Influence of Ultraviolet Radiation Exposure Time on Styrene-Ethylene-Butadiene-Styrene (SEBS) Copolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Exposure Conditions
2.3. Mechanical Characterization
2.4. Colorimetry
2.5. Optical Contact Angle Analysis (OCA)
2.6. Attenuated Total Reflectance Infrared Spectroscopy (FTIR–ATR) Surface Analysis
2.7. Surface Morphology Study
3. Results and Discussion
3.1. FTIR–ATR Surface Analysis
3.2. Surface Morphology Study
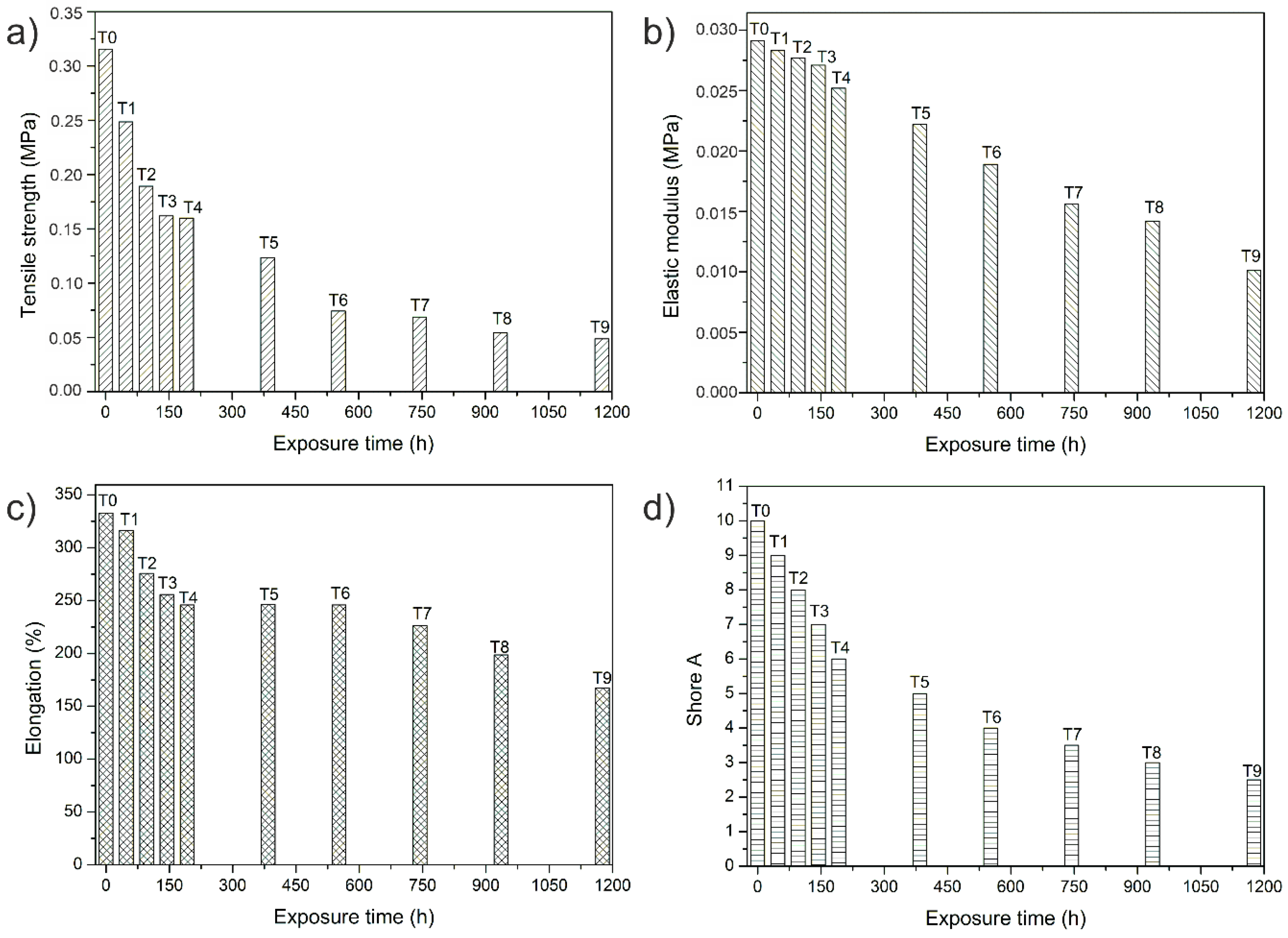
3.3. Mechanical Properties
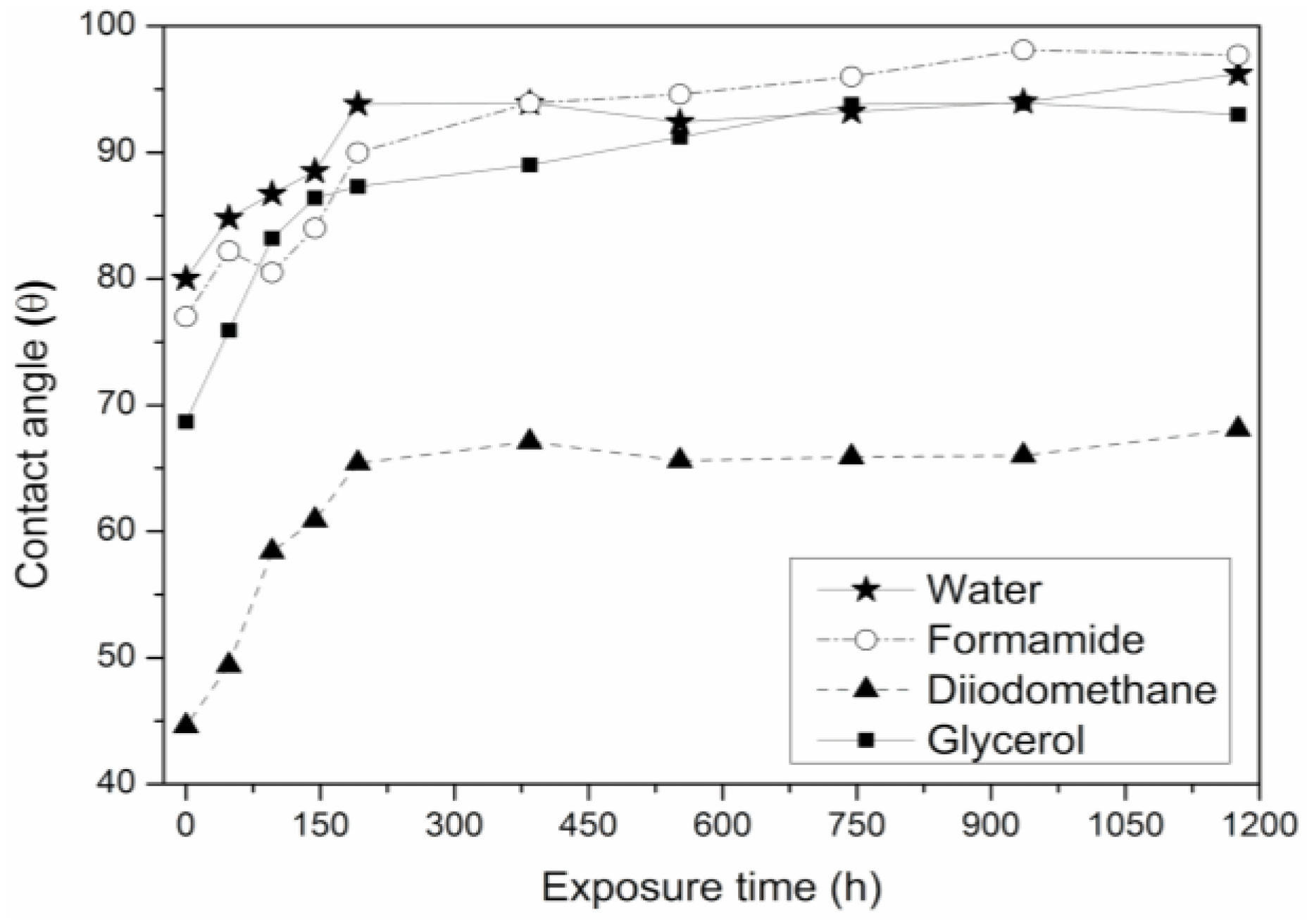
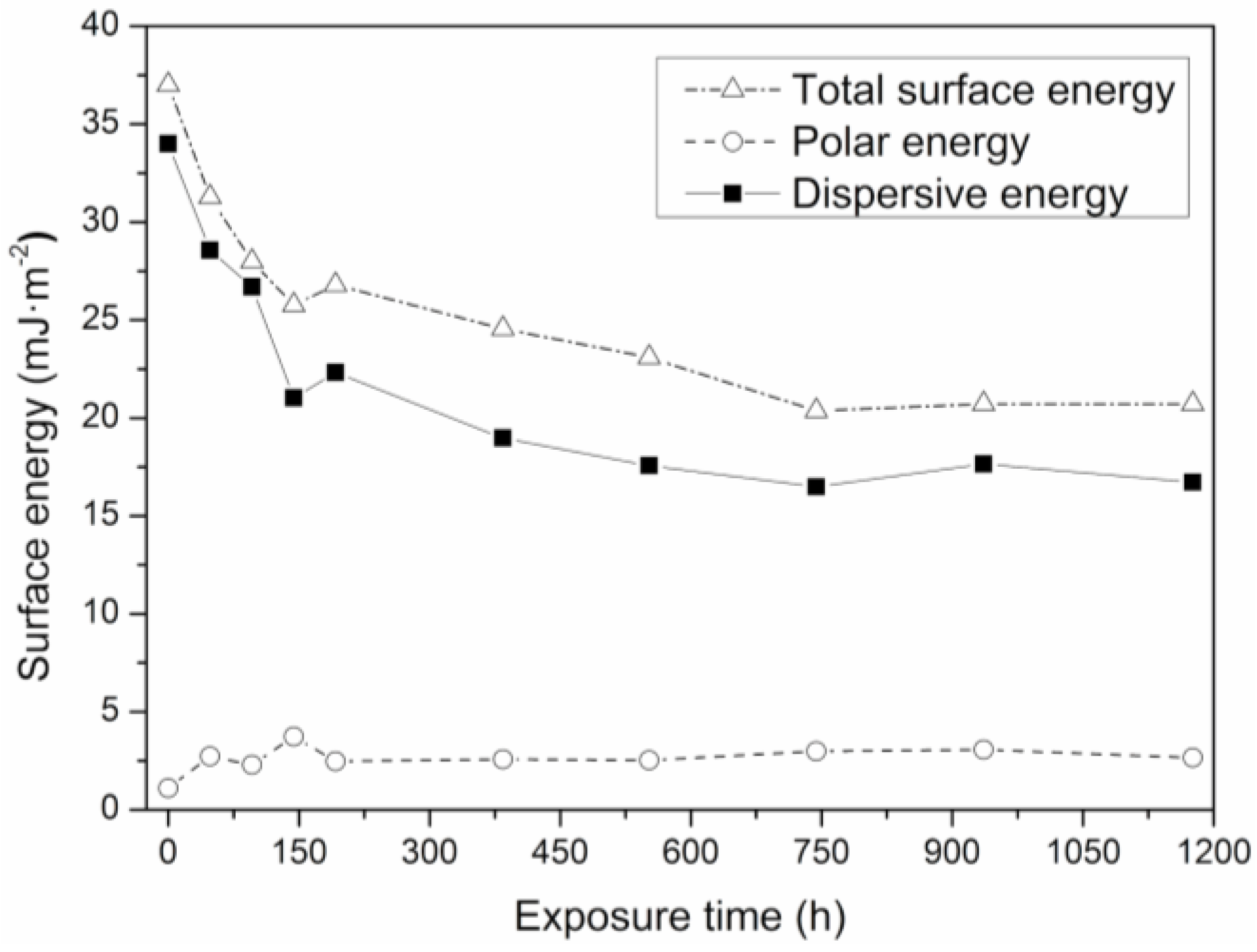
3.4. Analysis of Optical Contact Angle (OCA) and Surface Energy (γs)
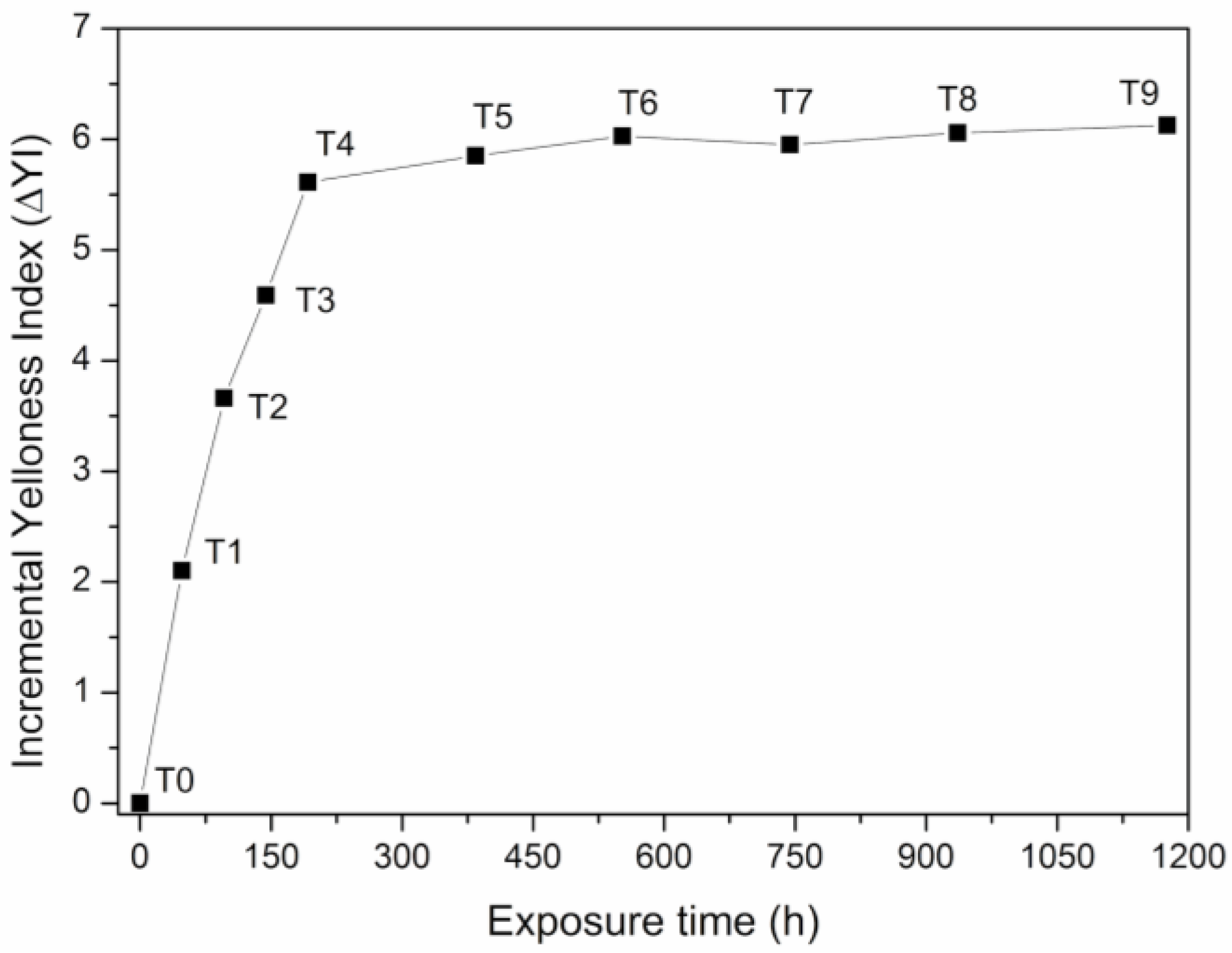
3.5. Yellowness Index (YI)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Holden, G. Thermoplastic elastomers. In Rubber Technology; Springer: Berlin, Germany, 1987; pp. 465–481. [Google Scholar]
- Picchioni, F.; Giorgi, I.; Passaglia, E.; Ruggeri, G.; Aglietto, M. Blending of styrene-block-butadiene-block-styrene copolymer with sulfonated vinyl aromatic polymers. Polym. Int. 2001, 50, 714–721. [Google Scholar] [CrossRef]
- Walker, B.M.; Rader, C.P. Handbook of Thermoplastic Elastomers; Van Nostrand Reinhold: New York, NY, USA, 1979. [Google Scholar]
- Zhu, J.; Birgisson, B.; Kringos, N. Polymer modification of bitumen: Advances and challenges. Eur. Polym. J. 2014, 54, 18–38. [Google Scholar] [CrossRef]
- Švec, P. Styrene-Based Plastics and Their Modification; Ellis Horwood: Hemel Hempstead, UK, 1990. [Google Scholar]
- Allen, N.S.; Edge, M. Fundamentals of Polymer Degradation and Stabilization; Springer: Berlin, Germany, 1992. [Google Scholar]
- Gupta, S.; Chandra, T.; Sikder, A.; Menon, A.; Bhowmick, A.K. Accelerated weathering behavior of poly (phenylene ether)-based tpe. J. Mater. Sci. 2008, 43, 3338–3350. [Google Scholar] [CrossRef]
- Mamodia, M.; Indukuri, K.; Atkins, E.T.; De Jeu, W.H.; Lesser, A.J. Hierarchical description of deformation in block copolymer tpes. J. Mater. Sci. 2008, 43, 7035–7046. [Google Scholar] [CrossRef]
- Mistarli, F.; Proni, A. Styrenic Block Copolymers; Applied Science: London, UK, 1982. [Google Scholar]
- Allen, N.S.; Edge, M.; Wilkinson, A.; Liauw, C.M.; Mourelatou, D.; Barrio, J.; Martínez-Zaporta, M.A. Degradation and stabilisation of styrene–ethylene–butadiene–styrene (SEBS) block copolymer. Polym. Degrad. Stab. 2000, 71, 113–122. [Google Scholar] [CrossRef]
- Costa, P.; Ribeiro, S.; Botelho, G.; Machado, A.; Mendez, S.L. Effect of butadiene/styrene ratio, block structure and carbon nanotube content on the mechanical and electrical properties of thermoplastic elastomers after UV ageing. Polym. Test. 2015, 42, 225–233. [Google Scholar] [CrossRef]
- Tomacheski, D.; Pittol, M.; Lopes, A.P.M.; Simões, D.N.; Ribeiro, V.F.; Santana, R.M.C. Effects of weathering on mechanical, antimicrobial properties and biodegradation process of silver loaded tpe compounds. J. Polym. Environ. 2018, 26, 73–82. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- White, C.; Tan, K.; Hunston, D.; Nguyen, T.; Benatti, D.; Stanley, D.; Chin, J. Laboratory accelerated and natural weathering of styrene–ethylene–butylene–styrene (SEBS) block copolymer. Polym. Degrad. Stab. 2011, 96, 1104–1110. [Google Scholar] [CrossRef]
- Allen, N.S.; Luengo, C.; Edge, M.; Wilkinson, A.; Parellada, M.D.; Barrio, J.A.; Santa Quiteria, V.R. Photooxidation of styrene–ethylene–butadiene–styrene (SEBS) block copolymer. J. Photochem. Photobiol. A Chem. 2004, 162, 41–51. [Google Scholar] [CrossRef]
- Flaris, V.; Stachurski, Z.H. The effects of processing on the mechanical properties of a polyolefin blend. Polym. Int. 1992, 27, 267–273. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, Y.; Zhao, S.; Xie, L.; Yao, S. Improving the aging resistance of styrene-butadiene-styrene tri-block copolymer and application in polymer-modified asphalt. J. Appl. Polym. Sci. 2010, 116, 754–761. [Google Scholar] [CrossRef]
- Xu, X.; Yu, J.; Xue, L.; Zhang, C.; He, B.; Wu, M. Structure and performance evaluation on aged sbs modified bitumen with bi-or tri-epoxy reactive rejuvenating system. Construct. Build. Mater. 2017, 151, 479–486. [Google Scholar] [CrossRef]
- Bachir, D.S.; Dekhli, S.; Mokhtar, K.A. Rheological evaluation of ageing properties of sebs polymer modified bitumens. Periodica Polytech. Civ. Eng. 2016, 60, 397–404. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Poisson, C.; Hervais, V.; Lacrampe, M.; Krawczak, P. Optimization of pe/binder/pa extrusion blow-molded films. Ii. Adhesion properties improvement using binder/eva blends. J. Appl. Polym. Sci. 2006, 101, 118–127. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, W.; Yu, Y.; Li, B.; Wu, C. Structure and properties of compatibilized recycled poly(ethylene terephthalate)/linear low density polyethylene blends. Eur. Polym. J. 2007, 43, 3662–3670. [Google Scholar] [CrossRef]
- Guerrica-Echevarria, G.; Eguiazábal, J.; Nazabal, J. Influence of compatibilization on the mechanical behavior of poly(trimethylene terephthalate)/poly(ethylene–octene) blends. Eur. Polym. J. 2007, 43, 1027–1037. [Google Scholar] [CrossRef]
- Chang, Y.W.; Shin, J.Y.; Ryu, S.H. Preparation and properties of styrene–ethylene/butylene–styrene (sebs)–clay hybrids. Polym. Int. 2004, 53, 1047–1051. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, S.M.; Chen, C.M. Preparation and properties of styrene–ethylene–butylene–styrene block copolymer/clay nanocomposites: I. Effect of clay content and compatibilizer types. Polym. Int. 2008, 57, 515–522. [Google Scholar] [CrossRef]
- Qin, R.-Y.; Schreiber, H. Adhesion at partially restructured polymer surfaces. Colloid Surf. A Physicochem. Eng. Aspects 1999, 156, 85–93. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, F.; Pen, Z.; Zhang, Y.; Zhang, Y.; Zhou, W. Effect of nucleating agent on the structure and properties of polypropylene/poly(ethylene–octene) blends. Eur. Polym. J. 2002, 38, 1–6. [Google Scholar] [CrossRef]
- Beholz, L.; Aronson, C.; Zand, A. Adhesion modification of polyolefin surfaces with sodium hypochlorite in acidic media. Polymer 2005, 46, 4604–4613. [Google Scholar] [CrossRef]
- Kinloch, A. The science of adhesion. J. Mater. Sci. 1980, 15, 2141–2166. [Google Scholar] [CrossRef]
- Lippert, T.; Dickinson, J.T. Chemical and spectroscopic aspects of polymer ablation: Special features and novel directions. Chem. Rev. 2003, 103, 453–486. [Google Scholar] [CrossRef]
- van der Leeden, M.C.; Frens, G. Surface properties of plastic materials in relation to their adhering performance. Adv. Eng. Mater. 2002, 4, 280–289. [Google Scholar] [CrossRef]
- Pukánszky, B. Interfaces and interphases in multicomponent materials: Past, present, future. Eur. Polym. J. 2005, 41, 645–662. [Google Scholar] [CrossRef]
- Tjong, S.C.; Xu, S.-A.; Mai, Y.-W. Tensile deformation mechanism of polyamide 6, 6/sebs-g-ma blend and its hybrid composites reinforced with short glass fibers. J. Mater. Sci. 2003, 38, 207–215. [Google Scholar] [CrossRef]
- Sanchis, R.; Fenollar, O.; García, D.; Sánchez, L.; Balart, R. Improved adhesion of ldpe films to polyolefin foams for automotive industry using low-pressure plasma. Int. J. Adhes. Adhes. 2008, 28, 445–451. [Google Scholar] [CrossRef]
- Brockmann, W.; Hüther, R. Adhesion mechanisms of pressure sensitive adhesives. Int. J. Adhes. Adhes. 1996, 16, 81–86. [Google Scholar] [CrossRef]
- Brovko, O.; Rosso, P.; Friedrich, K. Adhesion between differently treated fibers and a hybrid resin system. J. Mater. Sci. Lett. 2002, 21, 305–308. [Google Scholar] [CrossRef]
- Court, R.; Sutcliffe, M.; Tavakoli, S. Ageing of adhesively bonded joints—fracture and failure analysis using video imaging techniques. Int. J. Adhes. Adhes. 2001, 21, 455–463. [Google Scholar] [CrossRef]
- Komvopoulos, K. Adhesion and friction forces in microelectromechanical systems: Mechanisms, measurement, surface modification techniques, and adhesion theory. J. Adhes. Sci. Technol. 2003, 17, 477–517. [Google Scholar] [CrossRef]
- Pijpers, A.; Meier, R.J. Adhesion behaviour of polypropylenes after flame treatment determined by XPS (ESCA) spectral analysis. J. Electron Spectr. Relat. Phenom. 2001, 121, 299–313. [Google Scholar] [CrossRef]
- Yu, S.; Hu, H.; Zhang, Y.; Liu, Y. Effect of transfer film on tribological behavior of polyamide 66-based binary and ternary nanocomposites. Polym. Int. 2008, 57, 454–462. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Comparative study on the surface free energy of a solid calculated by different methods. Polym. Test. 2007, 26, 14–19. [Google Scholar] [CrossRef]
- Kumar, S.; Misra, R. Analysis of banana fibers reinforced low-density polyethylene/poly (ε-caprolactone) composites. Soft Mater. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Fowkes, F.; McCarthy, D.; Mostafa, M. Contact angles and the equilibrium spreading pressures of liquids on hydrophobic solids. J. Colloid Interf. Sci. 1980, 78, 200–206. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, S.; Myung, S.-W.; Lu, N.; Choi, H.-S. Effect of washing on surface free energy of polystyrene plate treated by rf atmospheric pressure plasma. Polym. Test. 2006, 25, 327–332. [Google Scholar] [CrossRef]
- Hänni-Ciunel, K.; Findenegg, G.H.; von Klitzing, R. Water contact angle on polyelectrolyte-coated surfaces: Effects of film swelling and droplet evaporation. Soft Mater. 2007, 5, 61–73. [Google Scholar] [CrossRef]
- Sheiko, S.S. Imaging of polymers using scanning force microscopy: From superstructures to individual molecules. In New Developments in Polymer Analytics II; Springer: Berlin, Germany, 2000; pp. 61–174. [Google Scholar]
- Radovanovic, E.; Carone, E., Jr.; Goncalves, M. Comparative afm and tem investigation of the morphology of nylon6-rubber blends. Polym. Test. 2004, 23, 231–237. [Google Scholar] [CrossRef]
- Drnovska, H.; Lapčík, L.; Buršíková, V.; Zemek, J.; Barros-Timmons, A.M. Surface properties of polyethylene after low-temperature plasma treatment. Colloid Polym. Sci. 2003, 281, 1025–1033. [Google Scholar] [CrossRef]
- Lehocký, M.; Drnovská, H.; Lapčíková, B.; Barros-Timmons, A.; Trindade, T.; Zembala, M.; Lapčík, L.R., Jr. Plasma surface modification of polyethylene. Colloid Surf. A Physicochem. Eng. Aspects 2003, 222, 125–131. [Google Scholar] [CrossRef]
- Ortiz-Magán, A.B.; Pastor-Blas, M.M.; Ferrándiz-Gómez, T.P.; Morant-Zacarés, C.; Martín-Martínez, J.M. Surface modifications produced by N2 and O2 rf plasma treatment on a synthetic vulcanized styrene-butadiene rubber. Plasmas Polym. 2001, 6, 81–105. [Google Scholar] [CrossRef]
- Mailhot, B.; Gardette, J.L. Polystyrene photooxidation. 2. A pseudo wavelength effect. Macromolecules 1992, 25, 4127–4133. [Google Scholar] [CrossRef]
- Mailhot, B.; Jarroux, N.; Gardette, J.-L. Comparative analysis of the photo-oxidation of polystyrene and poly (α-methylstyrene). Polym. Degrad. Stab. 2000, 68, 321–326. [Google Scholar] [CrossRef]
- Luengo, C.; Allen, N.S.; Edge, M.; Wilkinson, A.; Parellada, M.D.; Barrio, J.A.; Santa, V.R. Photo-oxidative degradation mechanisms in styrene–ethylene–butadiene–styrene (SEBS) triblock copolymer. Polym. Degrad. Stab. 2006, 91, 947–956. [Google Scholar] [CrossRef]
- Han, X.; Zhou, L.; Liu, H.; Hu, Y. Effect of in situ oxidization with potassium permanganate on the morphologies of sebs membranes. Polym. Degrad. Stab. 2007, 92, 75–85. [Google Scholar] [CrossRef]
- McKellar, J.F.; Allen, N.S. Photochemistry of Man-Made Polymers; Applied Science: London, UK, 1979. [Google Scholar]
- Allen, N.S.; Edge, M.; Mourelatou, D.; Wilkinson, A.; Liauw, C.M.; Parellada, M.D.; Barrio, J.A.; Santa Quiteria, V.R. Influence of ozone on styrene-ethylene-butylene-styrene (SEBS) copolymer. Polym. Degrad. Stab. 2003, 79, 297–307. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Bellobono, I.; Selli, E. Current Trends in Polymer Photochemistry; Allen, N.S., Edge, M., Bellobono, I., Selli, E., Eds.; Ellis Horwood Ltd.: Hemel Hempstead, UK, 1995. [Google Scholar]
- Fombuena, V.; Balart, J.; Boronat, T.; Sánchez-Nácher, L.; Garcia-Sanoguera, D. Improving mechanical performance of thermoplastic adhesion joints by atmospheric plasma. Mater. Des. 2013, 47, 49–56. [Google Scholar] [CrossRef]
- Sanchis, M.; Calvo, O.; Fenollar, O.; Garcia, D.; Balart, R. Characterization of the surface changes and the aging effects of low-pressure nitrogen plasma treatment in a polyurethane film. Polym. Test. 2008, 27, 75–83. [Google Scholar] [CrossRef]
- Sheikhy, H.; Shahidzadeh, M.; Ramezanzadeh, B.; Noroozi, F. Studying the effects of chain extenders chemical structures on the adhesion and mechanical properties of a polyurethane adhesive. J. Indust. Eng. Chem. 2013, 19, 1949–1955. [Google Scholar] [CrossRef]
- Svab, I.; Musil, V.; Smit, I.; Makarovic, M. Mechanical properties of wollastonite-reinforced polypropylene composites modified with sebs and SEBS-g-MA elastomers. Polym. Eng. Sci. 2007, 47, 1873. [Google Scholar] [CrossRef]
- Sanchis, M.; Blanes, V.; Blanes, M.; Garcia, D.; Balart, R. Surface modification of low density polyethylene (LDPE) film by low pressure O2 plasma treatment. Eur. Polym. J. 2006, 42, 1558–1568. [Google Scholar] [CrossRef]
- Ganguly, A.; Bhowmick, A.K. Effect of polar modification on morphology and properties of styrene-(ethylene-co-butylene)-styrene triblock copolymer and its montmorillonite clay-based nanocomposites. J. Mater. Sci. 2009, 44, 903–918. [Google Scholar] [CrossRef]
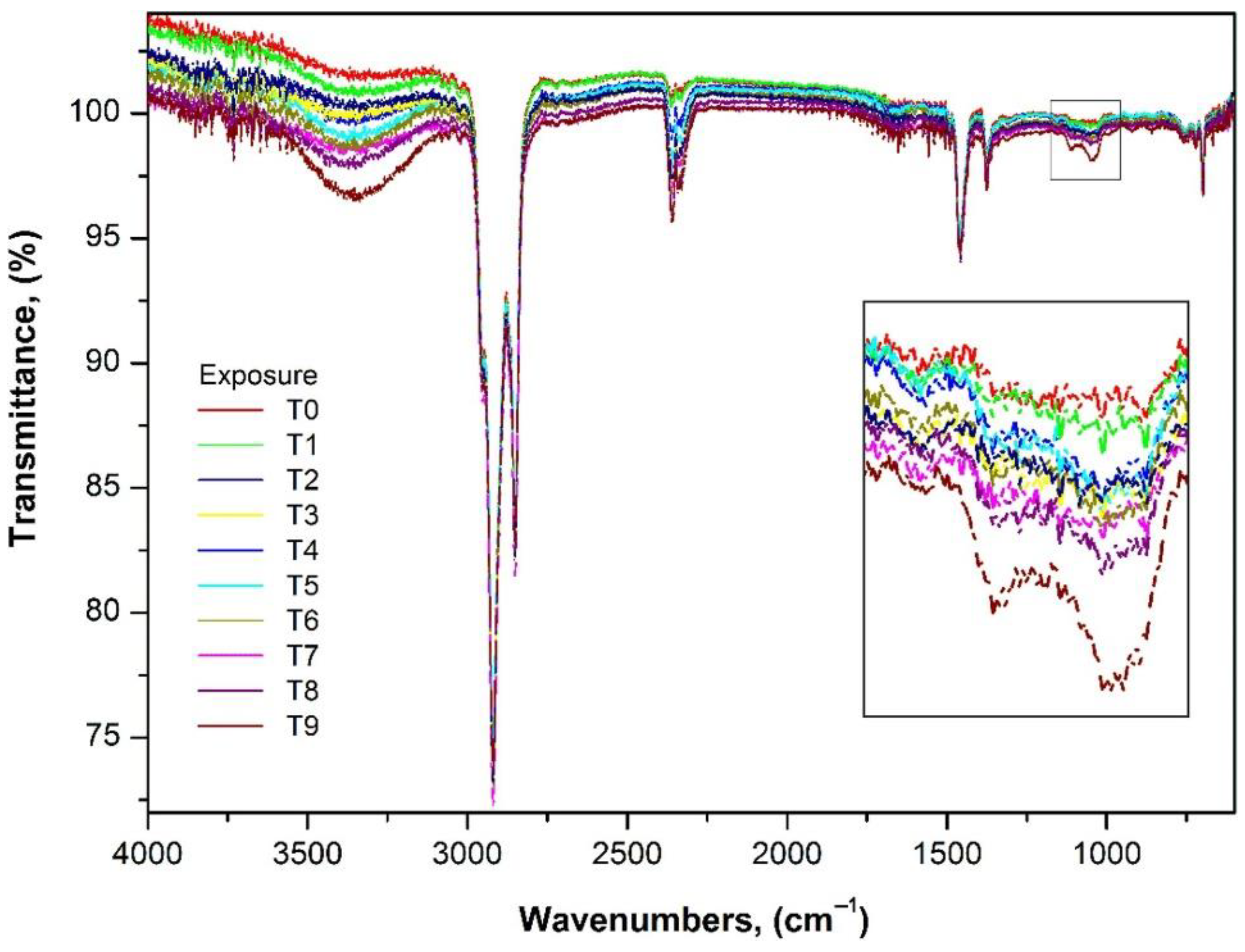
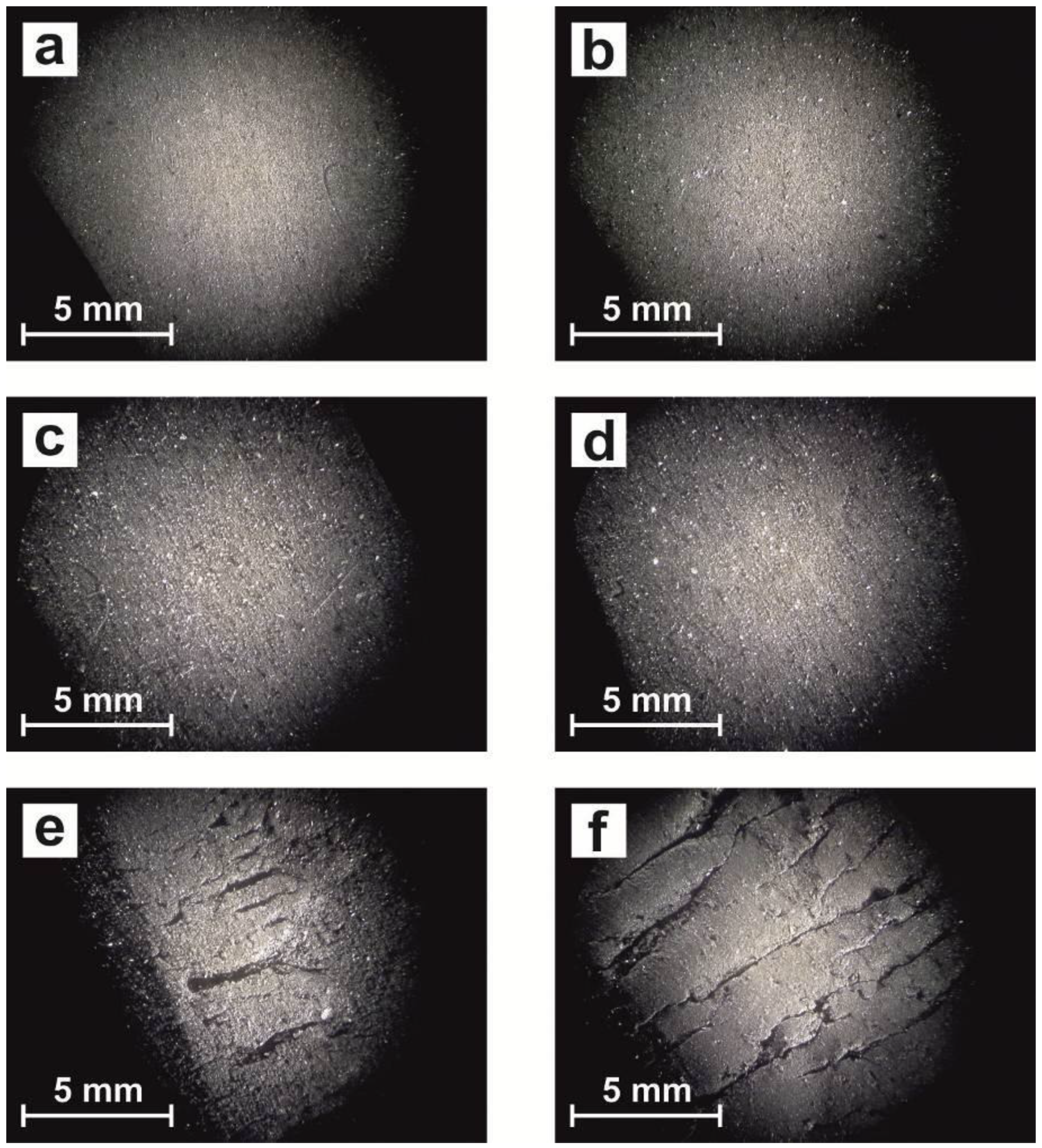
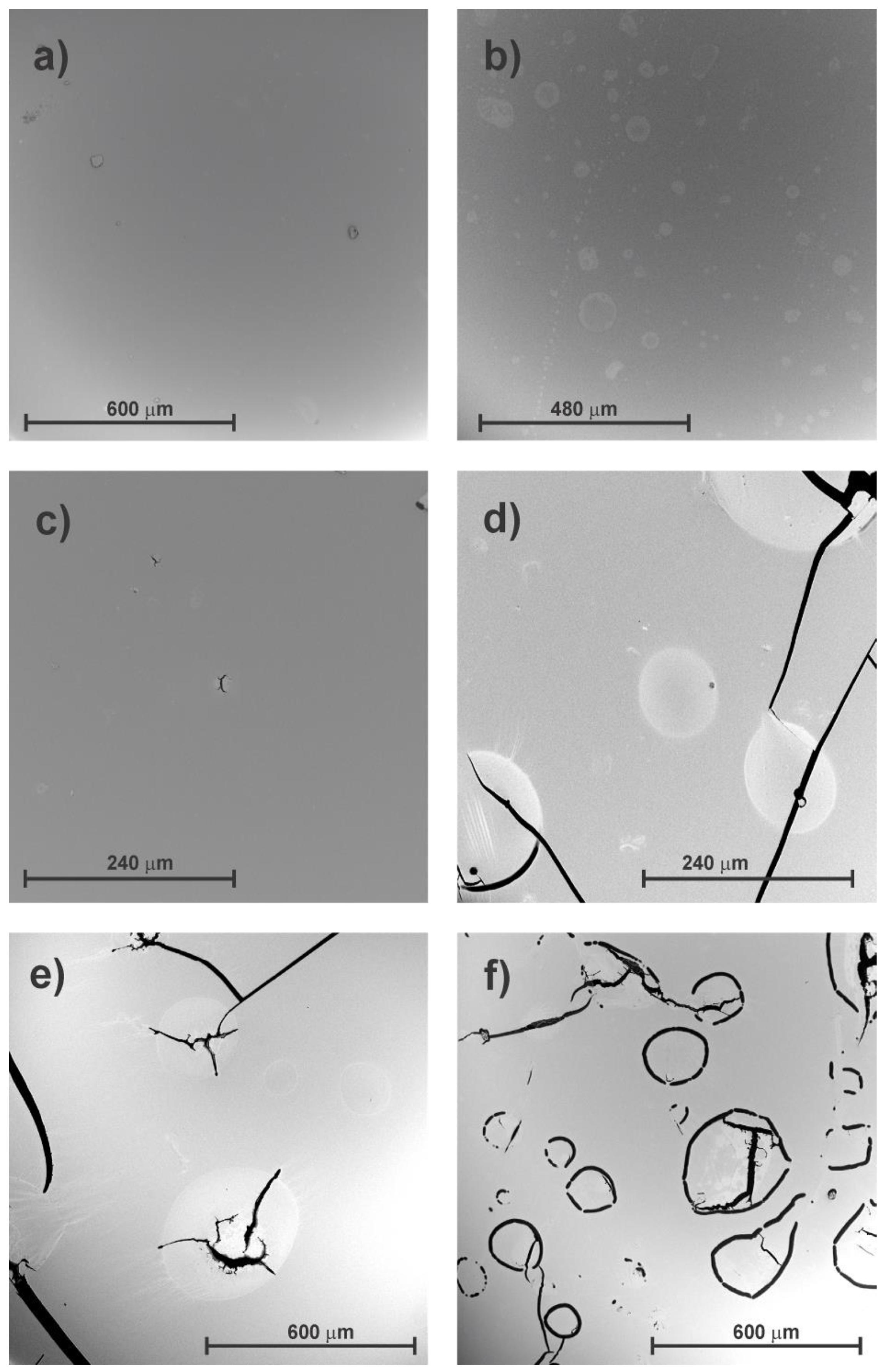
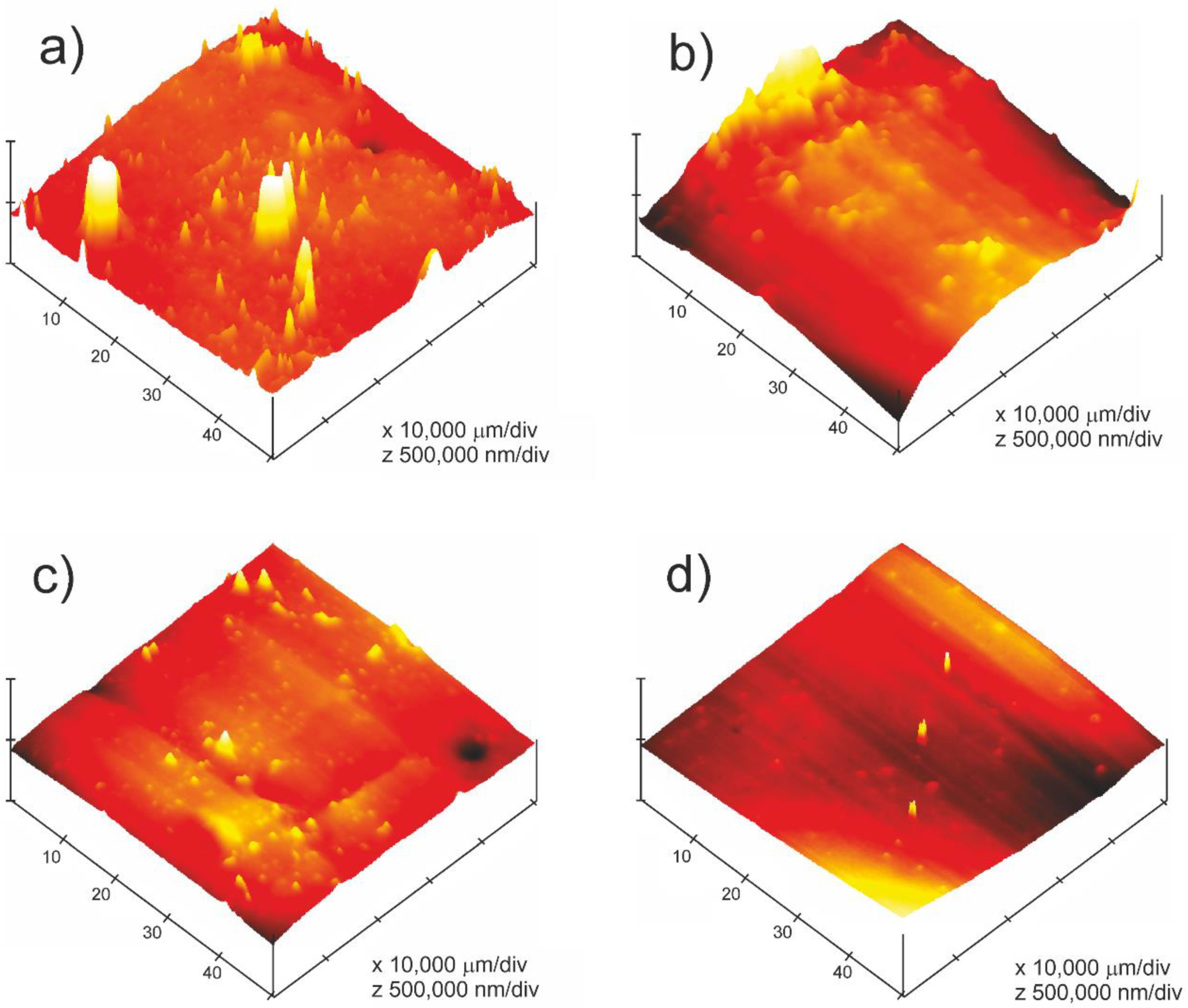
| Samples | UV Exposure Time (Hours) |
|---|---|
| T0 | 0 |
| T1 | 48 |
| T2 | 96 |
| T3 | 144 |
| T4 | 192 |
| T5 | 384 |
| T6 | 552 |
| T7 | 744 |
| T8 | 936 |
| T9 | 1176 |
| Liquid | γld (mJ·m−2) | γlp (mJ·m−2) | γl (mJ·m−2) |
|---|---|---|---|
| Water | 22.0 | 50.2 | 72.2 |
| Glycerol | 34.0 | 30.0 | 64.0 |
| Diiodomethane | 48.5 | 2.3 | 50.8 |
| Formamide | 32.3 | 26.0 | 58.3 |
| Samples | Rms (nm) | Rmax. (nm) |
|---|---|---|
| T0 | 221.68 | 938.10 |
| T1 | 195.36 | 825.69 |
| T2 | 106.34 | 607.52 |
| T3 | 78.29 | 589.64 |
| T4 | 63.12 | 368.96 |
| T5 | 46.31 | 254.24 |
| T6 | 38.45 | 178.12 |
| T7 | 20.21 | 102.23 |
| T8 | 16.47 | 88.26 |
| T9 | 9.49 | 74.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Garcia, D.; Crespo-Amorós, J.E.; Parres, F.; Samper, M.D. Influence of Ultraviolet Radiation Exposure Time on Styrene-Ethylene-Butadiene-Styrene (SEBS) Copolymer. Polymers 2020, 12, 862. https://doi.org/10.3390/polym12040862
Garcia-Garcia D, Crespo-Amorós JE, Parres F, Samper MD. Influence of Ultraviolet Radiation Exposure Time on Styrene-Ethylene-Butadiene-Styrene (SEBS) Copolymer. Polymers. 2020; 12(4):862. https://doi.org/10.3390/polym12040862
Chicago/Turabian StyleGarcia-Garcia, Daniel, José Enrique Crespo-Amorós, Francisco Parres, and María Dolores Samper. 2020. "Influence of Ultraviolet Radiation Exposure Time on Styrene-Ethylene-Butadiene-Styrene (SEBS) Copolymer" Polymers 12, no. 4: 862. https://doi.org/10.3390/polym12040862
APA StyleGarcia-Garcia, D., Crespo-Amorós, J. E., Parres, F., & Samper, M. D. (2020). Influence of Ultraviolet Radiation Exposure Time on Styrene-Ethylene-Butadiene-Styrene (SEBS) Copolymer. Polymers, 12(4), 862. https://doi.org/10.3390/polym12040862






