Performance of a Biodegradable Composite with Hydroxyapatite as a Scaffold in Pulp Tissue Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical statement
2.2. Cell Culture
2.3. Material Preparation and Releasing Assay
2.4. Lactate Dehydrogenase (LDH) Cell Cytotoxicity Assay
2.5. Proliferation Assay and Cell Morphology
2.6. Microstructure Analysis Using Scanning Electron Microscopy (SEM)
2.7. Pulp Capping Procedure
2.8. Three-Dimensional Micro-Computed Tomographic (Micro-CT) Analyses
2.9. Histological Evaluation
2.10. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
2.11. Statistical Analysis
3. Results
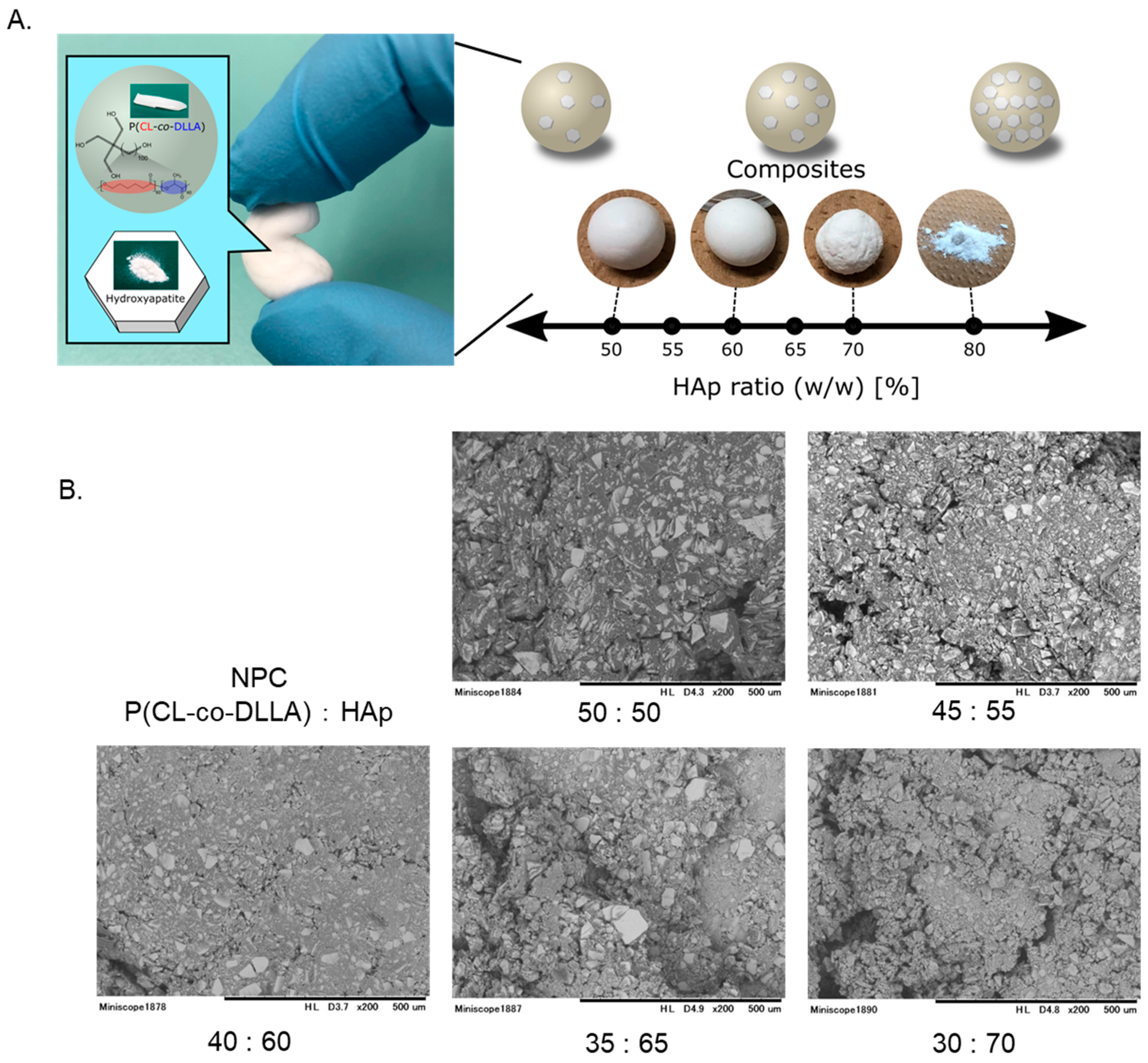
3.1. General Properties of Polymer Materials
3.2. Microstructural Analysis Using SEM
3.3. LDH Cytotoxicity and Proliferation Assay
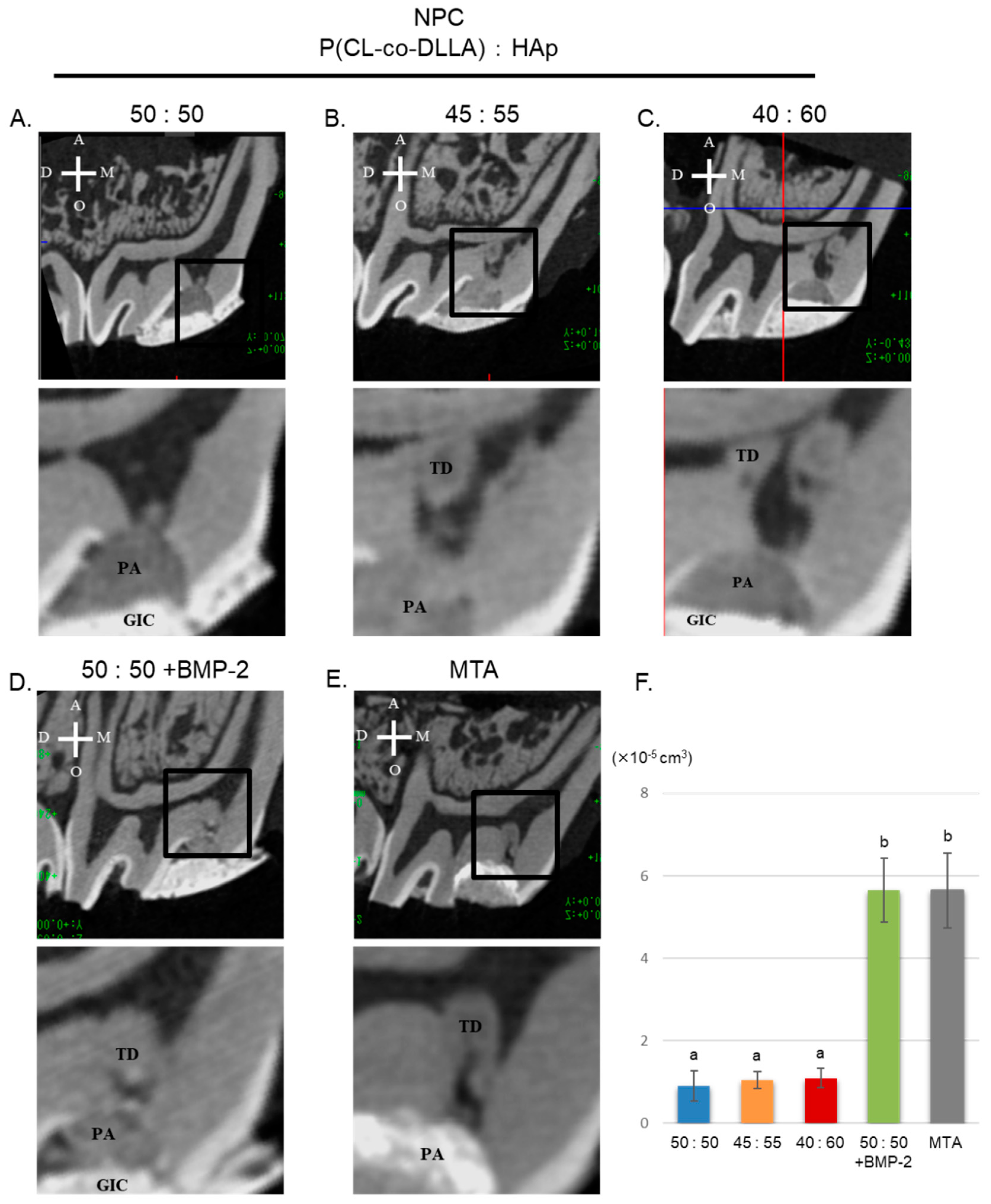
3.4. Three-Dimensional Micro-CT Analysis of Tertiary Dentin Formation
3.5. Histological Evaluation of Tertiary Dentin Formation
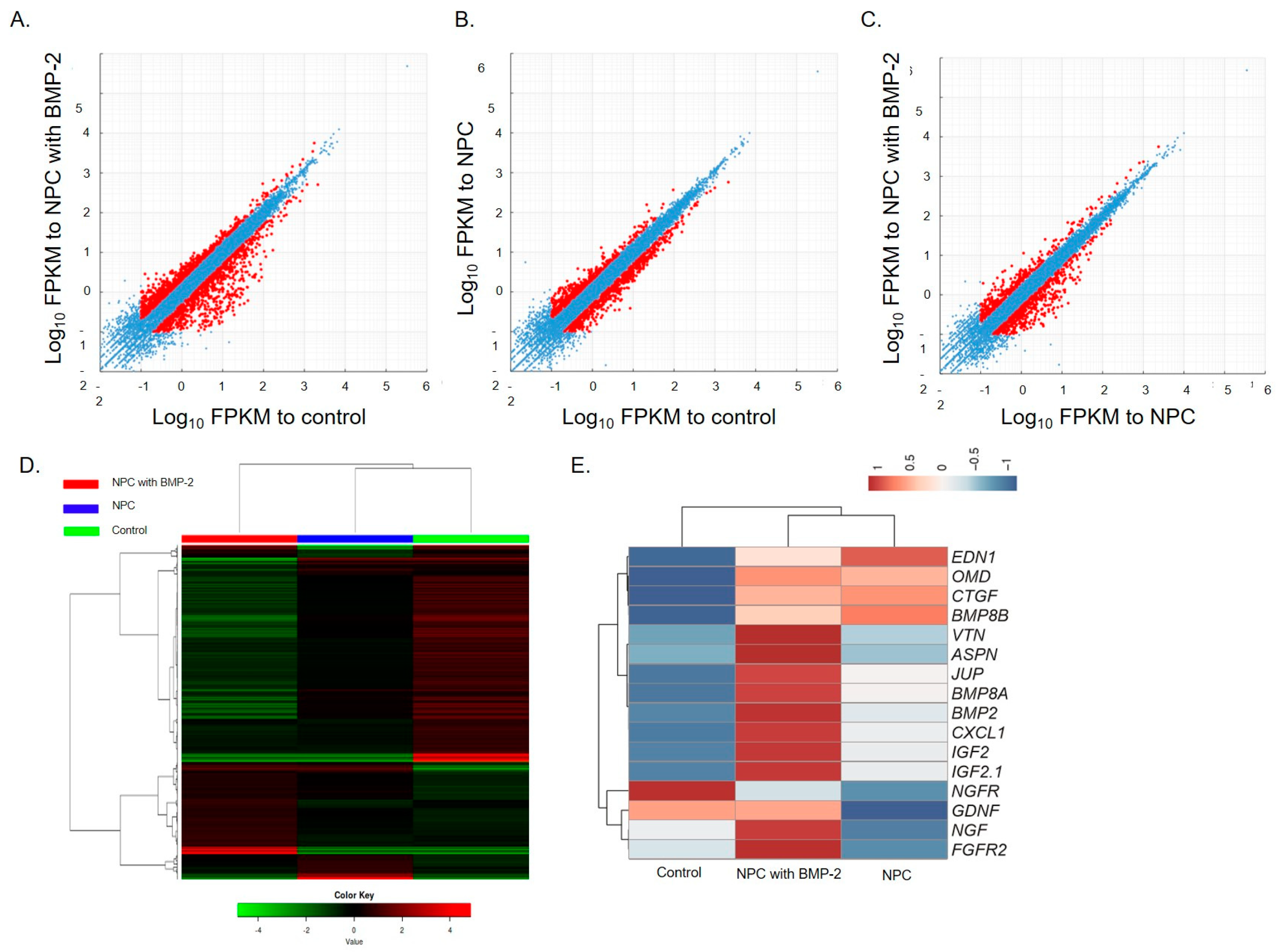
3.6. RNA Sequence Analysis
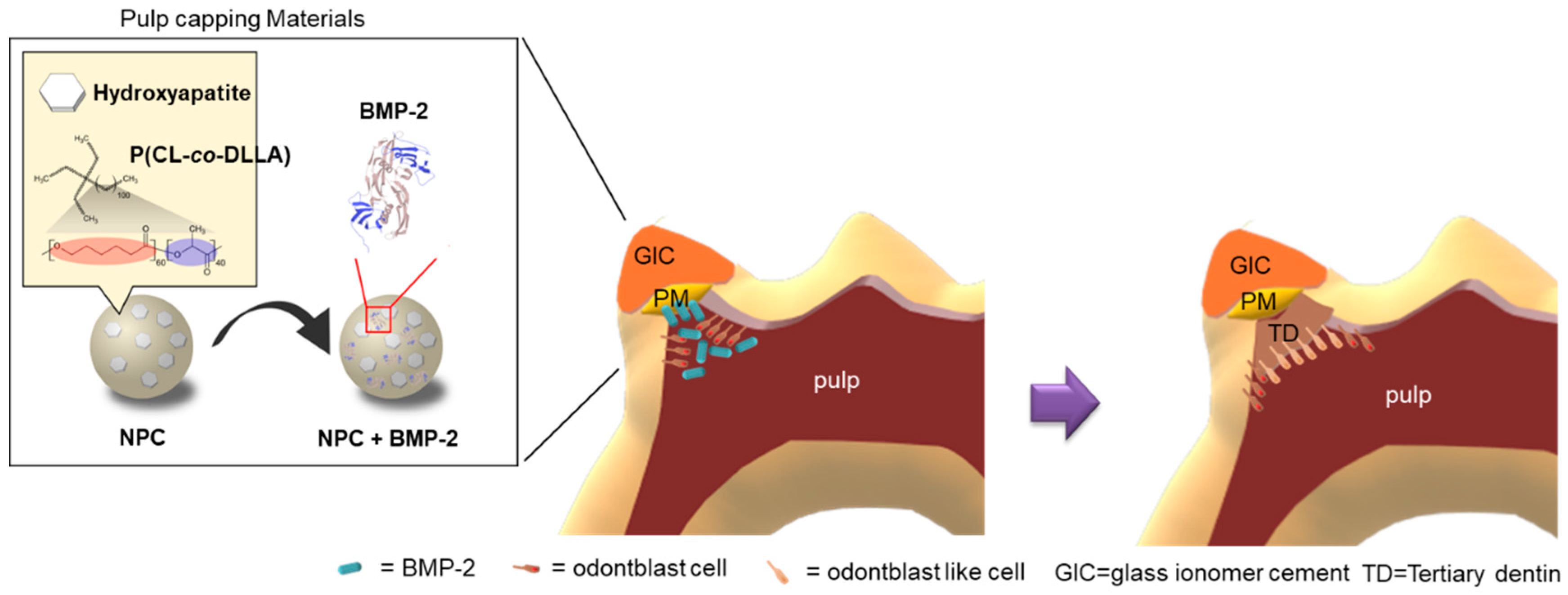
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, D.; Lynd, T.; Ho, D.; Chen, J.; Vines, J.; Jung, H.D.; Kim, J.H.; Zhang, P.; Wu, H.; Jun, H.W.; et al. Pulp-Dentin Tissue Healing Response: A Discussion of Current Biomedical Approaches. J. Clin. Med. 2020, 9, 434. [Google Scholar] [CrossRef]
- European Society of Endodontology (ESE) developed by; Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef]
- Elmsmari, F.; Ruiz, X.F.; Miró, Q.; Feijoo-Pato, N.; Durán-Sindreu, F.; Olivieri, J.G. Outcome of Partial Pulpotomy in Cariously Exposed Posterior Permanent Teeth: A Systematic Review and Meta-analysis. J. Endod. 2019, 45, 1296–1306. [Google Scholar] [CrossRef]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Nakashima, M. Induction of dentin formation on canine amputated pulp by recombinant human bone morphogenetic proteins (BMP)-2 and -4. J. Dent. Res. 1994, 73, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.K.; Gwon, G.J.; Neupane, S.; Sohn, W.J.; Kim, K.R.; Kim, J.Y.; An, S.Y.; Kwon, T.Y.; An, C.H.; Lee, Y.; et al. Bortezomib Facilitates Reparative Dentin Formation after Pulp Access Cavity Preparation in Mouse Molar. J. Endod. 2017, 43, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Luiz de Oliveira da Rosa, W.; Machado da Silva, T.; Fernando Demarco, F.; Piva, E.; Fernandes da Silva, A. Could the application of bioactive molecules improve vital pulp therapy success? A systematic review. J. Biomed. Mater. Res., A. 2017, 105, 941–956. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Q.; Han, Q.; Zhu, H.; Li, M.; Fang, Y.; Wang, X. Effects of Nel-like molecule-1 and bone morphogenetic protein 2 combination on rat pulp repair. J. Mol. Histol. 2019, 50, 253–261. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part I Chemical, physical, and antibacterial properties. J. Endod. 2010, 16–27. [Google Scholar] [CrossRef]
- Zaen El-Din, A.M.; Hamama, H.H.; Abo El-Elaa, M.A.; Grawish, M.E.; Mahmoud, S.H.; Neelakantan, P. The effect of four materials on direct pulp capping: An animal study. Aust. Endod. J. 2020, 4. [Google Scholar] [CrossRef] [PubMed]
- Karadzic, I.; Vucic, V.; Jokanovic, V.; Debeljak-Martacic, J.; Markovic, D.; Petrovic, S.; Glibetic, M. Effects of novel hydroxyapatite-based 3D biomaterials on proliferation and osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. A 2015, 103, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sancilio, S.; Gallorini, M.; Di Nisio, C.; Marsich, E.; Di Pietro, R.; Schweikl, H.; Cataldi, A. Alginate/Hydroxyapatite-Based Nanocomposite Scaffolds for Bone Tissue Engineering Improve Dental Pulp Biomineralization and Differentiation. Stem Cells Int. 2018, 2, 9643721. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, R.; Kanie, K.; Uto, K.; Kawai, S.; Hara, M.; Nagano, S.; Narita, Y.; Honda, H.; Naito, M.; Ebara, M.; et al. Combinational Effects of Polymer Viscoelasticity and Immobilized Peptides on Cell Adhesion to Cell-selective Scaffolds. Anal. Sci. 2016, 32, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 83, 590–595. [Google Scholar] [CrossRef]
- Nakashima, M. Induction of dentine in amputated pulp of dogs by recombinant human bone morphogenetic proteins-2 and -4 with collagen matrix. Arch. Oral Biol. 1994, 39, 1085–1899. [Google Scholar] [CrossRef]
- Uto, K.; Muroya, T.; Okamoto, M.; Tanaka, H.; Murase, T.; Ebara, M.; Aoyagi, T. Design of super-elastic biodegradable scaffolds with longitudinally oriented microchannels and optimization of the channel size for Schwann cell migration. Sci. Technol. Adv. Mater. 2012, 13, 064207. [Google Scholar] [CrossRef]
- Uto, K.; Mano, S.; Aoyagi, T.; Ebara, M. Substrate Fluidity Regulates Cell Adhesion and Morphology on Poly(ε-caprolactone)-Based Materials. ACS Biomater. Sci. Eng. 2016, 3, 446–453. [Google Scholar] [CrossRef]
- Okamoto, M.; Takahashi, Y.; Komichi, S.; Ali, M.; Yoneda, N.; Ishimoto, T.; Nakano, T.; Hayashi, M. Novel evaluation method of dentin repair by direct pulp capping using high-resolution micro-computed tomography. Clin. Oral Investig. 2018, 22, 2879–2887. [Google Scholar] [CrossRef]
- Okamoto, M.; Takahashi, Y.; Komichi, S.; Cooper, P.R.; Hayashi, M. Dentinogenic effects of extracted dentin matrix components digested with matrix metalloproteinases. Sci. Rep. 2018, 8, 10690. [Google Scholar] [CrossRef]
- Okamoto, M.; Takahashi, Y.; Komichi, S.; Ali, M.; Watanabe, M.; Hayashi, M. Effect of tissue inhibitor of metalloprotease 1 on human pulp cells in vitro and rat pulp tissue in vivo. Int. Endod. J. 2019, 52, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, L.; Hu, J.; Wang, L.; Li, N.; Wu, D.; Shi, X.; Yuan, M.; Hu, W.; Wang, X. Endothelial cells and endothelin-1 promote the odontogenic differentiation of dental pulp stem cells. Mol. Med. Rep. 2018, 18, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Farges, J.C.; Keller, J.F.; Carrouel, F.; Durand, S.H.; Romeas, A.; Bleicher, F.; Lebecque, S.; Staquet, M.J. Odontoblasts in the dental pulp immune response. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Muromachi, K.; Kamio, N.; Matsuki-Fukushima, M.; Nishimura, H.; Tani-Ishii, N.; Sugiya, H.; Matsushima, K. CCN2/CTGF expression via cellular uptake of BMP-1 is associated with reparative dentinogenesis. Oral Dis. 2015, 21, 778–784. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Brunetti, G.; Posa, F.; Ballini, A.; Grassi, F.R.; Colaianni, G.; Colucci, S.; Rossi, E.; Cavalcanti-Adam, E.A.; Lo Muzio, L.; et al. Osteogenic differentiation of mesenchymal stem cells from dental bud: Role of integrins and cadherins. Stem Cell Res. 2015, 15, 15,618–628. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, H.J.; Jeong, J.H.; Kim, Y.J.; Cha, D.W.; Kwon, D.K.; Lee, S.H.; Cho, J.Y. The role of asporin in mineralization of human dental pulp stem cells. J. Cell Physiol. 2011, 226, 1676–1682. [Google Scholar] [CrossRef]
- Komichi, S.; Takahashi, Y.; Okamoto, M.; Ali, M.; Watanabe, M.; Huang, H.; Nakai, T.; Cooper, P.; Hayashi, M. Protein S100-A7 Derived from Digested Dentin Is a Critical Molecule for Dentin Pulp Regeneration. Cells 2019, 8, 1002. [Google Scholar] [CrossRef]
- Yan, L.; Sun, S.; Qu, L. Insulin-like growth factor-1 promotes the proliferation and odontoblastic differentiation of human dental pulp cells under high glucose conditions. Int. J. Mol. Med. 2017, 40, 1253–1260. [Google Scholar] [CrossRef]
- Lin, W.; Gao, L.; Jiang, W.; Niu, C.; Yuan, K.; Hu, X.; Ma, R.; Huang, Z. The role of osteomodulin on osteo/odontogenic differentiation in human dental pulp stem cells. BMC Oral Health. 2019, 19, 22. [Google Scholar] [CrossRef]
- Xiao, N.; Yu, W.Y.; Liu, D. Glial cell-derived neurotrophic factor promotes dental pulp stem cell migration. J. Tissue Eng. Regen. Med. 2018, 12, 705–714. [Google Scholar] [CrossRef]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef] [PubMed]
- Vaseenon, S.; Chattipakorn, N.; Chattipakorn, S.C. The possible role of basic fibroblast growth factor in dental pulp. Arch. Oral Biol. 2020, 109, 104574. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, W.L.O.; Piva, E.; da Silva, A.F. Disclosing the physiology of pulp tissue for vital pulp therapy. Int. Endod. J. 2018, 51, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Toyono, T.; Murakami, T.; Akamine, A. Transforming growth factor-beta superfamily members expressed in rat incisor pulp. Arch. Oral Biol. 1998, 43, 745–751. [Google Scholar] [CrossRef]
- Otsuki, Y.; Ii, M.; Moriwaki, K.; Okada, M.; Ueda, K.; Asahi, M. W9 peptide enhanced osteogenic differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2018, 495, 904–910. [Google Scholar] [CrossRef]
- Kanie, K.; Kurimoto, R.; Tian, J.; Ebisawa, K.; Narita, Y.; Honda, H.; Kato, R. Screening of Osteogenic-Enhancing Short Peptides from BMPs for Biomimetic Material Applications. Materials (Basel) 2016, 9, 730. [Google Scholar] [CrossRef]



| Grade | Tertiary Dentin Formation |
|---|---|
| 2 | Consecutive hard tissue deposition without cellular contents or tunnel-like defect |
| 1 | Inconsecutive hard tissue deposition with cellular contents or tunnel-like defect |
| 0 | No hard tissue deposition |
| Group Samples | Samples | Teritiary Dentin | |||
|---|---|---|---|---|---|
| 4 weeks | 0 | 1 | 2 | ||
| NPC (50:50) | a | 8 | 5 | 3 | 0 |
| NPC (55:45) | a | 8 | 4 | 4 | 0 |
| NPC (60:40) | a | 8 | 4 | 4 | 0 |
| NPC (50:50) with BMP-2 | b | 8 | 0 | 1 | 7 |
| MTA | b | 8 | 0 | 1 | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, M.; Matsumoto, S.; Sugiyama, A.; Kanie, K.; Watanabe, M.; Huang, H.; Ali, M.; Ito, Y.; Miura, J.; Hirose, Y.; et al. Performance of a Biodegradable Composite with Hydroxyapatite as a Scaffold in Pulp Tissue Repair. Polymers 2020, 12, 937. https://doi.org/10.3390/polym12040937
Okamoto M, Matsumoto S, Sugiyama A, Kanie K, Watanabe M, Huang H, Ali M, Ito Y, Miura J, Hirose Y, et al. Performance of a Biodegradable Composite with Hydroxyapatite as a Scaffold in Pulp Tissue Repair. Polymers. 2020; 12(4):937. https://doi.org/10.3390/polym12040937
Chicago/Turabian StyleOkamoto, Motoki, Sayako Matsumoto, Ayato Sugiyama, Kei Kanie, Masakatsu Watanabe, Hailing Huang, Manahil Ali, Yuki Ito, Jiro Miura, Yujiro Hirose, and et al. 2020. "Performance of a Biodegradable Composite with Hydroxyapatite as a Scaffold in Pulp Tissue Repair" Polymers 12, no. 4: 937. https://doi.org/10.3390/polym12040937
APA StyleOkamoto, M., Matsumoto, S., Sugiyama, A., Kanie, K., Watanabe, M., Huang, H., Ali, M., Ito, Y., Miura, J., Hirose, Y., Uto, K., Ebara, M., Kato, R., Yamawaki-Ogata, A., Narita, Y., Kawabata, S., Takahashi, Y., & Hayashi, M. (2020). Performance of a Biodegradable Composite with Hydroxyapatite as a Scaffold in Pulp Tissue Repair. Polymers, 12(4), 937. https://doi.org/10.3390/polym12040937








