Melamine Foams Decorated with In-Situ Synthesized Gold and Palladium Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Functionalization of the ME Foams
2.3. Experimental Techniques
3. Results and Discussion
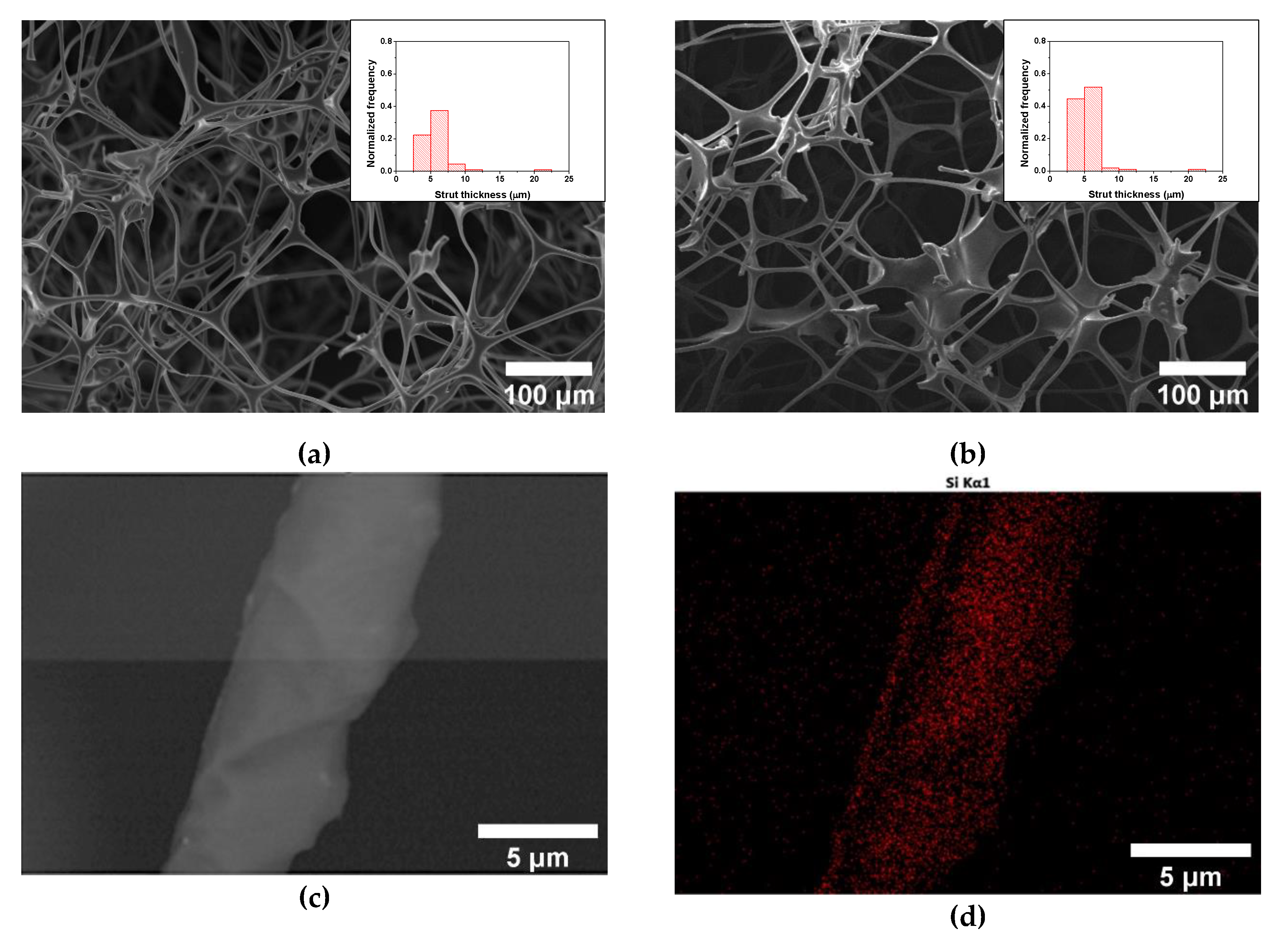
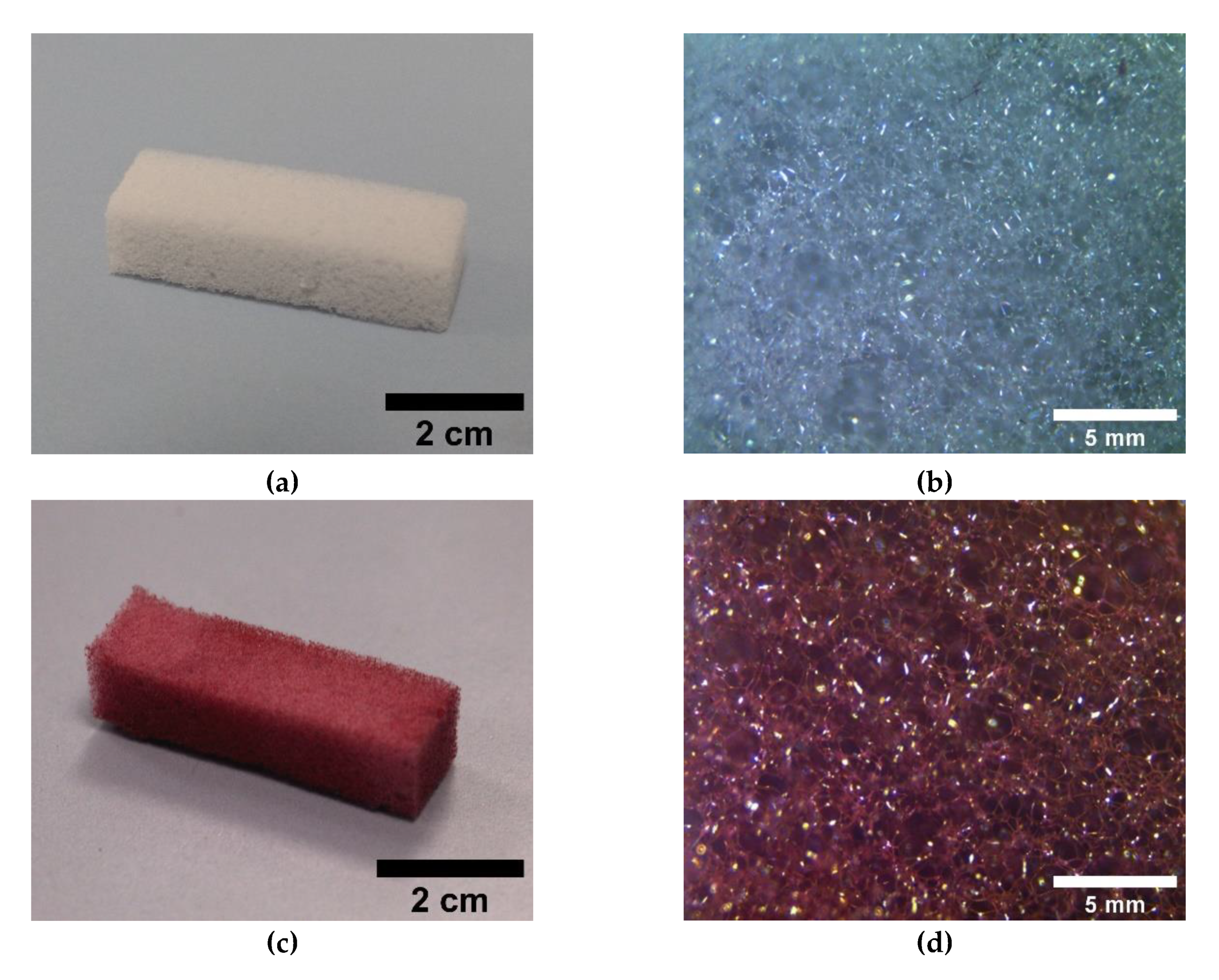
3.1. ME/PDMS Foams
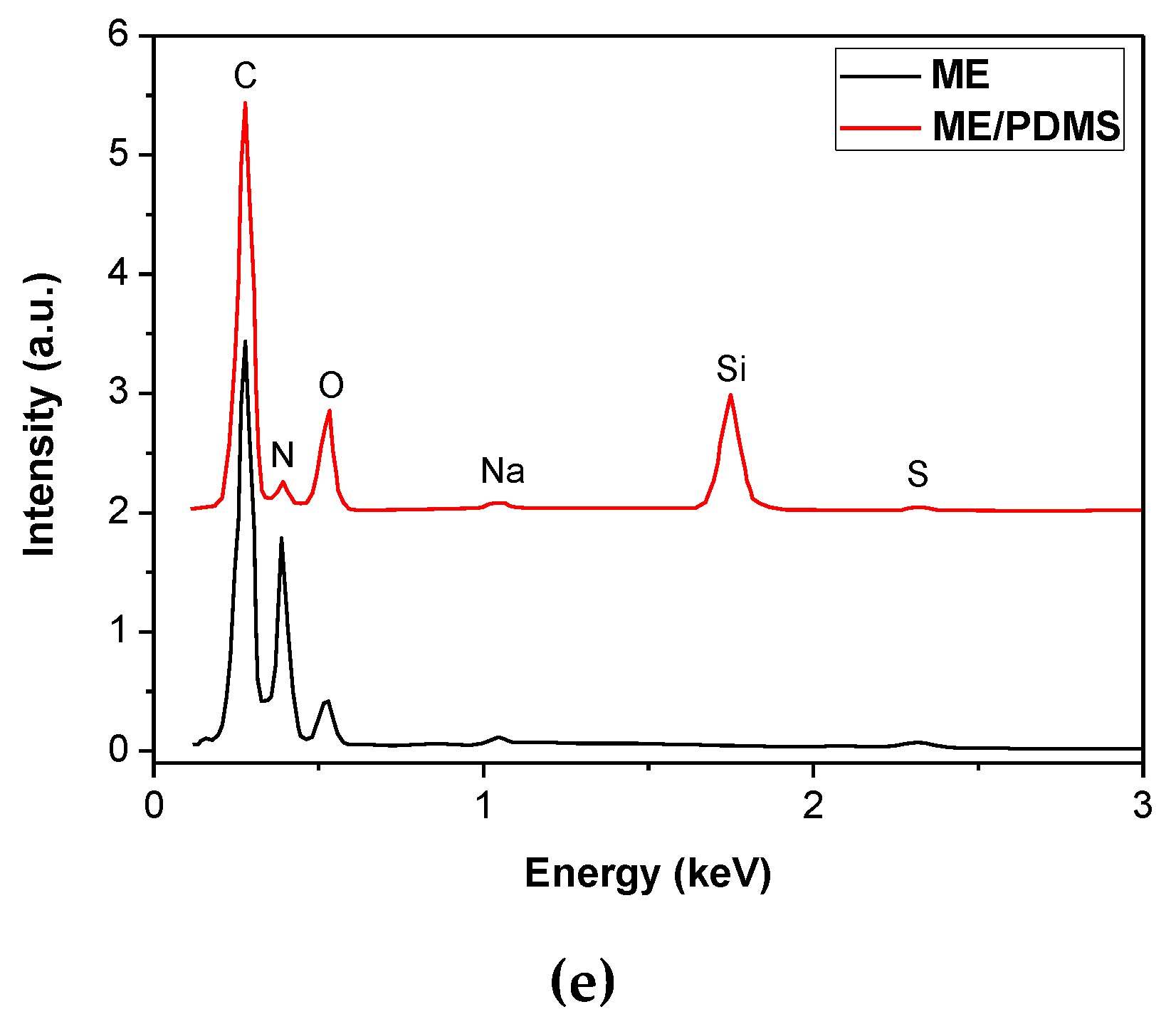
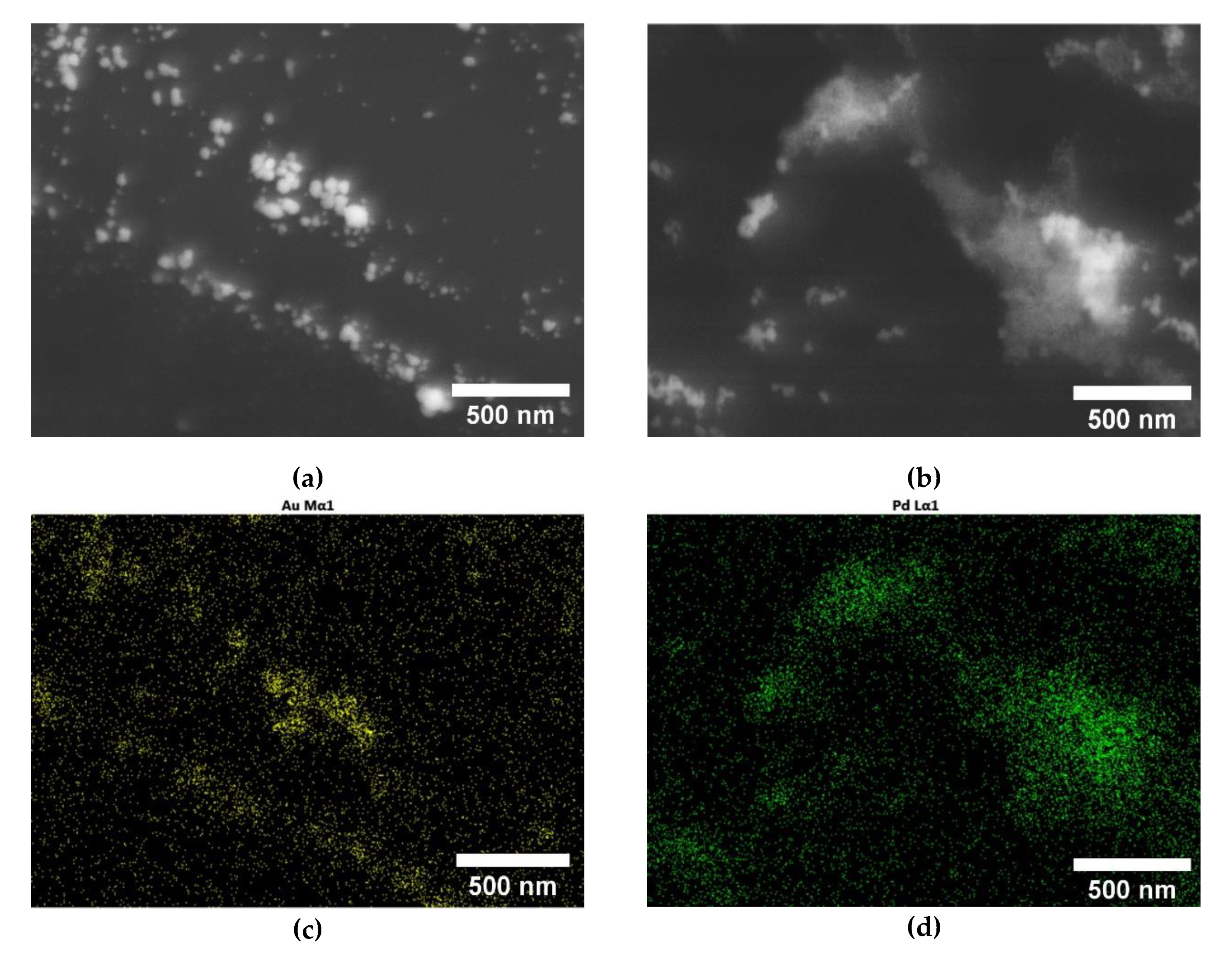
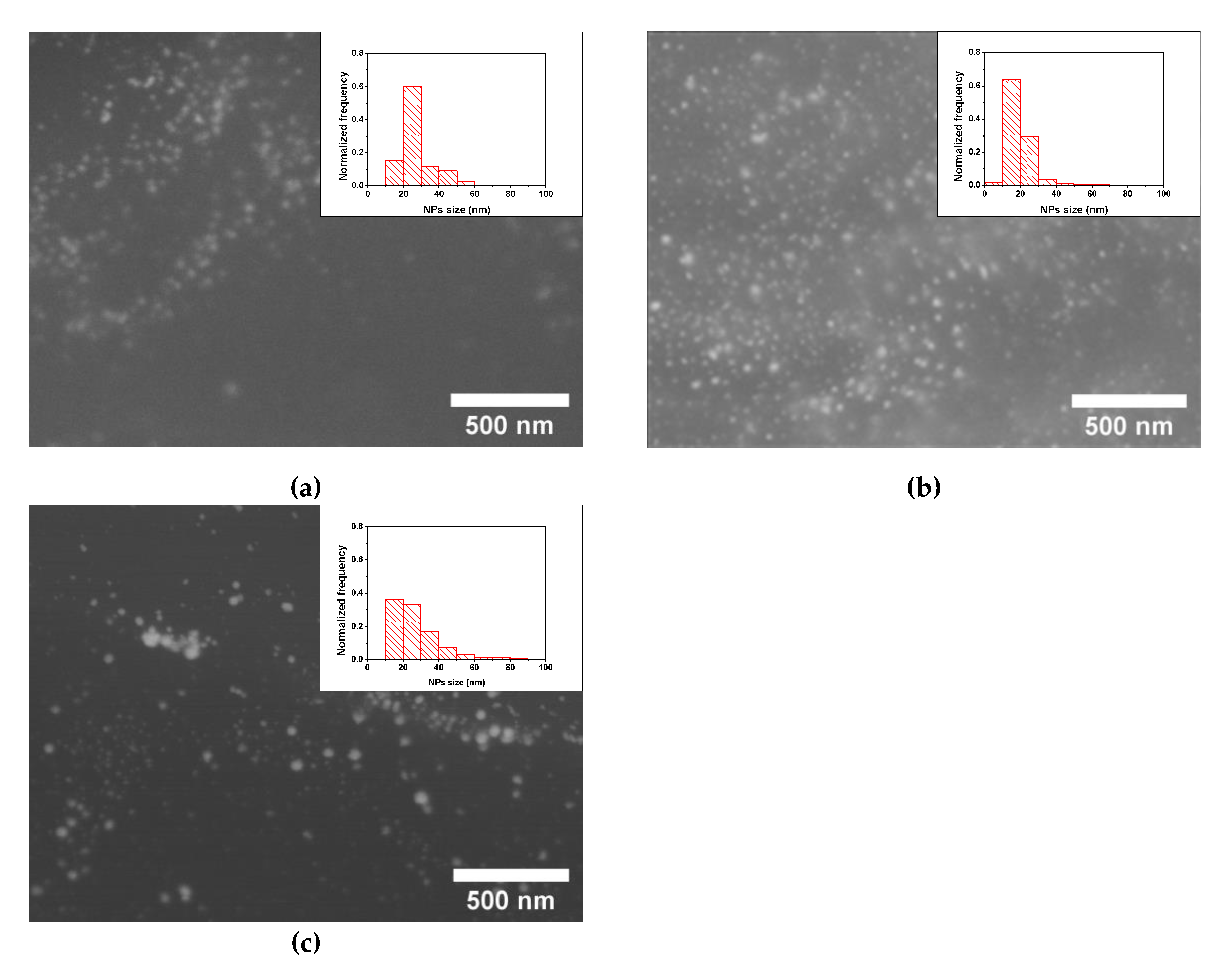
3.2. ME/PDMS/Au and ME/PDMS/Pd Foams
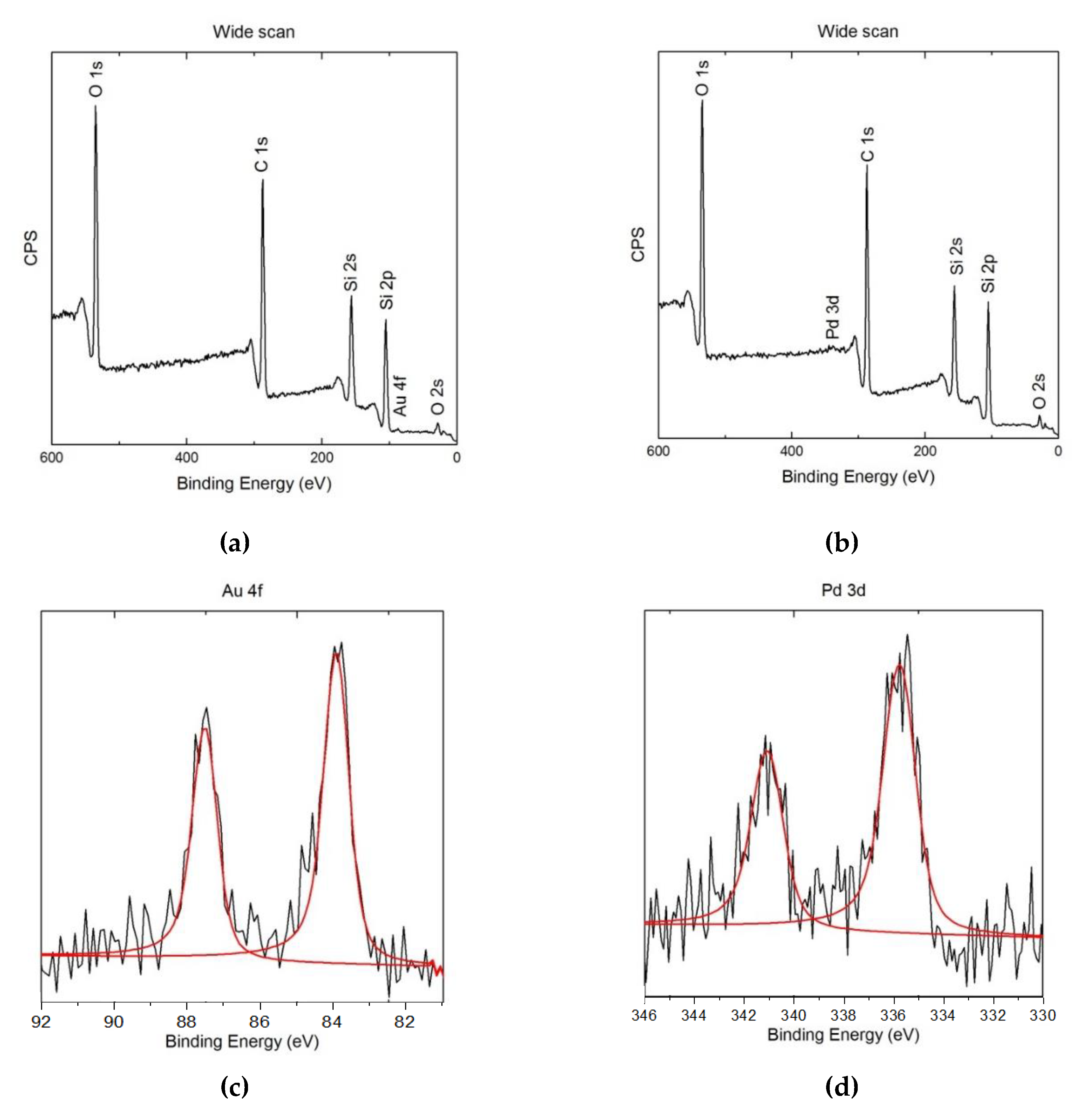
3.3. XPS Study of the ME/PDMS/Au and ME/PDMS/Pd Foams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, X.; Du, B.; Huang, Y.; Zheng, J. Ultrasmall noble metal nanoparticles: Breakthroughs and biomedical implications. Nano Today 2018, 21, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Chen, Z.; Wang, X.; Choo, J.; Chen, L. Plasmonic colorimetric sensors based on etching and growth of noble metal nanoparticles: Strategies and applications. Biosens. Bioelectron. 2018, 114, 52–65. [Google Scholar] [CrossRef]
- Vinci, G.; Rapa, M. Noble metal nanoparticles applications: Recent trends in food control. Bioengineering 2019, 6, 10. [Google Scholar] [CrossRef]
- Pinto, J.; Magrì, D.; Valentini, P.; Palazon, F.; Heredia-Guerrero, J.A.; Lauciello, S.; Barroso-Solares, S.; Ceseracciu, L.; Pompa, P.P.; Athanassiou, A.; et al. Antibacterial Melamine Foams Decorated with in Situ Synthesized Silver Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 16095–16104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, D.Y. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, J.; Shu, G.; Liu, Q.; Zeng, M. Heterogeneous catalytic composites from palladium nanoparticles in montmorillonite intercalated with poly (vinyl pyrrolidone) chains. Polymers 2018, 10, 669. [Google Scholar] [CrossRef]
- Padua, L.M.G.; Yeh, J.M.; Santiago, K.S. A novel application of electroactive polyimide doped with gold nanoparticles: As a chemiresistor sensor for hydrogen sulfide gas. Polymers 2019, 11, 1918. [Google Scholar] [CrossRef]
- Cobos, M.; De-la-pinta, I.; Quindos, G.; Fernandez, M.J.; Fernandez, M.D. Synthesis, Physical, Mechanical and Antibacterial Properties of Nanocomposites Based on Poly(vinyl alcohol)/Graphene Oxide–Silver Nanoparticles. Polymers 2020, 12, 723. [Google Scholar] [CrossRef]
- Scott, A.; Gupta, R.; Kulkarni, G.U. A Simple Water-Based Synthesis of Au Nanoparticle/PDMS Composites for Water Purification and Targeted Drug Release. Macromol. Chem. Phys. 2010, 211, 1640–1647. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef] [PubMed]
- Sreekumaran Nair, A.; Tom, R.T.; Pradeep, T. Detection and extraction of endosulfan by metal nanoparticles. J. Environ. Monit. 2003, 5, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, C.; Ji, Y.; Tian, Y.; Wei, H.; Li, C.; Li, Z.; Zhu, T.; Sun, Q.; Man, B.; et al. 3D Ultrasensitive Polymers-Plasmonic Hybrid Flexible Platform for In-Situ Detection. Polymers 2020, 12, 392. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Ma, L.; Zhang, L.; Zhao, W.; Li, Z.; Li, Q. A Green, Rapid and Efficient Dual-Sensors for Highly Selective and Sensitive Detection of Cation (Hg2+) and Anion (S2−) Ions Based on CMS/AgNPs Composites. Polymers 2020, 12, 113. [Google Scholar] [CrossRef]
- Zada, A.; Muhammad, P.; Ahmad, W.; Hussain, Z.; Ali, S.; Khan, M.; Khan, Q.; Maqbool, M. Surface Plasmonic-Assisted Photocatalysis and Optoelectronic Devices with Noble Metal Nanocrystals: Design, Synthesis, and Applications. Adv. Funct. Mater. 2020, 30, 1–29. [Google Scholar] [CrossRef]
- Pérez-Jiménez, L.E.; Solis-Cortazar, J.C.; Rojas-Blanco, L.; Perez-Hernandez, G.; Martinez, O.S.; Palomera, R.C.; Paraguay-Delgado, F.; Zamudio-Torres, I.; Morales, E.R. Enhancement of optoelectronic properties of TiO2 films containing Pt nanoparticles. Results Phys. 2019, 12, 1680–1685. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Xiao, S.L.; Shi, S.Q.; Cai, L.P. A one-pot synthesis and characterization of antibacterial silver nanoparticle-cellulose film. Polymers 2020, 12, 440. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Campagnolo, L.; Lauciello, S.; Athanassiou, A.; Fragouli, D. Au/ZnO hybrid nanostructures on electrospun polymeric mats for improved photocatalytic degradation of organic pollutants. Water 2019, 11, 1787. [Google Scholar] [CrossRef]
- Campbell, D.J.; Miller, J.D.; Andersh, B.J. Synthesis of palladium colloids within polydimethylsiloxane and their use as catalysts for hydrogenation. J. Colloid Interface Sci. 2011, 360, 309–312. [Google Scholar] [CrossRef]
- Saha, R.; Arunprasath, D.; Sekar, G. Surface enriched palladium on palladium-copper bimetallic nanoparticles as catalyst for polycyclic triazoles synthesis. J. Catal. 2019, 377, 673–683. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Chen, M.; Tang, L.; Tang, W.; Lai, C.; Cheng, M.; et al. Metal Organic Frameworks as Robust Host of Palladium Nanoparticles in Heterogeneous Catalysis: Synthesis, Application, and Prospect. ACS Appl. Mater. Interfaces 2019, 11, 32579–32598. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.H.; Gong, J.L.; Zhang, P.; Zeng, G.M.; Song, B.; Liu, H.Y. Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J. Colloid Interface Sci. 2017, 488, 26–38. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag+ ion release in the cytosol. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.C.; Cardoso, C.E.D.; Pereira, E.; Freitas, R. Toxic Effects of Metal Nanoparticles in Marine Invertebrates. In Nanostructured Materials for Treating Aquatic Pollution; Gonçalves, G.A.B., Marques, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 175–224. ISBN 978-3-030-33745-2. [Google Scholar]
- Song, J.Y.; Kwon, E.-Y.; Kim, B.S. Antibacterial latex foams coated with biologically synthesized silver nanoparticles using Magnolia kobus leaf extract. Korean J. Chem. Eng. 2012, 29, 1771–1775. [Google Scholar] [CrossRef]
- Phong, N.T.P.; Thanh, N.V.K.; Phuong, P.H. Fabrication of antibacterial water filter by coating silver nanoparticles on flexible polyurethane foams. J. Phys. Conf. Ser. 2009, 187, 12079. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Merillas, B.; Cimavilla-Román, P.; Rodriguez-Perez, M.A.; Pinto, J. Enhanced nitrates-polluted water remediation by polyurethane/sepiolite cellular nanocomposites. J. Clean. Prod. 2020, 254, 120038. [Google Scholar] [CrossRef]
- Goyal, A.; Kumar, A.; Patra, P.K.; Mahendra, S.; Tabatabaei, S.; Alvarez, P.J.J.; John, G.; Ajayan, P.M. In situ synthesis of metal nanoparticle embedded free standing multifunctional PDMS films. Macromol. Rapid Commun. 2009, 30, 1116–1122. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, D.; Xiao, X.; Zheng, M.; Ke, C.; Li, Y. Influence of organic modification on the structure and properties of polyurethane/sepiolite nanocomposites. Mater. Sci. Eng. A 2011, 528, 1656–1661. [Google Scholar] [CrossRef]
- Rajak, D.K.; Pagar, D.D.; Menezes, P.L.; Linul, E. Fiber-Reinforced Polymer Composites: Manufacturing, Properties, and Applications Dipen. Polymers 2019, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Apyari, V.V.; Arkhipova, V.V.; Gorbunova, M.V.; Volkov, P.A.; Isachenko, A.I.; Dmitrienko, S.G.; Zolotov, Y.A. Towards the development of solid-state platform optical sensors: Aggregation of gold nanoparticles on polyurethane foam. Talanta 2016, 161, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Calcagnile, P.; Fragouli, D.; Mele, E.; Ruffilli, R.; Athanassiou, A. Polymeric foams with functional nanocomposite cells. RSC Adv. 2014, 4, 19177. [Google Scholar] [CrossRef]
- Gupta, R.; Kulkarni, G.U. Removal of organic compounds from water by using a gold nanoparticle-poly(dimethylsiloxane) nanocomposite foam. ChemSusChem 2011, 4, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Desforges, A.; Deleuze, H.; Mondain-Monval, O.; Backov, R. Palladium nanoparticle generation within microcellular polymeric foam and size dependence under synthetic conditions. Ind. Eng. Chem. Res. 2005, 44, 8521–8529. [Google Scholar] [CrossRef]
- Suhaimi, H.S.M.; Khir, M.N.I.M.; Leo, C.P.; Ahmad, A.L. Preparation and characterization of polysulfone mixed-matrix membrane incorporated with palladium nanoparticles dispersed in polyvinylpyrrolidone for hydrogen separation. J. Polym. Res. 2014, 21, 1–8. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ Second Edition. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Pinto, J.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Reusable nanocomposite-coated polyurethane foams for the remediation of oil spills. Int. J. Environ. Sci. Technol. 2017, 14, 2055–2066. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, J.; Barroso-Solares, S.; Magrì, D.; Palazon, F.; Lauciello, S.; Athanassiou, A.; Fragouli, D. Melamine Foams Decorated with In-Situ Synthesized Gold and Palladium Nanoparticles. Polymers 2020, 12, 934. https://doi.org/10.3390/polym12040934
Pinto J, Barroso-Solares S, Magrì D, Palazon F, Lauciello S, Athanassiou A, Fragouli D. Melamine Foams Decorated with In-Situ Synthesized Gold and Palladium Nanoparticles. Polymers. 2020; 12(4):934. https://doi.org/10.3390/polym12040934
Chicago/Turabian StylePinto, Javier, Suset Barroso-Solares, Davide Magrì, Francisco Palazon, Simone Lauciello, Athanassia Athanassiou, and Despina Fragouli. 2020. "Melamine Foams Decorated with In-Situ Synthesized Gold and Palladium Nanoparticles" Polymers 12, no. 4: 934. https://doi.org/10.3390/polym12040934
APA StylePinto, J., Barroso-Solares, S., Magrì, D., Palazon, F., Lauciello, S., Athanassiou, A., & Fragouli, D. (2020). Melamine Foams Decorated with In-Situ Synthesized Gold and Palladium Nanoparticles. Polymers, 12(4), 934. https://doi.org/10.3390/polym12040934







