Design of Thermoplastic 3D-Printed Scaffolds for Bone Tissue Engineering: Influence of Parameters of “Hidden” Importance in the Physical Properties of Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Polymer
2.3. Scaffold Design and Fabrication
2.4. Scaffolds Characterization
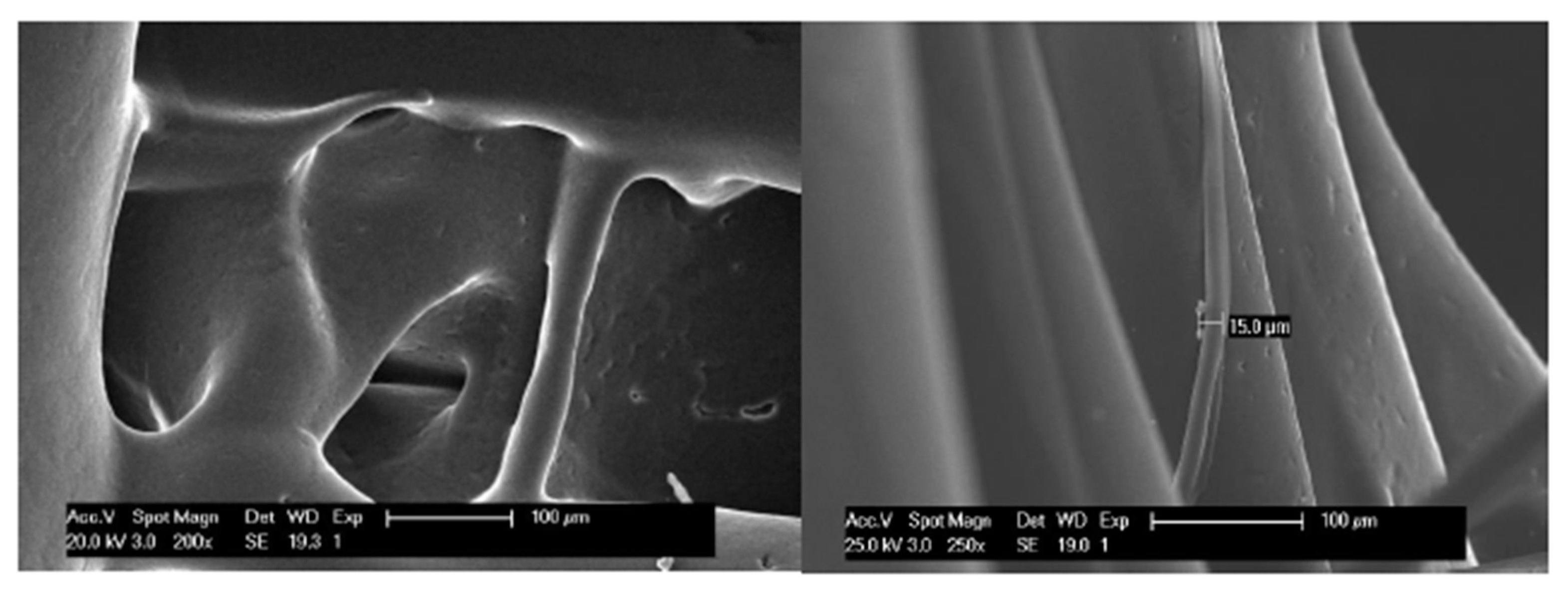
2.4.1. Microscopy: Optical and SEM
2.4.2. Mechanical Test
2.4.3. Dynamic Mechanical Analysis (DMA)
2.4.4. Statistical Analysis
3. Results
3.1. Polymer Characterization
3.2. Scaffolds Characterization
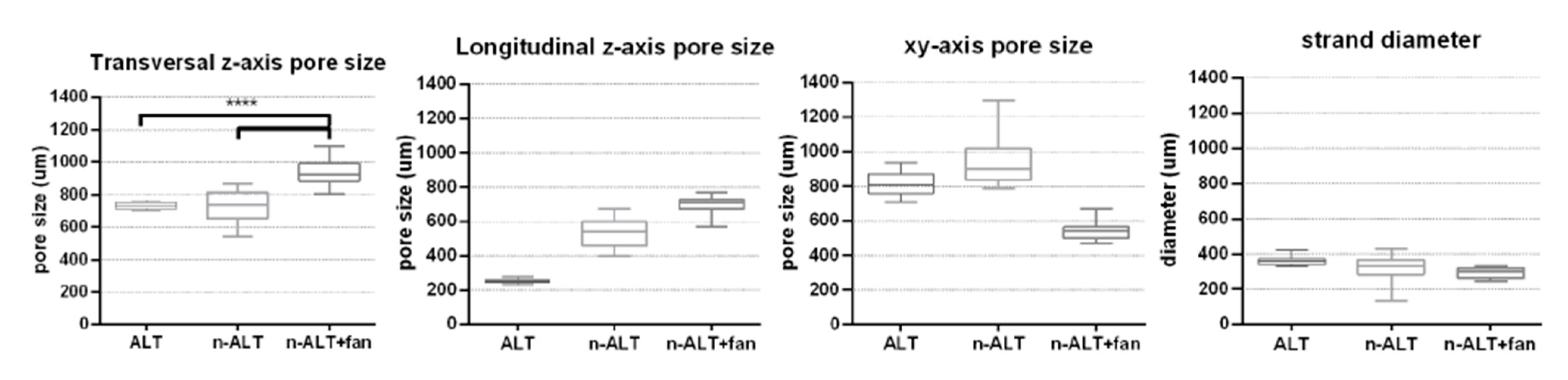
3.2.1. Pore Size and Distribution
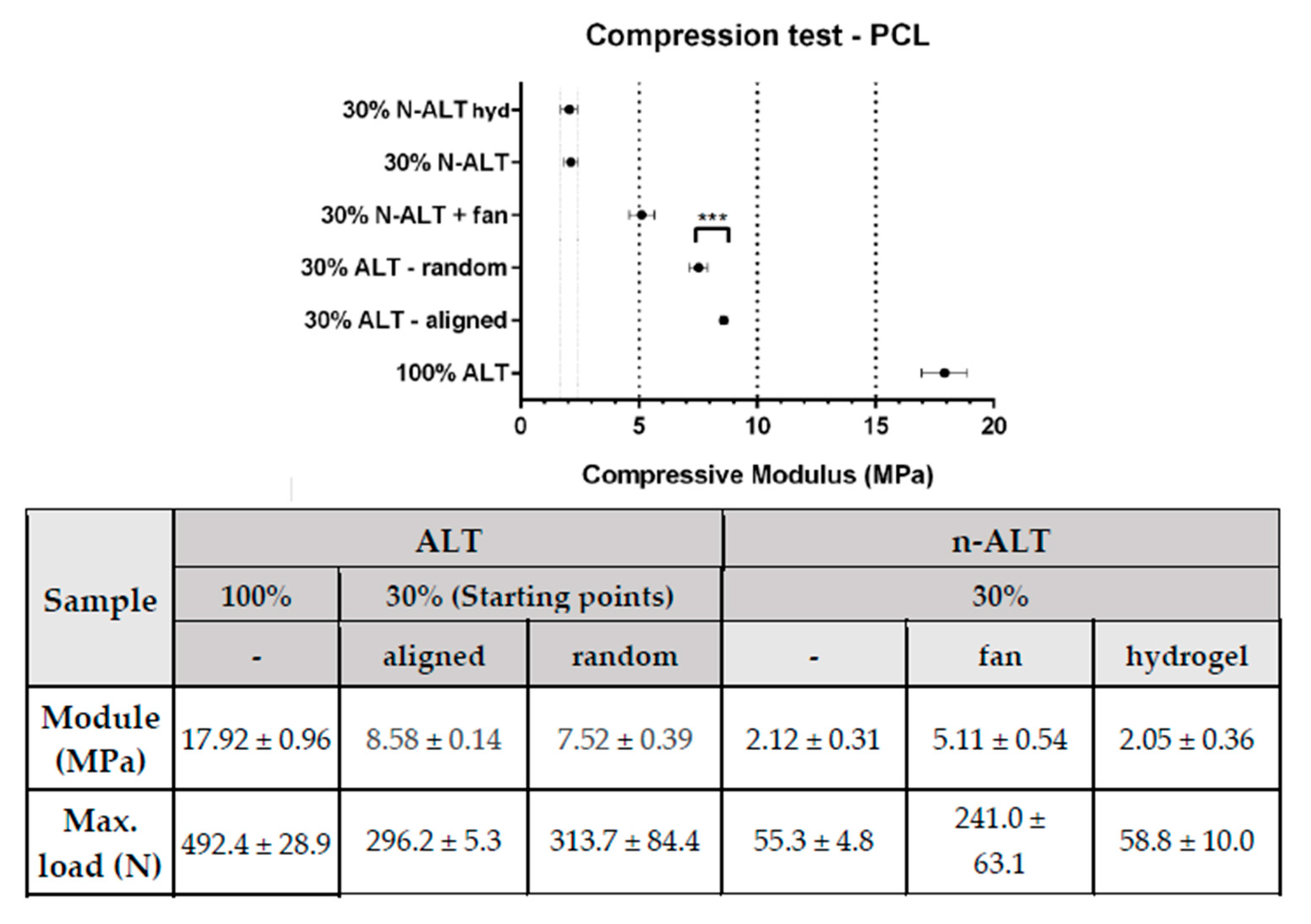
3.2.2. Compression Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuoco, T.; Ahlinder, A.; Jain, S.; Mustafa, K.; Finne-Wistrand, A. Poly(epsilon-caprolactone-co-p-dioxanone): A Degradable and Printable Copolymer for Pliable 3D Scaffolds Fabrication toward Adipose Tissue Regeneration. Biomacromolecules 2020, 21, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Rodriguez-Lorenzo, L.M. Biodegradable composite scaffolds with an interconnected spherical network for bone tissue engineering. Biomaterials 2004, 25, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Langford, C.R.; Rodriguez-Lorenzo, L.M.; Thissen, H.; Cameron, N.R. Bioceramic nanocomposite thiol-acrylate polyHIPE scaffolds for enhanced osteoblastic cell culture in 3D. Biomater. Sci. 2017, 5, 2035–2047. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.M.; Saldana, L.; Benito-Garzon, L.; Garcia-Carrodeguas, R.; de Aza, S.; Vilaboa, N.; Roman, J.S. Feasibility of ceramic-polymer composite cryogels as scaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 2012, 6, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J.; Flanagan, C.L.; Morrison, R.J.; Patel, J.J.; Wheeler, M.B.; Edwards, S.P.; Green, G.E. Integrating Image-Based Design and 3D Biomaterial Printing to create Patient Specific Devices within a Design Control Framework for Clinical Translation. ACS Biomater. Sci. Eng. 2016, 2, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Piard, C.; Baker, H.; Kamalitdinov, T.; Fisher, J. Bioprinted osteon-like scaffolds enhance in vivo neovascularization. Biofabrication 2019, 11, 025013. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fang, G.; Zhou, J. Additively Manufactured Scaffolds for Bone Tissue Engineering and the Prediction of their Mechanical Behavior: A Review. Materials 2017, 10, 50. [Google Scholar] [CrossRef]
- Pierantozzi, D.; Scalzone, A.; Jindal, S.; Stīpniece, L.; Šalma-Ancāne, K.; Dalgarno, K.; Gentile, P.; Mancuso, E. 3D printed Sr-containing composite scaffolds: Effect of structural design and material formulation towards new strategies for bone tissue engineering. Compos. Sci. Technol. 2020, 191, 108069. [Google Scholar] [CrossRef]
- Soufivand, A.A.; Abolfathi, N.; Hashemi, A.; Lee, S.J. The effect of 3D printing on the morphological and mechanical properties of polycaprolactone filament and scaffold. Polym. Adv. Technol. 2019. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- González-García, D.; Marcos-Fernández, Á.; Rodríguez-Lorenzo, L.; Jiménez-Gallegos, R.; Vargas-Becerril, N.; Téllez-Jurado, L. Synthesis and in Vitro Cytocompatibility of Segmented Poly(Ester-Urethane)s and Poly(Ester-Urea-Urethane)s for Bone Tissue Engineering. Polymers 2018, 10, 991. [Google Scholar] [CrossRef]
- Sánchez-Téllez, D.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef]
- Semba, J.A.; Mieloch, A.A.; Rybka, J.D. Introduction to the state-of-the-art 3D bioprinting methods, design, and applications in orthopedics. Bioprinting 2020, 18. [Google Scholar] [CrossRef]
- Liu, F.; Vyas, C.; Poologasundarampillai, G.; Pape, I.; Hinduja, S.; Mirihanage, W.; Bartolo, P. Structural Evolution of PCL during Melt Extrusion 3D Printing. Macromol. Mater. Eng. 2018, 303. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Fuoco, T.; Finne-Wistrand, A.; Pappalardo, D. A Route to Aliphatic Poly(ester)s with Thiol Pendant Groups: From Monomer Design to Editable Porous Scaffolds. Biomacromolecules 2016, 17, 1383–1394. [Google Scholar] [CrossRef]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.A.; Elisseeff, J.H.; Dorafshar, A.H.; Grayson, W.L. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef]
- Izadifar, Z.; Chang, T.; Kulyk, W.; Chen, X.; Eames, B.F. Analyzing Biological Performance of 3D-Printed, Cell-Impregnated Hybrid Constructs for Cartilage Tissue Engineering. Tissue Eng. Part. C Methods 2016, 22, 173–188. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.H.; Jang, J.; Kim, S.W.; Cho, D.W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Jang, K.M.; Hahn, S.K.; Park, J.Y.; Jung, H.; Oh, K.; Park, K.M.; Yeom, J.; Park, S.H.; Kim, S.W.; et al. Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint. Biofabrication 2016, 8, 014102. [Google Scholar] [CrossRef] [PubMed]
- Forrestal, D.P.; Klein, T.J.; Woodruff, M.A. Challenges in engineering large customized bone constructs. Biotechnol. Bioeng. 2017, 114, 1129–1139. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; Del Canizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef]
- Hernandez-Gonzalez, A.C.; Tellez-Jurado, L.; Rodriguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Liao, I.C.; Moutos, F.T.; Estes, B.T.; Zhao, X.; Guilak, F. Composite Three-Dimensional Woven Scaffolds with Interpenetrating Network Hydrogels to Create Functional Synthetic Articular Cartilage. Adv. Funct. Mater. 2013, 23, 5833–5839. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Téllez, D.A.; Rodríguez-Lorenzo, L.M.; Téllez-Jurado, L. Siloxane-inorganic chemical crosslinking of hyaluronic acid–based hybrid hydrogels: Structural characterization. Carbohydr. Polym. 2020, 230, 115590. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds–A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Moraczewski, K.; Stepczyńska, M.; Malinowski, R.; Budner, B.; Karasiewicz, T.; Jagodziński, B. Selected properties of polycaprolactone containing natural anti-aging compounds. Adv. Polym. Technol. 2018, 37, 3499–3510. [Google Scholar] [CrossRef]
- Choi, B.-H.; Jo, Y.K.; Zhou, C.; Jang, H.-S.; Ahn, J.-S.; Jun, S.H.; Cha, H.J. Sticky bone-specific artificial extracellular matrix for stem cell-mediated rapid craniofacial bone therapy. Appl. Mater. Today 2020, 18. [Google Scholar] [CrossRef]
- Uto, K.; Mano, S.S.; Aoyagi, T.; Ebara, M. Substrate Fluidity Regulates Cell Adhesion and Morphology on Poly(ε-caprolactone)-Based Materials. ACS Biomater. Sci. Eng. 2016, 2, 446–453. [Google Scholar] [CrossRef]
- Moroni, L.; de Wijn, J.R.; van Blitterswijk, C.A. 3D fiber-deposited scaffolds for tissue engineering: Influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006, 27, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Singh, A.K.; Kar, N.; Dravid, A.; Bellare, J. Modelling and optimization of NaOH-etched 3-D printed PCL for enhanced cellular attachment and growth with minimal loss of mechanical strength. Mater. Sci. Eng. C 2019, 98, 602–611. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.M.; Salinas, A.J.; Vallet-Regi, M.; San Roman, J. Composite biomaterials based on ceramic polymers. I. Reinforced systems based on Al2O3/PMMA/PLLA. J. Biomed. Mater. Res. 1996, 30, 515–522. [Google Scholar] [CrossRef]
- Samadi, K.; Francisco, M.; Hegde, S.; Diaz, C.A.; Trabold, T.A.; Dell, E.M.; Lewis, C.L. Mechanical, rheological and anaerobic biodegradation behavior of a Poly(lactic acid) blend containing a Poly(lactic acid)-co-poly(glycolic acid) copolymer. Polym. Degrad. Stab. 2019, 170. [Google Scholar] [CrossRef]
- Wang, S.-J.; Zhang, Z.-Z.; Jiang, D.; Qi, Y.-S.; Wang, H.-J.; Zhang, J.-Y.; Ding, J.-X.; Yu, J.-K. Thermogel-Coated Poly(ε-Caprolactone) Composite Scaffold for Enhanced Cartilage Tissue Engineering. Polymers 2016, 8, 200. [Google Scholar] [CrossRef]
- Ngadiman, N.; Yusof, N.; Idris, A.; Fallahiarezoudar, E.; Kurniawan, D. Novel Processing Technique to Produce Three Dimensional Polyvinyl Alcohol/Maghemite Nanofiber Scaffold Suitable for Hard Tissues. Polymers 2018, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Q.; Yang, J.-H.; Ding, X.; Ding, X.; Duan, S.; Xu, F.-J. Polycaprolactone/polysaccharide functional composites for low-temperature fused deposition modelling. Bioact. Mater. 2020, 5, 185–191. [Google Scholar] [CrossRef]
- Brown, T.D.; Slotosch, A.; Thibaudeau, L.; Taubenberger, A.; Loessner, D.; Vaquette, C.; Dalton, P.D.; Hutmacher, D.W. Design and Fabrication of Tubular Scaffolds via Direct Writing in a Melt Electrospinning Mode. Biointerphases 2012, 7. [Google Scholar] [CrossRef]
- Shahabipour, F.; Ashammakhi, N.; Oskuee, R.K.; Bonakdar, S.; Hoffman, T.; Shokrgozar, M.A.; Khademhosseini, A. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl. Res. 2020, 216, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.P.; Hutton, D.L.; Hung, B.P.; Huri, P.Y.; Cook, C.A.; Kondragunta, R.; Jia, X.; Grayson, W.L. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J. Biomed. Mater. Res. Part A 2014. [Google Scholar] [CrossRef]
- Grayson, W.L.; Bhumiratana, S.; Cannizzaro, C.; Chao, P.H.G.; Lennon, D.P.; Caplan, A.I.; Vunjak-Novakovic, G. Effects of Initial Seeding Density and Fluid Perfusion Rate on Formation of Tissue-Engineered Bone. Tissue Eng. Part A 2008, 14, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.J.; O’Brien, F.J. Influence of Shear Stress in Perfusion Bioreactor Cultures for the Development of Three-Dimensional Bone Tissue Constructs: A Review. Tissue Eng. Part B Rev. 2010, 16, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Mehrian, M.; Guyot, Y.; Papantoniou, I.; Olofsson, S.; Sonnaert, M.; Misener, R.; Geris, L. Maximizing neotissue growth kinetics in a perfusion bioreactor: An in silico strategy using model reduction and Bayesian optimization. Biotechnol. Bioeng. 2018, 115, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Duymaz, B.T.; Erdiler, F.B.; Alan, T.; Aydogdu, M.O.; Inan, A.T.; Ekren, N.; Uzun, M.; Sahin, Y.M.; Bulus, E.; Oktar, F.N.; et al. 3D bio-printing of levan/polycaprolactone/gelatin blends for bone tissue engineering: Characterization of the cellular behavior. Eur. Polym. J. 2019, 119, 426–437. [Google Scholar] [CrossRef]
- Yilmaz, B.; Tahmasebifar, A.; Baran, E.T. Bioprinting Technologies in Tissue Engineering. In Current Applications of Pharmaceutical Biotechnology; Springer: Berlin, Germany, 2019; pp. 279–319. [Google Scholar] [CrossRef]
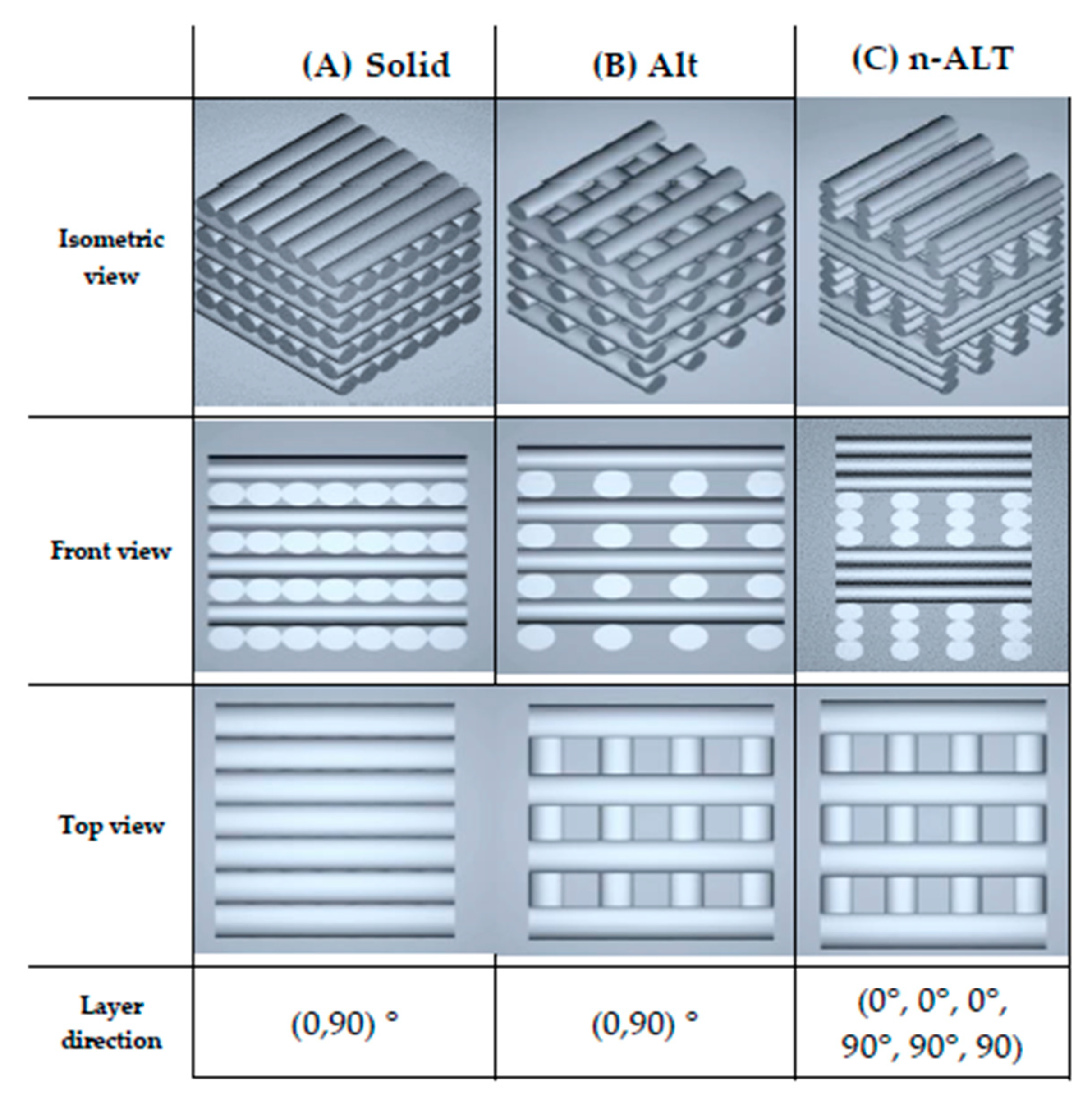


| n | Infill | Layers | Sample Abbrv. | Other | Notes |
|---|---|---|---|---|---|
| 1 | 100% | ALT | 100% ALT | solid | Original cylinder for comparison, no pores. |
| 2 | 30% | ALT | 30% ALT-aligned | aligned starting point | Porous scaffold, with anisotropy in the Z-axis Aligned start point (seam) |
| 3 | 30% ALT-random | random starting point | Porous scaffold, with anisotropy in the Z-axis Random start point | ||
| 4 | 30% | n-ALT | 30% n-ALT | RT cool down | Porous scaffold, with more isotropy in the Z-axis Slow cooling down (room temperature) |
| 5 | 30% n-ALT+fan | external fan | Porous scaffold, with isotropy in the Z-axis Forced cooling down (external fan) | ||
| 6 | 30% n-ALT+hyd | filled - hydrogel | Porous PCL scaffold, filled with crosslinked hydrogel |
| Tg | Tf | Tdeg | |
|---|---|---|---|
| Ref. T [7] | −62 °C | 55–60 °C | - |
| TGA | - | - | 392–435 °C |
| DSC | −64 °C | 51 °C | - |
| DMA (10 Hz) | −61 °C | 53 °C | - |
| Sample | z-axis | xy-axis Pore Size (μm) | Strand Diameter (μm) | Homogeneous Distribution? (Pore and Strand) | ||
|---|---|---|---|---|---|---|
| Pore Size (μm) | Trans. vs. Long. Ratio | |||||
| Transversal | Longitudinal | |||||
| 30% ALT | 733 ± 22 (3%) | 254 ± 13 (5%) | 2.90 ± 0.24 (8%) | 816 ± 61 (8%) | 361 ± 26 (7%) | ✓—huge anisotropy |
| 30% n-ALT+ fan | 938 ± 80 (9%) | 689 ± 60 (9%) | 1.37 ± 0.22 (16%) | 542 ± 48 (9%) | 290 ± 30 (10%) | ✓—more isotropic pores in z-axis |
| 30% n-ALT (RT) | 728 ± 90 (12%) | 532 ± 87 (16%) | 1.43 ± 0.40 (27%) | 930 ± 123 (13%) | 314 ± 87 (28%) | ✗—higher variability |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubo-Mateo, N.; Rodríguez-Lorenzo, L.M. Design of Thermoplastic 3D-Printed Scaffolds for Bone Tissue Engineering: Influence of Parameters of “Hidden” Importance in the Physical Properties of Scaffolds. Polymers 2020, 12, 1546. https://doi.org/10.3390/polym12071546
Cubo-Mateo N, Rodríguez-Lorenzo LM. Design of Thermoplastic 3D-Printed Scaffolds for Bone Tissue Engineering: Influence of Parameters of “Hidden” Importance in the Physical Properties of Scaffolds. Polymers. 2020; 12(7):1546. https://doi.org/10.3390/polym12071546
Chicago/Turabian StyleCubo-Mateo, Nieves, and Luis M. Rodríguez-Lorenzo. 2020. "Design of Thermoplastic 3D-Printed Scaffolds for Bone Tissue Engineering: Influence of Parameters of “Hidden” Importance in the Physical Properties of Scaffolds" Polymers 12, no. 7: 1546. https://doi.org/10.3390/polym12071546
APA StyleCubo-Mateo, N., & Rodríguez-Lorenzo, L. M. (2020). Design of Thermoplastic 3D-Printed Scaffolds for Bone Tissue Engineering: Influence of Parameters of “Hidden” Importance in the Physical Properties of Scaffolds. Polymers, 12(7), 1546. https://doi.org/10.3390/polym12071546






