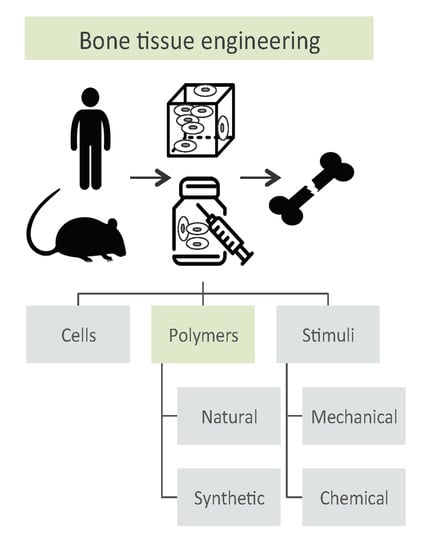
Natural and Synthetic Polymers for Bone Scaffolds Optimization
Abstract
1. Introduction
1.1. Anatomy
1.2. Cellular Components
1.3. Bone Mechanical Properties
1.4. Homeostasis
1.5. Spontaneous Repair
1.6. Bone Tissue Pathologies and Conventional Therapies
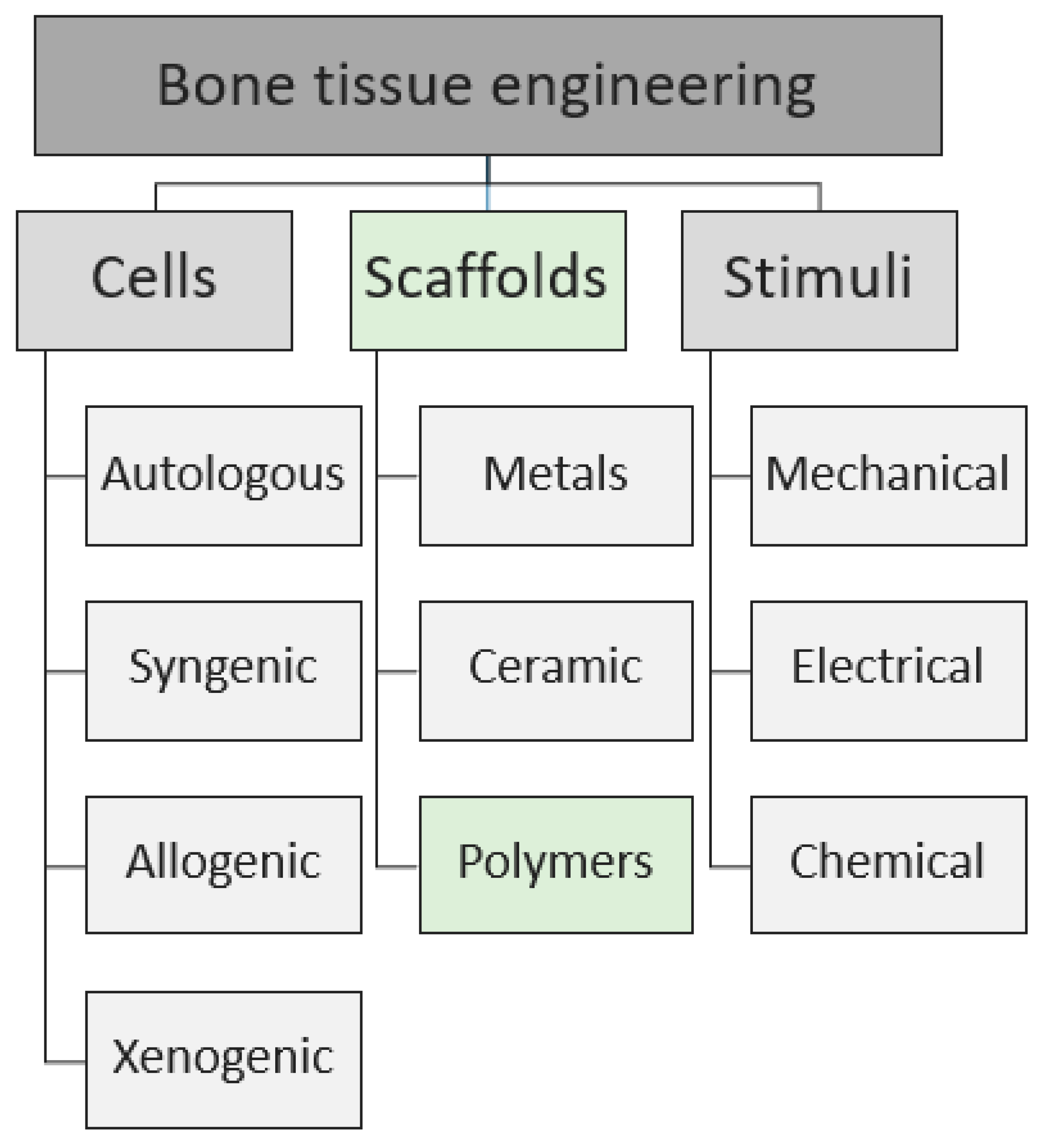
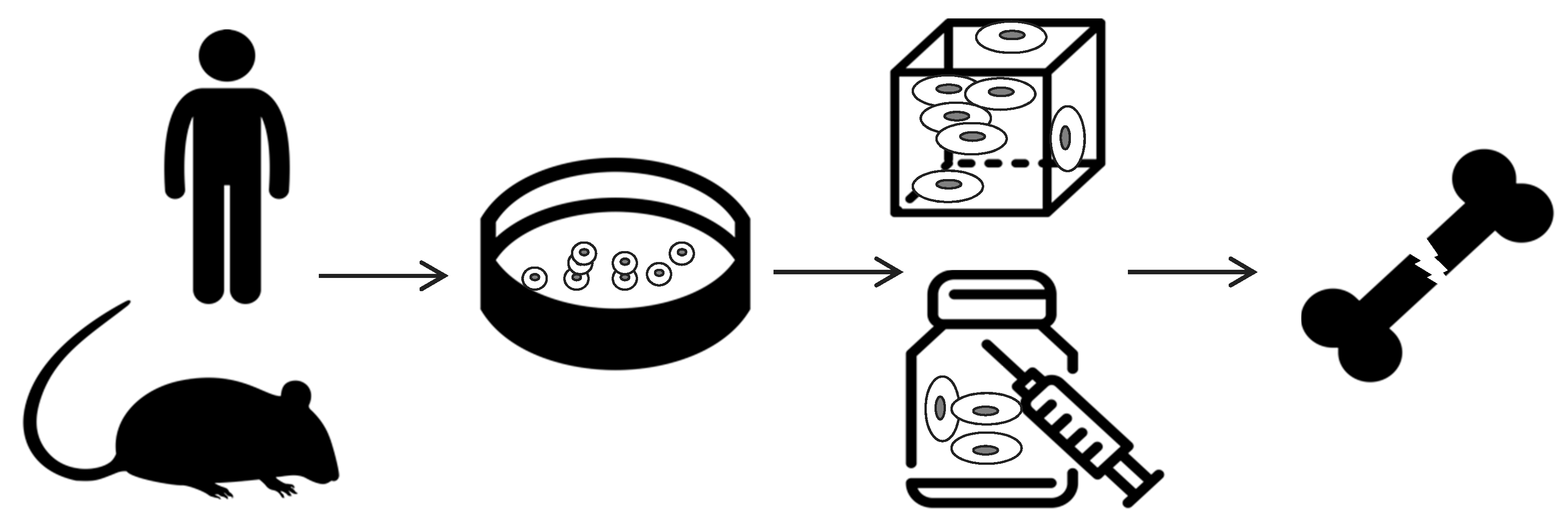
2. Scaffold-Based Regenerative Medicine
- cells harvesting from human being (named “autologous” source if patient and donor coincide or “allogenic” if they differ) or animals (termed “xenogenic” source), their in vitro expansion and, eventually, their differentiation;
- cells seeding on optimized scaffold, designed and developed to reproduce the in vivo bone feature;
- cellularized scaffold implantation into the patient damage site.
2.1. Cells Source
2.2. Stimuli
2.3. Scaffolds as Cell Support
3. Natural Polymers
4. Synthetic Polymers
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Colón, C.J.P.; Molina Vicenty, I.L.; Frontera-Rodríguez, M.; García-Ferré, A.; Rivera, B.P.; Cintrón-Vélez, G.; Frontera-Rodríguez, S. Muscle and Bone Mass Loss in the Elderly Population: Advances in diagnosis and treatment. J. Biomed. 2018, 3, 40–49. [Google Scholar] [CrossRef]
- Florencio-silva, R.; Rodrigues, G.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.P.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Adamo, S.; Comoglio, P.; Dolfi, A.; Molinaro, M.; Papaccio, G. Istologia Di Monesi, 6th ed.; Piccin-nuova Libraria: Padua, Italy, 2012. [Google Scholar]
- Mohamed, A.M. An Overview of Bone Cells and their Regulating Factors of Differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar]
- Karpiński, R.; Jaworski, Ł.; Czubacka, P. The structural and mechanical properties of the bone. J. Technol. Exploit. Mech. Eng. 2017, 3, 43–50. [Google Scholar] [CrossRef]
- Zarif, M.-E. A review of chitosan-, alginate-, and gelatin-based biocomposites for bone tissue engineering. Biomater. Tissue Eng. Bull. 2018, 5, 97–109. [Google Scholar]
- Lombardi, G.; Di Somma, C.; Rubino, M.; Faggiano, A.; Vuolo, L.; Guerra, E.; Contaldi, P.; Savastano, S.; Colao, A. The roles of parathyroid hormone in bone remodeling: Prospects for novel therapeutics. J. Endocrinol. Investig. 2011, 34, 18–22. [Google Scholar]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- McEwan, J.K.; Tribe, H.C.; Jacobs, N.; Hancock, N.; Qureshi, A.A.; Dunlop, D.G.; Oreffo, R.O. Regenerative medicine in lower limb reconstruction. Regen. Med. 2018, 13, 477–490. [Google Scholar] [CrossRef]
- Mehta, M.; Checa, S.; Lienau, J.; Hutmacher, D.; Duda, G.N. In vivo tracking of segmental bone defect healing reveals that callus patterning is related to early mechanical stimuli. Eur. Cells Mater. 2012, 24, 71. [Google Scholar] [CrossRef] [PubMed]
- Zanker, J.; Duque, G. Osteoporosis in Older Persons: Old and New Players. JAGS 2019, 67, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Acosta-olivo, C.A. Humeral Shaft Pseudoarthrosis Treated With Bone Autograft Versus Platelet Rich Plasma. NCT02520089. 2018. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02520089?view=record (accessed on 10 April 2020).
- Catanzano, A.A.; Fitch, R.D. The Use of Distraction Osteogenesis and a Taylor Spatial Frame in the Treatment of a Tibial Shaft Nonunion and Deformity in a Pediatric Patient with Osteopetrosis. J. Bone Jt. Surg. 2018, 8, e93–e94. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. P&T 2018, 43, 92–104. [Google Scholar]
- Rajani, R.; Gibbs, C.P. Treatment of Bone Tumors. Surg. Pathol. Clin. 2013, 5, 301–318. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 1–27. [Google Scholar] [CrossRef]
- Asnaghi, M.A.; Candiani, G.; Farè, S.; Fiore, G.B.; Petrini, P.; Raimondi, M.T.; Soncini, M.; Mantero, S. Trends in biomedical engineering: Focus on Regenerative Medicine. J. Appl. Biomater. Biomech. 2011, 9, 73–86. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Boeri, L.; Albani, D.; Jacchetti, E. Mechanical regulation of nucleocytoplasmic translocation in mesenchymal stem cells: Characterization and methods for investigation. Biophys. Rev. 2019, 11, 817–831. [Google Scholar] [CrossRef]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Wound Heal. Soc. 2013, 2, 306–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-S.; Feng, Z.-H.; Wu, G.-F.; Bai, S.-Z.; Dong, Y.; Chen, F.-M.; Zhao, Y.-M. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci. Rep. 2016, 6, 28126. [Google Scholar] [CrossRef]
- Wu, S.; Xiao, Z.; Song, J.; Li, M.; Li, W. Evaluation of BMP-2 Enhances the Osteoblast Differentiation of Human Amnion Mesenchymal Stem Cells Seeded on Nano- Hydroxyapatite/Collagen/Poly(L -Lactide ). Int. J. Mol. Sci. 2018, 19, 2171. [Google Scholar] [CrossRef] [PubMed]
- Namini, M.S.; Bayat, N.; Tajerian, R.; Ebrahimi-Barough, S.; Azami, M.; Irani, S.; Jangjoo, S.; Shirian, S.; Ai, J. A comparison study on the behavior of human endometrial stem cell-derived osteoblast cells on PLGA/HA nanocomposite scaffolds fabricated by electrospinning and freeze-drying methods. J. Orthop. Surg. Res. 2018, 13, 63. [Google Scholar] [CrossRef]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J. TNF- α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Bolander, J.; Ji, W.; Leijten, J.; Teixeira, L.M.; Bloemen, V.; Lambrechts, D.; Chaklader, M.; Luyten, F.P. Healing of a Large Long-Bone Defect through Serum-Free In Vitro Priming of Human Periosteum-Derived Cells. Stem Cell Rep. 2017, 8, 758–772. [Google Scholar] [CrossRef]
- Tokita, R.; Nakajima, K.; Inoue, K.; Al-wahabi, A.; Ser-od, T.; Matsuzaka, K. Differentiation behavior of iPS cells cultured on PLGA with osteoinduction medium. Dent. Mater. J. 2017, 36, 103–110. [Google Scholar] [CrossRef][Green Version]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef]
- De Witte, T.; Fratila-apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, F.; Jiang, X. The Effects of Platelet-Derived Growth Factor-BB on Human Dental Pulp Stem Cells Mediated Dentin-Pulp Complex Regeneration. Stem Cells Transl. Med. 2017, 6, 2126–2134. [Google Scholar] [CrossRef]
- Nava, M.M.; Fedele, R.; Raimondi, M.T. Computational prediction of strain-dependent diffusion of transcription factors through the cell nucleus. Biomech. Model. Mechanobiol. 2016, 15, 983–993. [Google Scholar] [CrossRef]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling Self-Renewal and Differentiation of Stem Cells via Mechanical Cues. J. Biomed. Biotechnol. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Hana, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Mao, J. Bone Tissue Engineering and Regeneration: From Discovery to the Clinic—An Overview. Tissue Eng. 2011, 17, 389–392. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.-W.; Tewari, M.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1–33. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 1–15. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Suchý, T.; Šupová, M.; Bartoš, M.; Sedláček, R.; Piola, M.; Soncini, M.; Fiore, G.B.; Sauerová, P.; Kalbáčová, M.H. Dry versus hydrated collagen scaffolds: Are dry states representative of hydrated states ? J. Mater. Sci. Mater. Med. 2018, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Polo-corrales, L.; Latorre-esteves, M.; Ramirez-vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Baldwin, P.; Ba, D.J.L.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 2017, 8, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, D.K.; Seong, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Silk Fibroin-Based Scaffold for Bone Tissue Engineering. In Novel Biomaterials for Regenerative Medicine. Advances in Experimental Medicine and Biology; Springer Nature Singapore Pte. Ltd.: Singapore, 2018. [Google Scholar]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-pawelska, A. Alginate-Based Hydrogels in Regenerative Medicine. In Alginates—Recent Uses of This Natural Polymer; Pereira, L., Cotas, J., Eds.; IntechOpen Limitid: London, UK, 2020. [Google Scholar]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 1–6. [Google Scholar]
- Zhu, Z.; Wang, Y.M.; Yang, J.; Luo, X.S. Hyaluronic acid: A versatile biomaterial in tissue engineering. Plast. Aesthetic Res. 2017, 4, 219–227. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2019, in press. [Google Scholar] [CrossRef]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Functional nanoparticles obtained from cellulose: Engineering the shape and size of 6- carboxycellulose. Chem. Commun. 2013, 78, 8818–8820. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Hatton, P.V. An Overview of Poly ( lactic- co -glycolic ) Acid ( PLGA ) -Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, J. Poly ( lactide- co -glycolide ) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Shindo, M.; Kobayashi, D.; Tsuruga, E.; Amemiya, A.; Kuboki, Y. Osteogenesis by bone marrow stromal cells maintained on type I collagen matrix gels in vivo. Bone 1997, 20, 101–107. [Google Scholar] [CrossRef]
- Chen, P.; Tao, J.; Zhu, S.; Cai, Y.; Mao, Q.; Yu, D.; Dai, J.; Ouyang, H. Biomaterials Radially oriented collagen scaffold with SDF-1 promotes osteochondral repair by facilitating cell homing. Biomaterials 2015, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Reddy, VJ.; Wong, SY.; Li, X.; Su, B.; Ramakrishna, S.; Lim, CT. Enhanced Biomineralization in Osteoblasts on a Novel Electrospun Biocomposite Nanofibrous Substrate of Hydroxyapatite/Collagen/Chitosan. Tissue Eng. 2010, 16, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.M.; Wang, L.; Huang, J.; Rowe, D.W.; Wei, M. Bone tissue engineering with a collagen–hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 243–253. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Plunkett, N.A.; Brien, F.J.O. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur. Cells Mater. 2010, 20, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Sauerova, P.; Suchy, T.; Supova, M.; Bartos, M.; Klima, J.; Juhasova, J.; Juhas, S.; Kubikova, T.; Tonar, Z.; Sedlacek, R.; et al. Positive impact of dynamic seeding of mesenchymal stem cells on bone-like biodegradable scaffolds with increased content of calcium phosphate nanoparticles. Mol. Biol. Rep. 2019, 46, 4483–4500. [Google Scholar] [CrossRef]
- Kane, R.J.; Weiss-Bilka, H.E.; Meagher, M.J.; Liu, Y.; Gargac, J.A.; Niebur, G.L.; Wagner, D.R.; Roeder, R.K. Hydroxyapatite reinforced collagen scaffolds with improved architecture and mechanical properties. Acta Biomater. 2015, 17, 16–25. [Google Scholar] [CrossRef]
- Perdisa, F.; Filardo, G.; Sessa, A.; Busacca, M.; Zaffagnini, S.; Marcacci, M. One-Step Treatment for Patellar Cartilage Defects With a Cell-Free Osteochondral Scaffold. Am. J. Sports Med. 2017, 45, 1581–1588. [Google Scholar] [CrossRef]
- Wang, L.; Stegemann, J.P. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissue engineering. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2019, 10, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Gawlitta, D.; Benders, K.E.; Toma, S.M.; Pouran, B.; van Weeren, P.R.; Dhert, W.J.; Malda, J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef]
- Kim, S.; Yi, S.; Choi, S.; Kim, K.; Lee, Y. Gelatin-layered and multi-sized porous b-tricalcium phosphate for tissue engineering scaffold. Nanoscale Res. Lett. 2012, 7, 1–5. [Google Scholar] [CrossRef]
- Rahmanian, M.; Seyfoori, A.; Dehghan, M.M.; Eini, L.; Naghib, S.M.; Gholami, H.; Mohajeri, S.F.; Mamaghani, K.R.; Majidzadeh-A, K. Multifunctional gelatin—Tricalcium phosphate porous nanocomposite sca ff olds for tissue engineering and local drug delivery: In vitro and in vivo studies. J. Taiwan Inst. Chem. Eng. 2019, 101, 214–220. [Google Scholar] [CrossRef]
- Fu, Y.T.; Sheu, S.Y.; Chen, Y.S.; Chen, K.Y.; Yao, C.H. Porous gelatin/tricalcium phosphate/genipin composites containing lumbrokinase for bone repair. Bone 2015, 78, 15–22. [Google Scholar] [CrossRef]
- Park, S.Y.; Ki, C.S.; Park, Y.H.; Jung, H.M.; Woo, K.M.; Kim, H.J. Electrospun Silk Fibroin Scaffolds with Macropores for Bone Regeneration: An In Vitro and In Vivo Study. Tissue Eng. 2010, 16, 1271–1279. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, A.; Liu, Y.; Jiao, D.; Zeng, D.; Wang, X.; Cao, L.; Jiang, X. Enhanced bone regeneration of the silk fibroin electrospun scaffolds through the modification of the graphene oxide functionalized by BMP-2 peptide. Int. J. Nanomed. 2019, 14, 733–751. [Google Scholar] [CrossRef]
- Park, J.-Y.; Yang, C.; Jung, I.-H.; Lim, H.-C.; Lee, J.-S.; Jung, U.-W.; Seo, Y.-K.; Park, J.-K.; Choi, S.-H. Regeneration of rabbit calvarial defects using cells-implanted nano- hydroxyapatite coated silk scaffolds. Biomater. Res. 2015, 19, 7. [Google Scholar] [CrossRef]
- Mcnamara, S.L.; Rnjak-kovacina, J.; Schmidt, D.F.; Lo, T.J.; Kaplan, D.L. Silk as a biocohesive sacrificial binder in the fabrication of hydroxyapatite load bearing scaffolds. Biomaterials 2014, 35, 6941–6953. [Google Scholar] [CrossRef]
- Cao, C.; Li, H.; Li, J.; Liu, C. Mechanical reinforcement of injectable calcium phosphate cement/silk fibroin (SF) composite by mineralized SF Chengbin. Ceram. Int. 2014, 40, 13987–13993. [Google Scholar] [CrossRef]
- Li, J.J.; Gil, E.S.; Hayden, R.S.; Li, C.; Kaplan, D.L.; Zreiqat, H. Multiple Silk Coatings on Biphasic Calcium Phosphate Scaffolds: Effect on Physical and Mechanical Properties and In Vitro Osteogenic Response of Human Mesenchymal Stem Cells. Biomacromolecules 2013, 14, 2179–2188. [Google Scholar] [CrossRef]
- Kweon, H.; Lee, S.; Hahn, B.; Lee, Y.; Kim, S.G. Hydroxyapatite and Silk Combination-Coated Dental Implants Result in Superior Bone Formation in the Peri-Implant Area Compared With Hydroxyapatite and Collagen Combination- Coated Implants. J. Oral Maxillofac. Surg. 2014, 75, 1928–1936. [Google Scholar] [CrossRef]
- Maji, K.; Dasgupta, S. Hydroxyapatite-Chitosan and Gelatin Based Scaffold for Bone Tissue Engineering. Trans. Indian Ceram. Soc. 2014, 73, 37–41. [Google Scholar] [CrossRef]
- Shi, S.; Cheng, X.; Wang, J.; Zhang, W.; Peng, L.; Zhang, Y. RhBMP-2 Microspheres-Loaded Enhanced Osseointegration: An Experiment in Dog. J. Biomater. Appl. 2009, 23, 331–346. [Google Scholar]
- Hou, J.; Wang, J.; Cao, L.; Qian, X.; Xing, W.; Lu, J.; Liu, C. Segmental bone regeneration using rhBMP-2-loaded collagen/chitosan microspheres composite scaffold in a rabbit model. Biomed. Mater. 2012, 7, 035002. [Google Scholar] [CrossRef]
- Wu, H.; Lei, P.; Liu, G.; Zhang, Y.S.; Yang, J.; Zhang, L.; Xie, J.; Niu, W.; Liu, H.; Ruan, J.; et al. Reconstruction of Large-scale Defects with a Novel Hybrid Scaffold Made from Poly ( L-lactic acid )/ Nanohydroxyapatite/Alendronate-loaded Chitosan Microsphere: In vitro and in vivo Studies. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. Bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Bi, Y.; Lin, Z.; Deng, S. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 576–583. [Google Scholar] [CrossRef]
- Zhao, L.; Weir, M.D.; Xu, H.H.K. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef]
- Lin, H.R.; Yeh, Y.J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: Preparation, characterization, andin vitro studies. J. Biomed. Mater. Res. 2004, 71, 52–65. [Google Scholar] [CrossRef]
- De Paula, F.L.; Barreto, I.C.; Rocha-Leão, M.H.; Borojevic, R.; Rossi, A.M.; Rosa, F.P.; Farina, M. Hydroxyapatite-alginate biocomposite promotes bone mineralization in different length scales in vivo. Front. Mater. Sci. China 2009, 3, 145–153. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.; Padalhin, A.R.; Lee, B. Materials Science & Engineering C Incorporation of chitosan-alginate complex into injectable calcium phosphate cement system as a bone graft material. Mater. Sci. Eng. C 2019, 94, 385–392. [Google Scholar]
- Florczyk, S.J.; Leung, M.; Li, Z.; Huang, J.I.; Zhang, M. Evaluation of three-dimensional porous chitosan–alginate scaffolds in rat calvarial defects for bone regeneration applications. J. Biomed. Mater. Res. Part A 2013, 101, 2974–2983. [Google Scholar] [CrossRef]
- Ghosh, M.; Halperin-sternfeld, M.; Grinberg, I.; Adler-abramovich, L. Injectable Alginate-Peptide Composite Hydrogel as a Scaffold for Bone Tissue Regeneration. Nanomaterials 2019, 9, 497. [Google Scholar] [CrossRef]
- Hernández-gonzález, A.C.; Téllez-jurado, L.; Rodríguez-lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Rubert, M.; Alonso-Sande, M.; Monjo, M.; Ramis, J.M. Evaluation of Alginate and Hyaluronic Acid for Their Use in Bone Tissue Engineering. Biointerphases 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Ba, T.; Nguyen, L.; Lee, B. A Combination of Biphasic Calcium Phosphate Scaffold with Hyaluronic Acid-Gelatin Hydrogel. Tissue Eng. A 2014, 20, 13–14. [Google Scholar]
- Xu, C.; Wang, Y.; Yu, X.; Chen, X.; Li, X.; Yang, X.; Li, S.; Zhang, X.; Xiang, A.P. Evaluation of human mesenchymal stem cells response to biomimetic bioglass-collagen-hyaluronic acid-phosphatidylserine composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. 2008, 88, 264–273. [Google Scholar] [CrossRef]
- Kim, E.; Yoon, S.J.; Noh, K.; Lee, D. Dual Effect of Curcumin/BMP-2 Loaded in HA/PLL Hydrogels on Osteogenesis In Vitro and In Vivo. J. Nanosci. Nanotechnol. 2017, 17, 143–152. [Google Scholar] [CrossRef]
- Todeschi, M.R.; Backly, E.L.; Varghese, O.P.; Hilborn, J.; Cancedda, R.; Mastrogiacomo, M. Host cell recruitment patterns by bone morphogenetic protein-2 releasing hyaluronic acid hydrogels in a mouse subcutaneous environment. Regen. Med. 2017, 12, 525–539. [Google Scholar] [CrossRef]
- Zanchetta, P.; Lagarde, N.; Uguen, A.; Marcorelles, P. Mixture of hyaluronic acid, chondroitin 6 sulphate and dermatan sulphate used to completely regenerate bone in rat critical size defect model. J. Cranio Maxillofac. Surg. 2012, 40, 783–787. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef]
- Rajan Unnithan, A.; Ramachandra Kurup Sasikala, A.; Park, C.H.; Kim, C.S. A unique scaffold for bone tissue engineering: An osteogenic combination of graphene oxide–hyaluronic acid–chitosan with simvastatin. J. Ind. Eng. Chem. 2017, 46, 182–191. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef]
- Lee, Y.-J.; An, S.-J.; Bae, E.-B.; Gwon, H.-J.; Park, J.-S.; Jeong, S.I.; Jeon, Y.-C.; Lee, S.-H.; Lim, Y.-M.; Huh, J.-B. The Effect of Thickness of Resorbable Bacterial Cellulose Membrane on Guided Bone Regeneration. Materials 2017, 10, 320. [Google Scholar] [CrossRef]
- Ekholm, E.; Tommila, M.; Forsback, A.-P.; Märtson, M.; Holmbom, J.; Ääritalo, V.; Finnberg, C.; Kuusilehto, A.; Salonen, J.; Yli-Urpo, A.; et al. Hydroxyapatite coating of cellulose sponge does not improve its osteogenic potency in rat bone. Acta Biomater. 2005, 1, 535–544. [Google Scholar] [CrossRef]
- Torres-Rendon, J.G.; Femmer, T.; De Laporte, L.; Tigges, T.; Rahimi, K.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M.; et al. Bioactive Gyroid Scaffolds Formed by Sacrificial Templating of Nanocellulose and Nanochitin Hydrogels as Instructive Platforms for Biomimetic Tissue Engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef]
- Rodríguez, K.; Sundberg, J.; Gatenholm, P.; Renneckar, S. Electrospun nanofibrous cellulose scaffolds with controlled microarchitecture. Carbohydr. Polym. 2014, 100, 143–149. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun Bio-Nanocomposite Scaffolds for Bone Tissue Engineering by Cellulose Nanocrystals Reinforcing Maleic Anhydride Grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Salickd, M.R.; Cordiee, T.M.; Ellingham, T.; Dan, Y.; Turng, L.S. Incorporation of poly ( ethylene glycol ) grafted cellulose nanocrystals in poly ( lactic acid ) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef]
- Gorgieva, S.; Girandon, L.; Kokol, V. Mineralization potential of cellulose-nanofibrils reinforced gelatine sca ff olds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng. C 2017, 73, 478–489. [Google Scholar] [CrossRef]
- Si, J.; Cui, Z.; Wang, Q.; Liu, Q.; Liu, C. Biomimetic composite scaffolds based on mineralization of hydroxyapatite on electrospun poly(ɛ-caprolactone )/ nanocellulose fibers. Carbohydr. Polym. 2016, 143, 270–278. [Google Scholar] [CrossRef]
- Eftekhari, S.; El Sawi, I.; Bagheri, Z.S.; Turcotte, G.; Bougherara, H. Fabrication and characterization of novel biomimetic PLLA/cellulose/hydroxyapatite nanocomposite for bone repair applications. Mater. Sci. Eng. C 2014, 39, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, D.; Shin, S.; Hyun, J. Effect of negatively charged cellulose nanofibers on the dispersion of hydroxyapatite nanoparticles for scaffolds in bone tissue engineering. Colloids Surf. B Biointerfaces 2015, 130, 130–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C 2017, 75, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Sukul, M.; Min, Y.; Lee, S.; Lee, B. Osteogenic potential of simvastatin loaded gelatin-nanofibrillar cellulose- β tricalcium phosphate hydrogel scaffold in critical-sized rat calvarial defect. Eur. Polym. J. 2015, 73, 308–323. [Google Scholar] [CrossRef]
- Chen, Q.; Pérez Garcia, R.; Munoz, J.; Pérez de Larraya, U.; Garmendia, N.; Yao, Q.; Boccaccini, A.R. Cellulose Nanocrystals—Bioactive Glass Hybrid Coating as Bone Substitutes by Electrophoretic Co-deposition: In Situ Control of Mineralization of Bioactive Glass and Enhancement of Osteoblastic Performance. ACS Appl. Mater. Interfaces 2015, 7, 24715–24725. [Google Scholar] [CrossRef]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Yang, H.; Wang, Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef]
- Luo, H.; Li, J.; Zhou, F. Advances in Hard Tissue Engineering Materials—Nanocellulose-based Composites. PBM·Nanocellulose Based Compos. 2018, 3, 62–76. [Google Scholar]
- Kim, M.S.; Kim, G. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 2014, 114, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Cosme, J.G.L.; Xu, T.; Miszuk, J.M.; Picciani, P.H.S.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J. Biomed. Mater. Res. Part A 2010, 94, 241–251. [Google Scholar] [CrossRef]
- Chuenjitkuntaworn, B.; Osathanon, T.; Nowwarote, N.; Supaphol, P.; Pavasant, P. The efficacy of polycaprolactone/hydroxyapatite scaffold in combination with mesenchymal stem cells for bone tissue engineering. J. Biomed. Mater. Res. Part A 2015, 104, 264–271. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, W.-Z.; Shi, D.; Wu, M.; Xiong, X.-L.; Chen, Z.-G.; Wei, S.-C. Bioinspired and osteopromotive polydopamine nanoparticle-incorporated fibrous membranes for robust bone regeneration. NPG Asia Mater. 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chen, Y.W.; Wangde, K.; Shie, M.Y. Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(e-caprolactone) scaffolds by stereolithography. J. Mater. Chem. 2016, 4, 6307–6315. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B 2016, 104, 1210–1219. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.-C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 887–894. [Google Scholar]
- Holmes, B.; Bulusu, K.; Plesniak, M.; Zhang, L.G. A synergistic approach to the design, fabrication and evaluation of 3D printed micro and nano featured scaffolds for vascularized bone tissue repair. Nanotechnology 2016, 27, 064001. [Google Scholar] [CrossRef]
- Ren, Z.; Ma, S.; Jin, L.; Liu, Z.; Liu, D.; Zhang, X.; Cai, Q.; Yang, X. Repairing a bone defect with a three-dimensional cellular construct composed of a multi-layered cell sheet on electrospun mesh. Biofabrication 2017, 9, 025036. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hu, Y.; Wang, C.; Binks, B.P. Fabrication of Hierarchical Macroporous Biocompatible Scaffolds by Combining Pickering High Internal Phase Emulsion Templates with Three-Dimensional Printing. ACS Appl. Mater. Interfaces 2017, 9, 22950–22958. [Google Scholar] [CrossRef]
- Kim, S.S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Y.; Cui, Y.; Jing, X.; Zhang, P.; Chen, X. The nanocomposite scaffold of poly ( lactide-co-glycolide ) and hydroxyapatite surface-grafted with L -lactic acid oligomer for bone repair. Acta Biomater. 2009, 5, 2680–2692. [Google Scholar] [CrossRef]
- Ebrahimian-Hosseinabadi, M.; Ashrafizadeh, F.; Etemadifar, M.; Venkatraman, S.S. Evaluating and Modeling the Mechanical Properties of the Prepared PLGA/nano-BCP Composite Scaffolds for Bone Tissue Engineering. J. Mater. Sci. Technol. 2011, 27, 1105–1112. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, B.; Peng, S.; Li, Z. Development of composite porous scaffolds based via selective laser sintering. Int. J. Adv. Manuf. Technol. 2013, 69, 51–57. [Google Scholar] [CrossRef]
- Yun, Y.P.; Kim, S.E.; Kwon, I.K. Comparison of osteogenic differentiation from adipose-derived stem cells, mesenchymal stem cells, and pulp cells on PLGA/hydroxyapatite nanofiber. Tissue Eng. Regen. Med. 2009, 6, 336–345. [Google Scholar]
- Haider, A.; Gupta, K.C.; Kang, I. Morphological Effects of HA on the Cell Compatibility of Electrospun HA/PLGA Composite Nanofiber Scaffolds. Biomed. Res. Int. 2014, 2014, 308306. [Google Scholar] [CrossRef]
- Kang, S.; Yang, H.; Seo, S.; Han, D.; Kim, B. Apatite-coated poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for bone tissue. J. Biomed. Mater. Res. A 2007, 85, 747–756. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Ren, L.; Gong, Y.; Wang, D. Enhancing Alendronate Release from a Novel PLGA/Hydroxyapatite Microspheric System for Bone Repairing Applications. Pharm. Res. 2009, 26, 422–430. [Google Scholar] [CrossRef]
- Jakus, A.E.; Rutz, A.L.; Jordan, S.W.; Kannan, A.; Mitchell, S.M.; Yun, C.; Koube, K.D.; Yoo, S.C.; Whiteley, H.E.; Richter, C.-P.; et al. Hyperelastic ‘bone’: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Biomaterials 2016, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Cui, W.; Yang, F.; Min, C.; Shen, H.; Bei, J.; Wang, S. The effect of oxygen plasma pretreatment and incubation in modified simulated body fluids on the formation of bone-like apatite on poly ( lactide- co -glycolide ) ( 70/30 ). Biomaterials 2007, 28, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.C.; Lee, J.H.; Kim, M.J.; Park, J.H.; Kim, S.E.; Kim, J.S.; Oh, J.-W.; Han, D.-W. Biomimetic Hybrid Nanofiber Sheets Composed of RGD Peptide-Decorated PLGA as Cell-Adhesive Substrates. J. Funct. Biomater. 2015, 6, 367–378. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.-H.; Cho, H.-J.; Kim, H.K.; Yoon, T.R.; Shin, H. Electrospun fibers immobilized with bone forming peptide-1 derived from BMP7 for guided bone regeneration. Biomaterials 2013, 34, 5059–5069. [Google Scholar] [CrossRef] [PubMed]
| Bone | Young’s Modulus [8] | Compressive Strength [3] | Porosity [3,4] |
|---|---|---|---|
| Cortical | 15–20 GPa | 100–230 MPa | 5–30% |
| Trabecular | 0.1–2 GPa | 2–12 MPa | 30–95% |
| Acronym | Cell Type | Feature |
|---|---|---|
| BMSC | Bone marrow stem cell | Largely available from the body, they enhance osteoblasts differentiation. Invasive extraction procedure. |
| ADSC | Adipose-derived stem cells | Largely available, they recruit other cells from the bone. Lower osteogenic potential than BMSC. |
| UMSC | Umbilical cord mesenchymal stem cells | Largely available and non-invasive procedures. Ethical problems correlated to their usage. |
| DPSC | Dental pulp stem cells | Easy harvesting. Fast proliferation and possible differentiation in different types of cells. Lower ostogenic potential than BMSC. |
| PDLSC | Periodontal ligament stem cells | Reduction of proinflammarory cytokines. They induce both osteoblast commitment and vascularization. They need conditioned medium. |
| GMSC | Gingival mesenchymal stem cell | Reduction of proinflammarory cytokines. They need conditioned medium. |
| DFSC | Endometrial stem cell | High proliferation rate but weak osteogenic potential. |
| JBMSC | Jaw bone mesenchymal stem cells | Highly expandable. Good osteogenic potential. |
| AMSC | Amnion mesenchymal stem cells | Anti-inflammatory multipotent cells. Non-invasive harvest. Limited availability. |
| MDSC | Muscle-derived stromal cells | Good osteogenic potential. Contrasting results between human and animal cells. |
| PDSC | Periosteum-derived stem cells | Large availability, they produce functional tissue. Difficult extraction procedure. |
| iPS | Induced-pluripotent stem cells | Possible teratogenic cells in vivo. |
| Requirement | Description |
|---|---|
| Cytocompatibility | The released products should be non-toxic and non-inflammatory. |
| Bioactivity | Scaffold should interact with the tissue according to osteoinductive and osteoconductive principles. |
| Biodegradability | An ideal scaffold should degrade in a controlled way by external-enzymatic/biological process. |
| Suitable porosity | Interconnected pores are necessary for cell diffusion and migration. The scaffold should present micro porosity to guarantee enough surface area for its interaction with the tissue. Macro porosity is required for cell migration and cell growth. On the other hand, the porosity should not affect the mechanical stability. |
| Mechanical features | Scaffold should reproduce elastic and fatigue strength of the bones tissue site. |
| Tunable properties | Scaffold should have customizable properties. |
| Easy manufacturing, processing and handling | Scaffold should be easy to be fabricated and sterilized. Easy clinical manipulation is required. |
| Scaffold | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Natural polymers | Bioactivity Biomimetic surface Natural remodelling | Immunogenic response Microbial contamination Weak mechanical strength Lack of tunability Uncontrollable degradation rate | [39,44] |
| Collagen | Similar to ECM Cytocompatibility Enzymatic biodegradability Cytocompatibility and cell-binding properties Versatility in being processed in different physical forms Possible injectability FDA approved | Low mechanical strength Difficult disinfection Difficult handling | [48,49] |
| Gelatin | Cytocompatibility Biodegradability Porosity tunability Osteoconductivity | Poor mechanical properties Low stability in physiological conditions | [8,50] |
| Silk fibroin | Cytocompatibility Immunogenicity Flexible processability Limited biological adhesion High mechanical strength Thermal stability Easy chemical modification | [51,52] | |
| Chitosan | Cytocompatibility Biodegradability Cell-binding, differentiation and migration properties Antibacterial properties Mucoadhesivity Easy properties tunability | Poor mechanical strength and stability Rapid in vivo degradation rate | [8,53] |
| Alginate | Cytocompatibility Cytocompatibility Tuneable properties Easy gelling | Difficult to sterilize Low cell adhesion | [54,55] |
| Hyaluronic acid | Cytocompatibility Biodegradability Enzymatic biodegradability Viscoelasticity Easy manipulation Easy chemical functionalization | Poor mechanical strength Very rapid degradation | [56,57,58] |
| Cellulose | Hydrophilicity Cytocompatibility Bioactivity Optical transparency Tuneable properties | [38,59,60,61] | |
| Synthetic Aliphatic polymers | Tailored structure Predictable and reproducibility properties Water solubility Tuneable crystallinity Tuneable physical and mechanical properties FDA approved | Reduced bioactivity No cell recognition sites Low osteoconductivity Possible adverse tissue reaction for acid degradation product Lack of cellular adhesion | [39,44] |
| PCL | Cytocompatibility Biodegradability Slow degradation rate | Hydrophobicity Low bioactivity | [62] |
| PLA | Cytocompatibility Thermal stability Tuneable properties | [63] | |
| PLGA | Wide range of degradation rate Tunability | Suboptimal mechanical properties Poor osteoconductivity | [64,65] |
| Strategy | Inorganic Material Addition | Chemical Factor/Drug Addition | As Minor Component | Other Strategies |
|---|---|---|---|---|
| Main Aim | To increase the mechanical properties | To enhance osteoactivities | To increase cytocompatibility of other materials | |
| Collagen | Facilitate pore interconnectivity good porosity, cell infiltration, cell differentiation, angiogenesis and osteogenesis [69,70,71,72,73,74] | Appropriate scaffold porosity, swelling, and drug release [75,76]1 [90,91,104] | Increased osteogenesis, and osteoblast differentiation [68] | [66,67][75]1 |
| Gelatin | Relevant osteoconductive properties [80,81,89] [79,103,122] 1 | New bone formation [78,80][122] 1 | [77] | |
| Silk fibroin | Good stem cells differentiation, cells attachment, and osteogenesis [84,85,88][86] 1 | Increased osteogenic potential [83] | Increased compressive strenght of bone cement; increased prolifertion and osteogenic differantiation [87,88] | [82] |
| Chitosan | Good osteogenic cell differentiation [89,92,94][93] 1 | Suitable drug release and enhanced osteogenic differentiation [90,91,92] [75,93]1 [94,99,109] | [98] 1 | |
| Alginate | Osteodifferentiation, increased cell adhesion and osteogenesis [95] 1 [94,96,97] | Good results in cell viability, osteogenic differentiationand cell adhesion and controlled drugs release [94,95,99] | Increased osteogenesis in bone cement [98]1 | [100,101,102]1 |
| Cellulose | Increased osteoblast proliferation, differentiation, and osteoconductivity [118,119,121][120,122]1 | Controlled drug release, osteoblastic differantiation, and new bone formation [122]1 [117,124] | Increased compressive strenght and in vitro stability; improved cell attachement, viability, proliferation, and calcium deposition [115,116,117,123] | [111,112,114] [113] 1 |
| Hyaluronic acid | Good porosity, proliferation and mineralization [103,105] 1 | High cell adhesion, proliferaration and migration, vibility, and calcium deposition [104][105,105,106,107,108,109] 1 | [102] | |
| PCL | Increased cell attachment, proliferation, differentiation, calcium deposition, and bone formation [128,129,130,131,132] | Increased cellular attachment, angiogenesys, and osteogenesis [130,131] | [126,127] | |
| PLA | Good cell adhesion proliferation, and osteo-differentiation [134,136] | Rapid and complete drug release, good cell viability, proliferation, and osteogenic differentiation [136] | [133,135] | |
| PLGA | Good porosity, osteogenic potential, and mineralization activity, higher cellular adhesion/proliferatio, and new bone formation [137,138,139,140,141,142,144,145][143] 1 | Higher initial adhesion, increased proliferation, new bone formation, and suitable scaffold integration; controlled drug release. [144,147,148] | Good cell viability, proliferation, and osteogenic differentiation [145] | [146] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. https://doi.org/10.3390/polym12040905
Donnaloja F, Jacchetti E, Soncini M, Raimondi MT. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers. 2020; 12(4):905. https://doi.org/10.3390/polym12040905
Chicago/Turabian StyleDonnaloja, Francesca, Emanuela Jacchetti, Monica Soncini, and Manuela T. Raimondi. 2020. "Natural and Synthetic Polymers for Bone Scaffolds Optimization" Polymers 12, no. 4: 905. https://doi.org/10.3390/polym12040905
APA StyleDonnaloja, F., Jacchetti, E., Soncini, M., & Raimondi, M. T. (2020). Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers, 12(4), 905. https://doi.org/10.3390/polym12040905