H.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27, 1573–1589. [Google Scholar] [CrossRef]
- Auvinen, V.; Virtanen, J.; Merivaara, A.; Virtanen, V.; Laurén, P.; Tuukkanen, S.; Laaksonen, T. Modulating sustained drug release from nanocellulose hydrogel by adjusting the inner geometry of implantable capsules. J. Drug Deliv. Sci. Technol. 2020, 57, 101625. [Google Scholar] [CrossRef]
- Islam, K.; Zuki, B.; Mustapha, M.; Mohd, Z.; Norshazlirah, S.; Eaqub, A. Characterisation of calcium carbonate and its polymorphs from cockle Shells. Powder Technol. 2011, 213, 188–191. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Sharifi, S.; Ahmadian, E.; Eftekhari, A.; Adibkia, K.; Lotfipour, F. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin. Drug Deliv. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Pachuau, L. Application of Nanocellulose for Controlled Drug Delivery; Wiley-VCH: Weinheim, Germany, 2017; ISBN 978-3-527-80383-5. [Google Scholar]
- Sajab, M.S.; Mohan, D.; Santanaraj, J.; Chia, C.H.; Kaco, H.; Harun, S.; Kamarudin, N.H.N. Telescopic synthesis of cellulose nanofibrils with a stable dispersion of Fe(0) nanoparticles for synergistic removal of 5-fluorouracil. Sci. Rep. 2019, 9, 11703. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sajab, M.S.; Kaco, H.; Bakarudin, S.B.; Mohamed Noor, A. 3D Printing of UV-Curable Polyurethane Incorporated with Surface-Grafted Nanocellulose. Nanomaterials 2019, 9, 1726. [Google Scholar] [CrossRef]
- Santanaraj, J.; Sajab, M.S.; Mohammad, A.W.; Harun, S.; Chia, C.H.; Zakari, S.; Kaco, H. Enhanced delignification of oil palm empty fruit bunch fibers with in situ fenton-oxidation. BioResources 2017, 12, 5223–5235. [Google Scholar] [CrossRef]
- Qurratu, W.N.; Wan Manan, A.; Santanaraja, J.; Sajab, M.S.; Wan Isahak, W.N.R.; Hua Chia, C. Discoloration of Batik Effluent by Chemically Modified Oil Palm Empty Fruit Bunch Fibers. J. Kejuruter. 2018, 1, 87–92. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Wang, W.; Zhang, J.; Wang, Y.; Yu, S.-H. Controlled crystallization of hierarchical and porous calcium carbonate crystals using polypeptide type block copolymer as crystal growth modifier in a mixed solution. CrystEngComm 2011, 13, 2054–2061. [Google Scholar] [CrossRef]
- Salomão, R.; Costa, L.M.M.; Olyveira, G.M. Precipitated calcium carbonate nano-microparticles: Applications in drug delivery. Adv. Tissue Eng. Regen. Med. 2017, 3, 00059. [Google Scholar] [CrossRef]
- Palamaea, S.; Dechatiwongse, P.; Choorita, W.; Chistid, Y.; Prasertsan, P. Cellulose and hemicellulose recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carbohydr. Polym. 2017, 155, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.H.; Vinoba, M.; Bhagiyalakshmi, M.; Baek, I.H.; Nam, S.C.; Yoon, Y.; Kim, S.H.; Jeong, S.K. CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system. RSC Adv. 2013, 3, 21722–21729. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, J.; Teng, H.; Becker, U. Growth process and crystallographic properties of ammonia-induced vaterite. Am. Mineral 2012, 97, 1437–1445. [Google Scholar] [CrossRef]
- Donnelly, F.C.; Purcell-Milton, F.; Framont, V.; Cleary, O.; Dunne, P.W.; Gun’ko, Y.K. Synthesis of CaCO3 nano- and micro-particles by dry ice carbonation. Chem. Commun. 2017, 53, 6657–6660. [Google Scholar] [CrossRef]
- Azali, N.S.; Kamarudin, N.H.N.; Rahim, A.R.A.; Nasir, N.S.A.J.; Timmiati, S.N.; Jaafar, N.F. Adsorption and Release of 5-Fluorouracil (5FU) from Mesoporous Silica Nanoparticles. Mater. Today Proc. 2019, 19, 1722–1729. [Google Scholar] [CrossRef]
- Sulekova, M.; Vahovska, L.; Hudak, A.; Zid, L.; Zelenak, V. A Study of 5-fluorouracil desorption from mesoporous silica by RP-UHPLC. Molecules 2019, 24, 1317. [Google Scholar] [CrossRef]
- Chong, K.Y.; Chia, C.H.; Zakaria, S.; Sajab, M.S. Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions. J. Environ. Chem. Eng. 2014, 2, 2156–2161. [Google Scholar] [CrossRef]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of nanocellulose and its applications in drug delivery: A critical review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Quennouz, N.; Hashmi, S.M.; Choi, H.S.; Kim, J.W.; Osuji, C.O. Rheology of cellulose nanofibrils in the presence of surfactants. Soft Matter. 2016, 12, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Okuzono, T.; Kobayashi, M.; Doi, M. Final shape of a drying thin film. Phys. Rev. E 2009, 80, 021603. [Google Scholar] [CrossRef] [PubMed]
- Dalei, G.; Swain, S.; Das, S.; Das, S.P. Controlled Release of 5-Fluorouracil from Alginate Hydrogels by Cold HMDSO-Plasma Surface Engineering. ChemistrySelect 2020, 5, 2168–2178. [Google Scholar] [CrossRef]
- Ibrahim, R.A.A.; Shareef, F.; Suhail, A.; Al-hakeim, H.K. Stability of Anticancer Drug 5-Fluorouracil in Aqueous Solution: An Assessment of Kinetic Behavior. Nano Biomed. Eng. 2018, 10, 224–234. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Jiang, H.B.; Ryu, J.H.; Kang, H.; Kim, K.M.; Kwon, J.S. Comparing properties of variable pore-sized 3D-printed PLA membrane with conventional PLA membrane for guided bone/tissue regeneration. Materials 2019, 12, 1718. [Google Scholar] [CrossRef]
- Priya, P.; Raja, A.; Raj, V. Interpenetrating polymeric networks of chitosan and egg white with dual crosslinking agents polyethylene glycol/polyvinylpyrrolidone as a novel drug carrier. Cellulose 2016, 23, 699–712. [Google Scholar] [CrossRef]
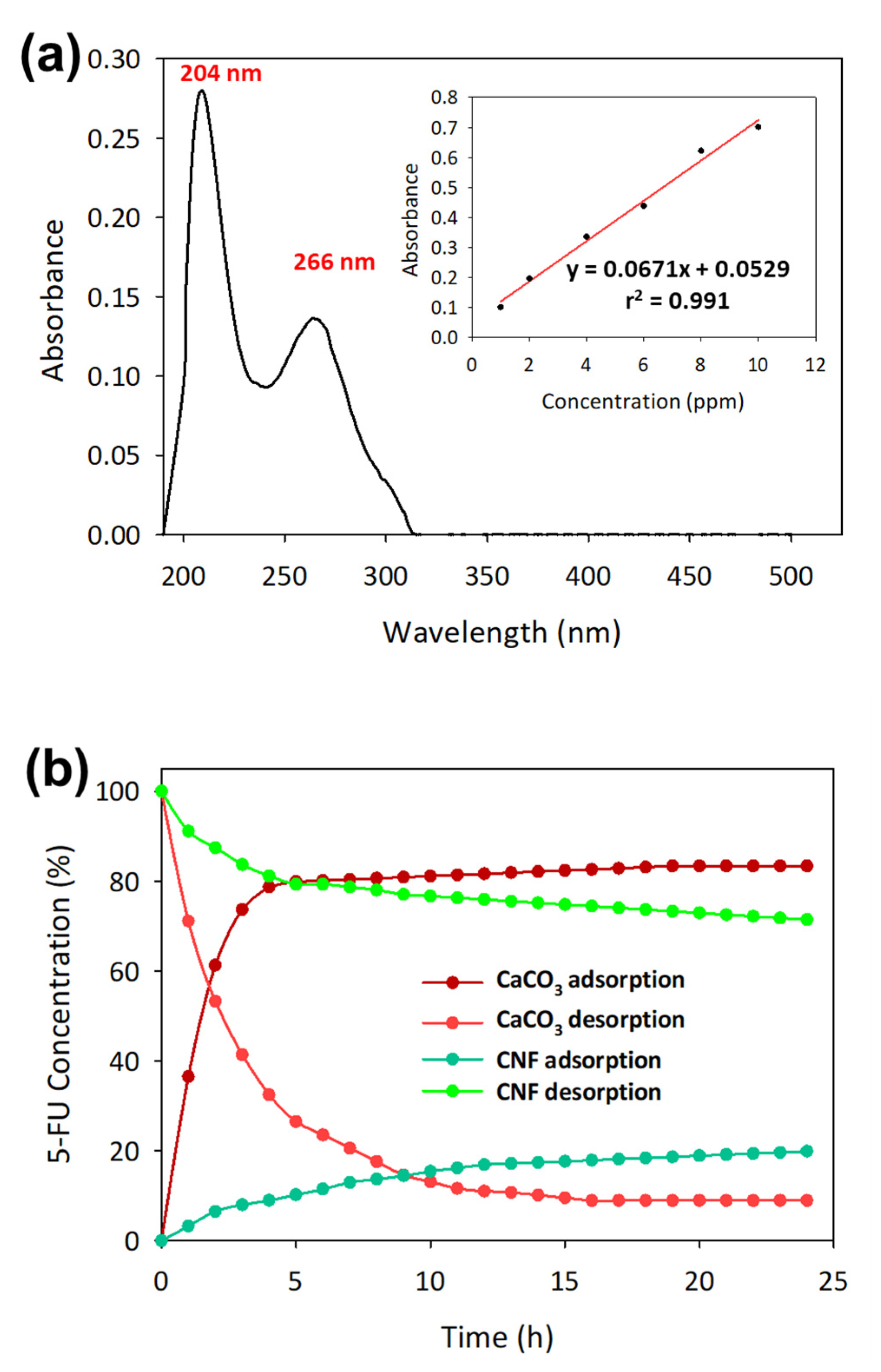

| Samples | C0 (ppm) | Ce (ppm) | qe (mg/g) | Uptake (%) | Release (%) |
|---|---|---|---|---|---|
| CaCO3 | 1 | 0.21 | 0.24 | 78.57 ± 0.34 | 80.52 ± 0.35 |
| 2 | 0.42 | 0.47 | 79.10 ± 0.34 | 84.91 ± 0.18 | |
| 4 | 0.71 | 0.99 | 82.16 ± 0.41 | 87.77 ± 0.47 | |
| 6 | 1.01 | 1.50 | 83.19 ± 0.35 | 91.20 ± 0.24 | |
| 8 | 1.49 | 1.95 | 81.35 ± 0.41 | 86.92 ± 0.46 | |
| 10 | 1.90 | 2.43 | 80.96 ± 0.26 | 86.51 ± 0.42 | |
| CNF | 1 | 0.84 | 0.05 | 15.12 ± 0.21 | 24.91 ± 0.18 |
| 2 | 1.67 | 0.09 | 16.71 ± 0.19 | 26.12 ± 0.22 | |
| 4 | 3.25 | 0.23 | 18.12 ± 0.20 | 27.54 ± 0.23 | |
| 6 | 4.83 | 0.35 | 19.86 ± 0.26 | 28.59 ± 0.21 | |
| 8 | 6.30 | 0.51 | 21.37 ± 0.24 | 29.12 ± 0.27 | |
| 10 | 7.67 | 0.69 | 23.11 ± 0.17 | 30.86 ± 0.32 | |
| CaCO3–CNF | 1 | 0.26 | 0.22 | 73.94 ± 0.37 | 77.42 ± 0.26 |
| 2 | 0.47 | 0.46 | 76.45 ± 0.38 | 80.12 ± 0.34 | |
| 4 | 0.78 | 0.97 | 80.43 ± 0.36 | 83.38 ± 0.28 | |
| 6 | 1.09 | 1.47 | 81.83 ± 0.34 | 86.45 ± 0.37 | |
| 8 | 1.58 | 1.93 | 80.25 ± 0.40 | 82.67 ± 0.36 | |
| 10 | 1.99 | 2.40 | 80.12 ± 0.33 | 82.47 ± 0.35 |
| Time (h) | 5-FU Release (%) | ||||
|---|---|---|---|---|---|
| CaCO3 | CNF | CaCO3–CNF–M | CaCO3–CNF–L | CaCO3–CNF–DL | |
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 1 | 28.94 ± 0.09 a | 8.86 ± 0.11 e | 15.44 ± 0.07 d | 24.04 ± 0.13 c | 26.80 ± 0.11 b |
| 2 | 46.83 ± 0.12 a | 12.61 ± 0.15 e | 30.20 ± 0.13 d | 39.62 ± 0.17 c | 40.96 ± 0.14 b |
| 3 | 58.75 ± 0.24 a | 16.35 ± 0.17 e | 41.68 ± 0.23 d | 53.65 ± 0.27 b | 47.73 ± 0.16 c |
| 4 | 67.70 ± 0.20 a | 18.85 ± 0.18 d | 51.51 ± 0.27 c | 63.00 ± 0.37 b | 52.04 ± 0.21 c |
| 5 | 73.66 ± 0.18 a | 20.72 ± 0.12 e | 58.73 ± 0.28 c | 69.24 ± 0.38 b | 52.96 ± 0.24 d |
| 6 | 76.64 ± 0.21 a | 20.72 ± 0.16 e | 62.99 ± 0.32 c | 73.92 ± 0.27 b | 53.88 ± 0.21 d |
| 7 | 79.62 ± 0.25 a | 21.35 ± 0.14 e | 65.28 ± 0.22 c | 76.41 ± 0.31 b | 54.80 ± 0.26 d |
| 8 | 82.60 ± 0.28 a | 21.97 ± 0.13 e | 67.25 ± 0.26 c | 78.59 ± 0.20 b | 55.11 ± 0.30 d |
| 9 | 85.58 ± 0.18 a | 22.97 ± 0.12 e | 69.55 ± 0.21 c | 80.15 ± 0.23 b | 55.73 ± 0.31 d |
| 10 | 87.07 ± 0.27 a | 23.35 ± 0.11 e | 71.19 ± 0.20 c | 81.71 ± 0.21 b | 56.65 ± 0.34 d |
| 11 | 88.56 ± 0.28 a | 23.72 ± 0.15 e | 72.83 ± 0.21 c | 83.27 ± 0.29 b | 57.27 ± 0.24 d |
| 12 | 89.16 ± 0.18 a | 24.09 ± 0.16 e | 74.47 ± 0.22 c | 84.83 ± 0.30 b | 57.88 ± 0.26 d |
| 13 | 89.46 ± 0.29 a | 24.47 ± 0.21 e | 76.11 ± 0.23 c | 86.39 ± 0.34 b | 60.65 ± 0.27 d |
| 14 | 90.05 ± 0.31 a | 24.84 ± 0.24 e | 77.75 ± 0.19 c | 86.70 ± 0.31 b | 65.27 ± 0.21 d |
| 15 | 90.65 ± 0.16 a | 25.22 ± 0.35 e | 79.38 ± 0.32 c | 87.01 ± 0.38 b | 69.88 ± 0.28 d |
| 16 | 91.19 ± 0.17 a | 25.59 ± 0.26 e | 80.37 ± 0.42 c | 87.32 ± 0.40 b | 74.50 ± 0.21 d |
| 17 | 91.21 ± 0.25 a | 25.97 ± 0.21 e | 81.35 ± 0.41 c | 87.63 ± 0.33 b | 77.58 ± 0.31 d |
| 18 | 91.21 ± 0.19 a | 26.09 ± 0.19 e | 81.37 ± 0.24 c | 87.75 ± 0.21 b | 78.45 ± 0.24 d |
| 19 | 91.22 ± 0.17 a | 26.21 ± 0.26 e | 81.39 ± 0.18 c | 87.83 ± 0.24 b | 79.11 ± 0.21 d |
| 20 | 91.22 ± 0.21 a | 26.34 ± 0.24 d | 81.39 ± 0.27 c | 87.94 ± 0.35 b | 80.65 ± 0.40 c |
| 21 | 91.26 ± 0.24 a | 26.72 ± 0.26 e | 81.46 ± 0.21 d | 88.26 ± 0.39 b | 83.73 ± 0.41 c |
| 22 | 91.33 ± 0.19 a | 27.09 ± 0.27 e | 81.68 ± 0.21 d | 88.57 ± 0.38 b | 85.88 ± 0.34 c |
| 23 | 91.35 ± 0.23 a | 27.47 ± 0.28 d | 82.01 ± 0.24 c | 88.61 ± 0.31 b | 88.35 ± 0.36 b |
| 24 | 91.37 ± 0.16 a | 27.84 ± 0.26 e | 82.06 ± 0.21 d | 88.63 ± 0.35 c | 89.88 ± 0.37 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, D.; Khairullah, N.F.; How, Y.P.; Sajab, M.S.; Kaco, H. 3D Printed Laminated CaCO3-Nanocellulose Films as Controlled-Release 5-Fluorouracil. Polymers 2020, 12, 986. https://doi.org/10.3390/polym12040986
Mohan D, Khairullah NF, How YP, Sajab MS, Kaco H. 3D Printed Laminated CaCO3-Nanocellulose Films as Controlled-Release 5-Fluorouracil. Polymers. 2020; 12(4):986. https://doi.org/10.3390/polym12040986
Chicago/Turabian StyleMohan, Denesh, Nur Fatin Khairullah, Yan Ping How, Mohd Shaiful Sajab, and Hatika Kaco. 2020. "3D Printed Laminated CaCO3-Nanocellulose Films as Controlled-Release 5-Fluorouracil" Polymers 12, no. 4: 986. https://doi.org/10.3390/polym12040986
APA StyleMohan, D., Khairullah, N. F., How, Y. P., Sajab, M. S., & Kaco, H. (2020). 3D Printed Laminated CaCO3-Nanocellulose Films as Controlled-Release 5-Fluorouracil. Polymers, 12(4), 986. https://doi.org/10.3390/polym12040986