Dynamics and Rheological Behavior of Chitosan-Grafted-Polyacrylamide in Aqueous Solution upon Heating
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Rheological Measurements.
2.4. Dynamic Light Scattering (DLS) and Ultraviolet and Visible Spectrum (UV)
3. Results and Discussion
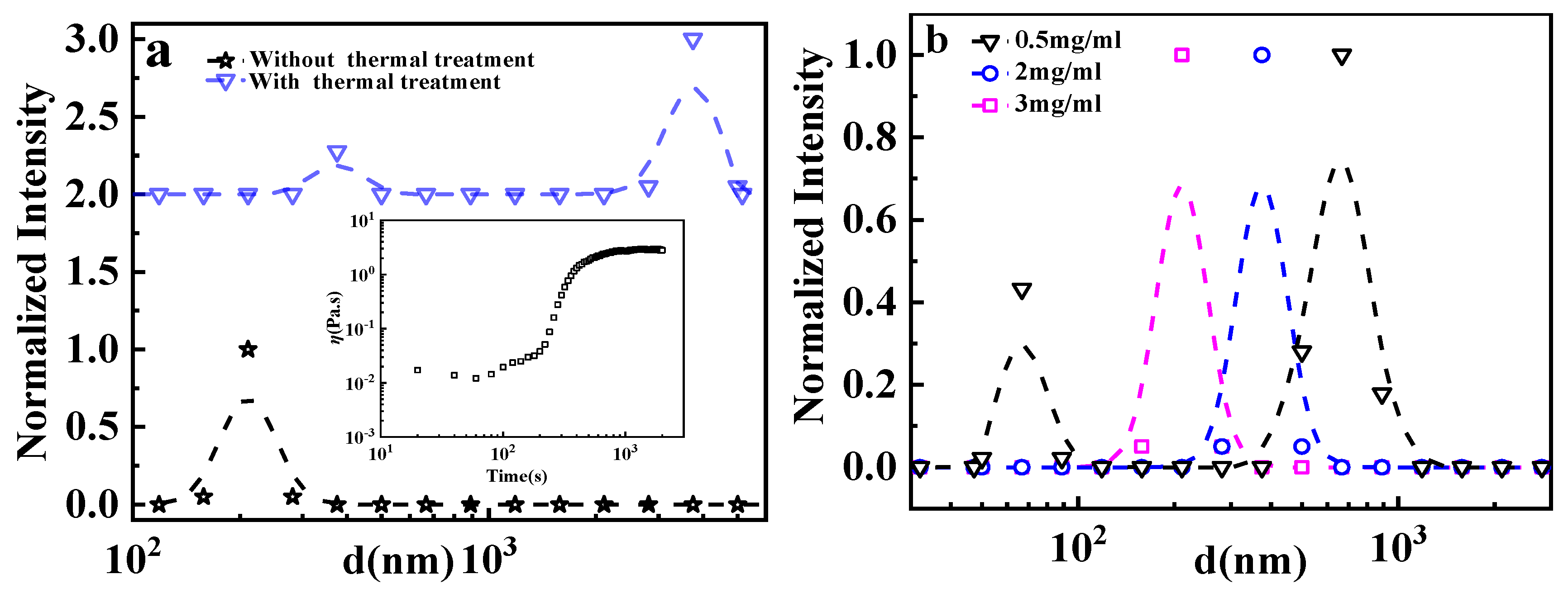
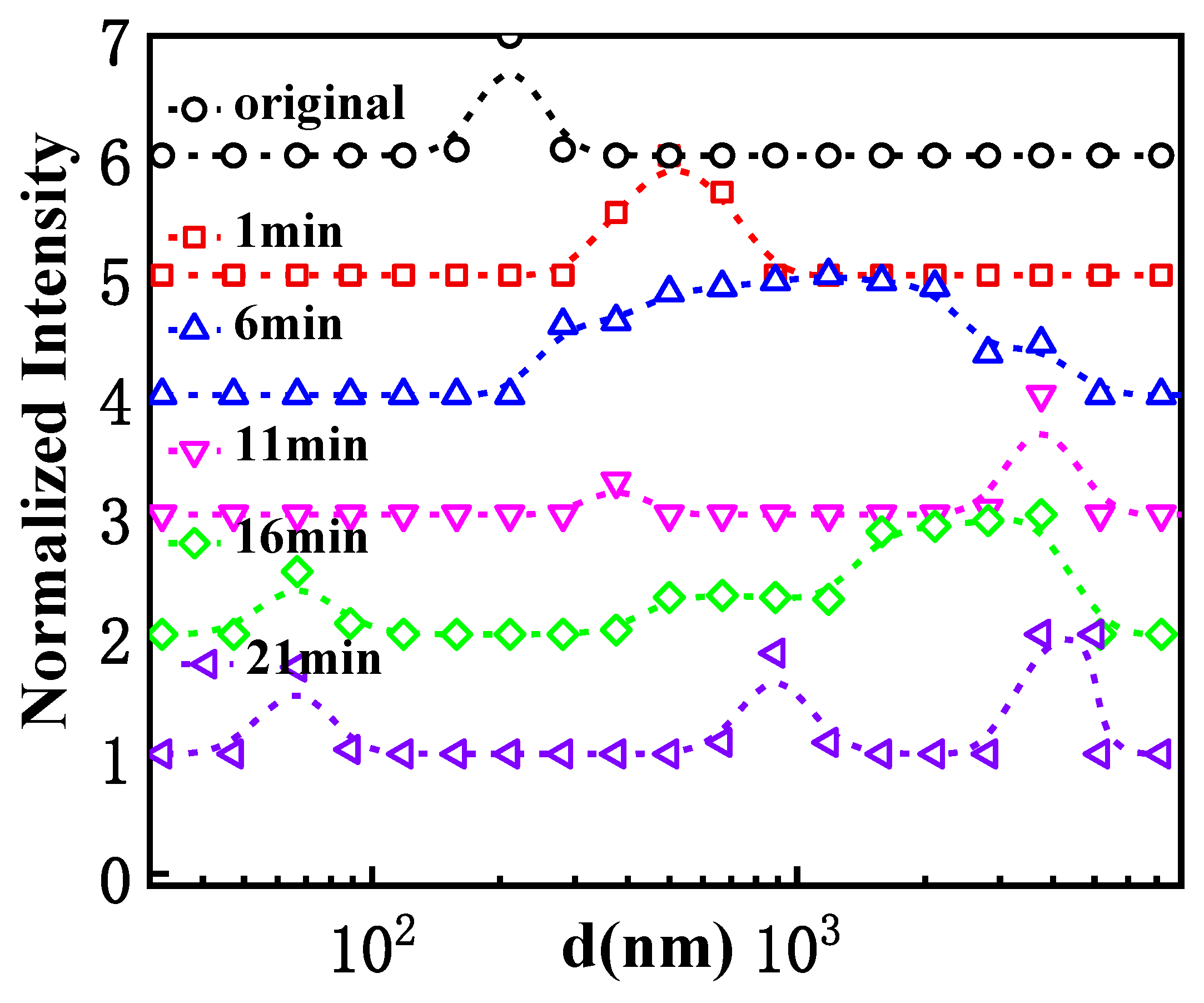
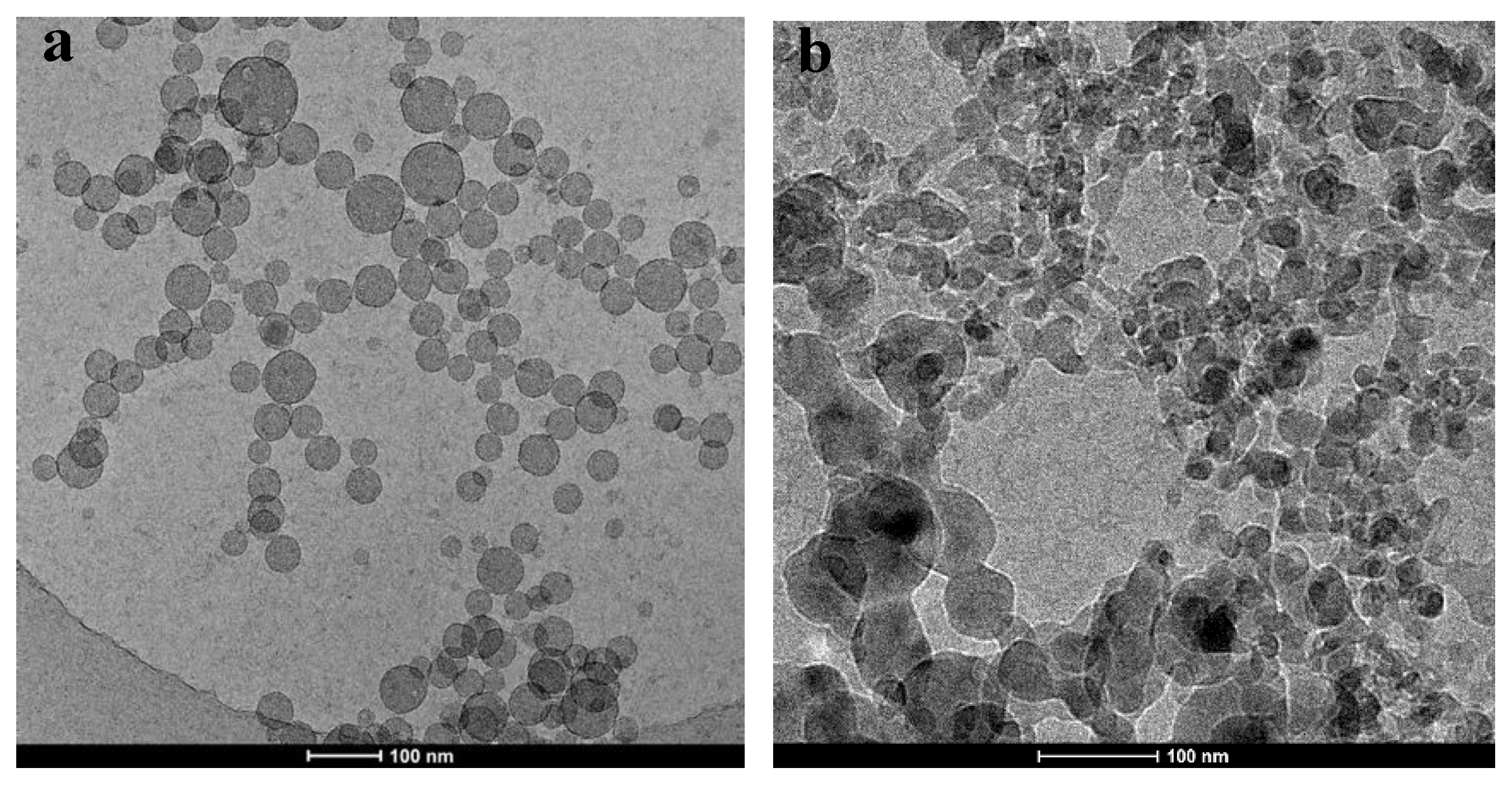
3.1. Transformation of GPAM Aggregates upon Heating
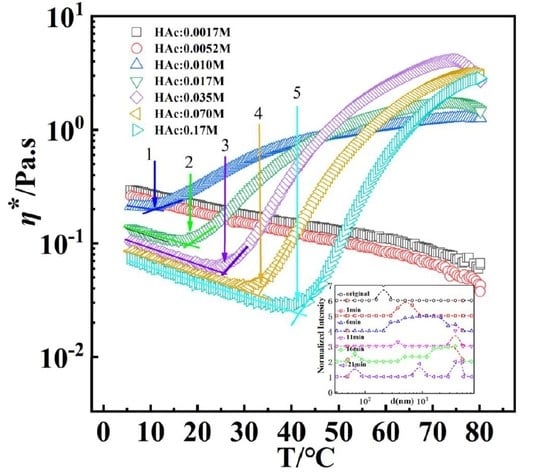
3.2. Effects of HAc on GPAM Solution
3.3. Influence of Heating Rate and Shear Rate
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cammas, S.; Suzuki, K.; Sone, C.; Sakurai, Y.; Kataoka, K.; Okano, T. Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J. Control. Release 1997, 48, 157–164. [Google Scholar] [CrossRef]
- Censi, R.; Vermonden, T.; van Steenbergen, M.J.; Deschout, H.; Braeckmans, K.; De Smedt, S.C.; van Nostrum, C.F.; di Martino, P.; Hennink, W.E. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control. Release 2009, 140, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shull, K.R. Thermothickening behavior of self-stabilized colloids formed from associating polymers. Macromolecules 2019, 52, 4926–4933. [Google Scholar] [CrossRef]
- Hourdet, D.; L’Alloret, F.; Audebert, R. Reversible thermothickening of aqueous polymer solutions. Polymer 1994, 35, 2624–2630. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Q.; Zhang, L.; Zhuo, R.; Jiang, X. Synthesis of carboxymethyl chitin in aqueous solution and its thermo- and pH-sensitive behaviors. Carbohydr. Polym. 2016, 137, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, W.; Pang, Y.; Zhu, X.; Zhou, Y.; Yan, D. Self-assembled micelles from an amphiphilic hyperbranched copolymer with polyphosphate arms for drug delivery. Langmuir 2010, 26, 10585–10592. [Google Scholar] [CrossRef]
- McCormick, C.L.; Nonaka, T.; Johnson, C.B. Water-soluble copolymers: 27. Synthesis and aqueous solution behaviour of associative acrylamideN-alkylacrylamide copolymers. Polymer 1988, 29, 731–739. [Google Scholar] [CrossRef]
- Hourdet, D.; Gadgil, J.; Podhajecka, K.; Badiger, M.V.; Brûlet, A.; Wadgaonkar, P.P. Thermoreversible behavior of associating polymer solutions: Thermothinning versus thermothickening. Macromolecules 2005, 38, 8512–8521. [Google Scholar] [CrossRef]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Characterization of acrylamide polymers for enhanced oil recovery. J. Appl. Polym. Sci. 2003, 87, 1869–1878. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure–property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Shangguan, Y.G.; Liu, M.G.; Jin, L.; Wang, M.J.; Wang, Z.K.; Wu, Q.; Zheng, Q. Thermo-thickening behavior and its mechanism in a chitosan-graft-polyacrylamide aqueous solution. Soft Matter 2018, 14, 6667–6677. [Google Scholar] [CrossRef] [PubMed]
- Seetapan, N.; Mai-ngam, K.; Plucktaveesak, N.; Sirivat, A. Linear viscoelasticity of thermoassociative chitosan-g-poly(N-isopropylacrylamide) copolymer. Rheol. Acta 2006, 45, 1011–1018. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Mauk, M.; Hong, K.S.; Li, M.; Yang, S.; Bau, H.H. Self-actuated, thermo-responsive hydrogel valves for lab on a chip. Biomed. Microdevices 2005, 7, 313–322. [Google Scholar] [CrossRef]
- Huang, X.; Pang, Y.; Liu, Y.; Zhou, Y.; Wang, Z.; Hu, Q. Green synthesis of silver nanoparticles with high antimicrobial activity and low cytotoxicity using catechol-conjugated chitosan. RSC Adv. 2016, 6, 64357–64363. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.; Sandeman, S. Nano carriers for drug transport across the blood–brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Tong, Z.; Kong, X.; Yao, J. Preparation of chitosan-based multifunctional nanocarriers overcoming multiple barriers for oral delivery of insulin. Mater. Sci. Eng. C 2017, 70, 278–286. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Ho, K.W.; Tey, B.T.; Chan, E.-S. Effects of environmental factors on the physical stability of pickering-emulsions stabilized by chitosan particles. Food Hydrocolloid 2016, 60, 543–550. [Google Scholar] [CrossRef]
- Subramani, S.E.; Thinakaran, T. Isotherm, kinetic and thermodynamic studies on the adsorption behaviour of textile dyes onto chitosan. Process Saf. Environ. Prot. 2017, 106, 1–10. [Google Scholar]
- Shariful, M.I.; Sharif, S.B.; Lee, J.J.L.; Habiba, U.; Ang, B.C.; Amalina, M.A. Adsorption of divalent heavy metal ion by mesoporous-high surface area chitosan/poly (ethylene oxide) nanofibrous membrane. Carbohydr. Polym. 2017, 157, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Schuetz, Y.B.; Gurny, R.; Jordan, O. A novel thermoresponsive hydrogel based on chitosan. Eur. J. Pharm. Biopharm. 2008, 68, 19–25. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. Determination of phase behavior of poly(ethylene oxide) and chitosan solution blends using rheometry. Macromolecules 2012, 45, 7621–7633. [Google Scholar] [CrossRef]
- Chenite, A.; Buschmann, M.; Wang, D.; Chaput, C.; Kandani, N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001, 46, 39–47. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Effect of urea on solution behavior and heat-induced gelationof chitosan-β-glycerophosphate. Carbohydr. Polym. 2006, 63, 507–518. [Google Scholar] [CrossRef]
- Recillas, M.; Silva, L.L.; Peniche, C.; Goycoolea, F.M.; Rinaudo, M.; Argüelles-Monal, W.M. Thermoresponsive behavior of chitosan-g-n-isopropylacrylamide copolymer solutions. Biomacromolecules 2009, 10, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Niang, P.M.; Huang, Z.; Dulong, V.; Souguir, Z.; Le Cerf, D.; Picton, L. Thermo-controlled rheology of electro-assembled polyanionic polysaccharide (alginate) and polycationic thermo-sensitive polymers. Carbohydr. Polym. 2016, 139, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, L.; Leong, W.C.; Gan, L.H. Thermo-responsive association of chitosan-graft-poly(n-isopropylacrylamide) in aqueous solutions. J. Phys. Chem. B 2010, 114, 10666–10673. [Google Scholar] [CrossRef]
- Lu, Y.; Shang, Y.; Huang, X.; Chen, A.; Yang, Z.; Jiang, Y.; Cai, J.; Gu, W.; Qian, X.; Yang, H.; et al. Preparation of strong cationic chitosan-graft-polyacrylamide flocculants and their flocculating properties. Ind. Eng. Chem. Res. 2011, 50, 7141–7149. [Google Scholar] [CrossRef]
- Sokker, H.H.; El-Sawy, N.M.; Hassan, M.A.; El-Anadouli, B.E. Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization. J. Hazard. Mater. 2011, 190, 359–365. [Google Scholar] [CrossRef]
- Wang, J.-P.; Chen, Y.-Z.; Zhang, S.-J.; Yu, H.-Q. A chitosan-based flocculant prepared with gamma-irradiation-induced grafting. Bioresour. Technol. 2008, 99, 3397–3402. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, B.; Huang, X.; Zhou, J.; Cai, J.; Yang, H.; Li, A.; Cheng, R. Evaluation of the flocculation performance of carboxymethyl chitosan-graft-polyacrylamide, a novel amphoteric chemically bonded composite flocculant. Water Res. 2012, 46, 107–114. [Google Scholar] [CrossRef]
- Yuan, B.; Shang, Y.; Lu, Y.; Qin, Z.; Jiang, Y.; Chen, A.; Qian, X.; Wang, G.; Yang, H.; Cheng, R. The flocculating properties of chitosan-graft-polyacrylamide flocculants (I)—Effect of the grafting ratio. J. Appl. Polym. Sci. 2010, 117, 1876–1882. [Google Scholar] [CrossRef]
- Dubochet, J.; Lepault, J.; Freeman, R.; Berriman, J.A.; Homo, J.-C. Electron microscopy of frozen water and aqueous solutions. J. Microsc. 1982, 128, 219–237. [Google Scholar] [CrossRef]
- Milne, J.L.S.; Borgnia, M.J.; Bartesaghi, A.; Tran, E.E.H.; Earl, L.A.; Schauder, D.M.; Lengyel, J.; Pierson, J.; Patwardhan, A.; Subramaniam, S. Cryo-electron microscopy—A primer for the non-microscopist. FEBS J. 2013, 280, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Korchagina, E.V.; Philippova, O.E. Multichain aggregates in dilute solutions of associating polyelectrolyte keeping a constant size at the increase in the chain length of individual macromolecules. Biomacromolecules 2010, 11, 3457–3466. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]

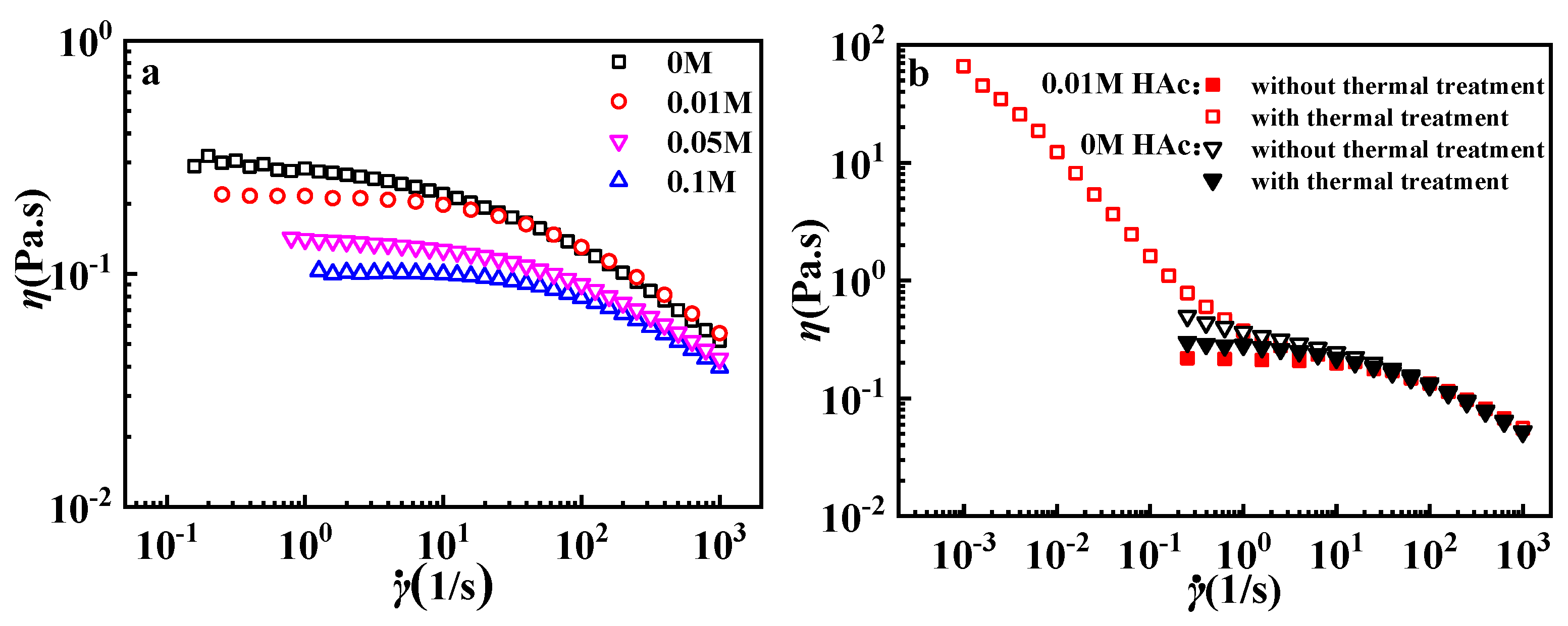
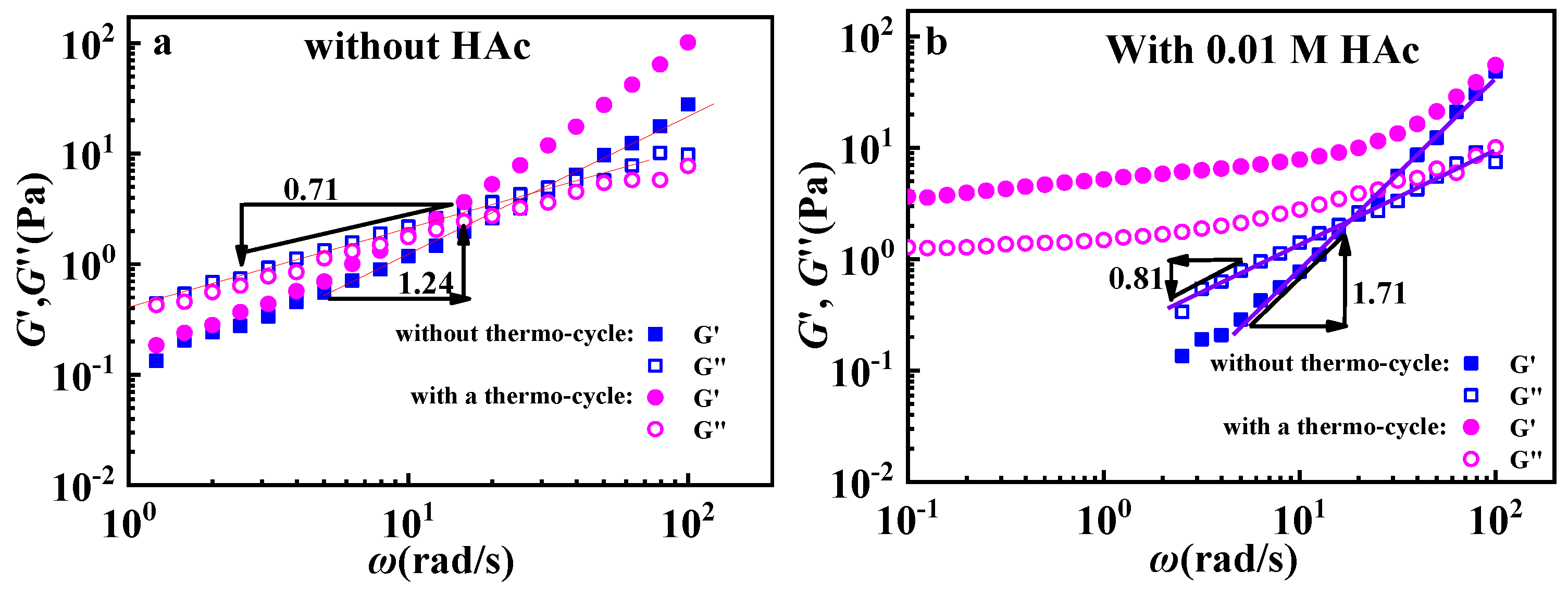

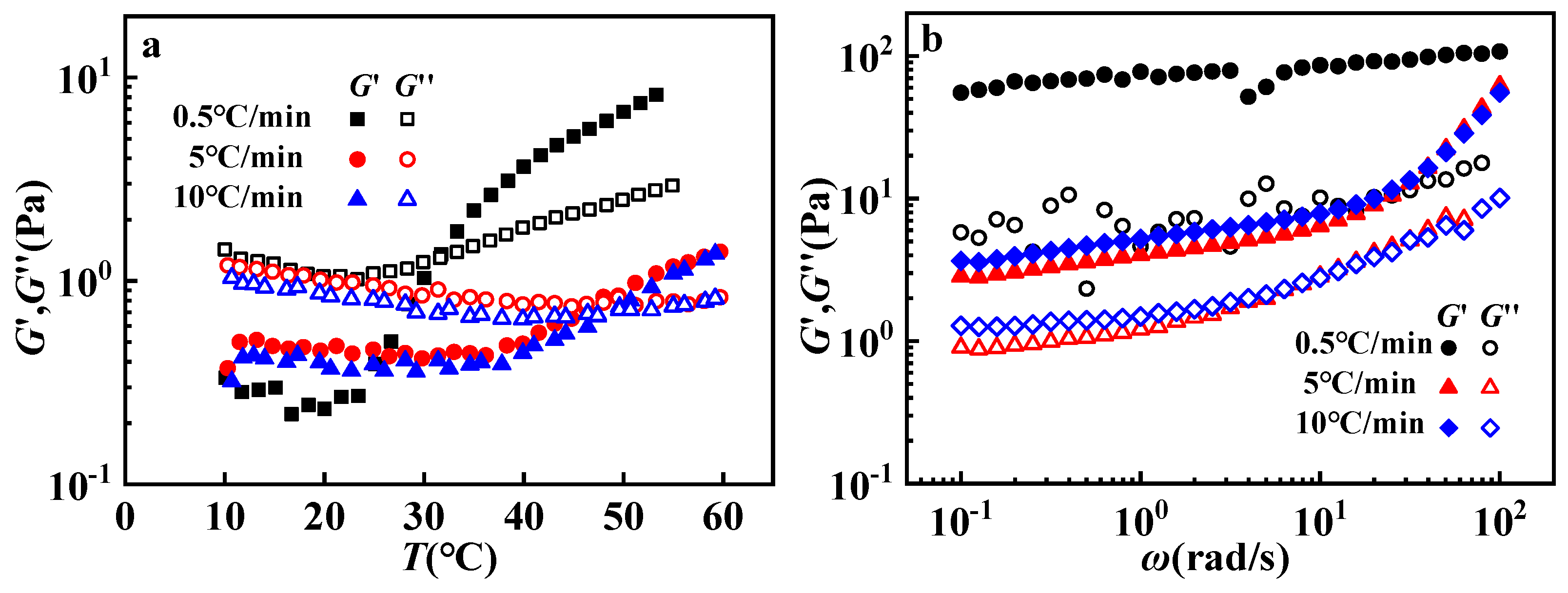

| HAc (mol/L) | η0 (Pa·s) |
|---|---|
| 0 | 0.28 |
| 0.01 | 0.22 |
| 0.05 | 0.15 |
| 0.1 | 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Shangguan, Y.; Zheng, Q. Dynamics and Rheological Behavior of Chitosan-Grafted-Polyacrylamide in Aqueous Solution upon Heating. Polymers 2020, 12, 916. https://doi.org/10.3390/polym12040916
Wang M, Shangguan Y, Zheng Q. Dynamics and Rheological Behavior of Chitosan-Grafted-Polyacrylamide in Aqueous Solution upon Heating. Polymers. 2020; 12(4):916. https://doi.org/10.3390/polym12040916
Chicago/Turabian StyleWang, Mengjie, Yonggang Shangguan, and Qiang Zheng. 2020. "Dynamics and Rheological Behavior of Chitosan-Grafted-Polyacrylamide in Aqueous Solution upon Heating" Polymers 12, no. 4: 916. https://doi.org/10.3390/polym12040916
APA StyleWang, M., Shangguan, Y., & Zheng, Q. (2020). Dynamics and Rheological Behavior of Chitosan-Grafted-Polyacrylamide in Aqueous Solution upon Heating. Polymers, 12(4), 916. https://doi.org/10.3390/polym12040916