Sorting Liquid Droplets by Surface Tension Using Devices with Quasi-Superamphiphobic Coatings
Abstract
1. Introduction
2. Experimental Section
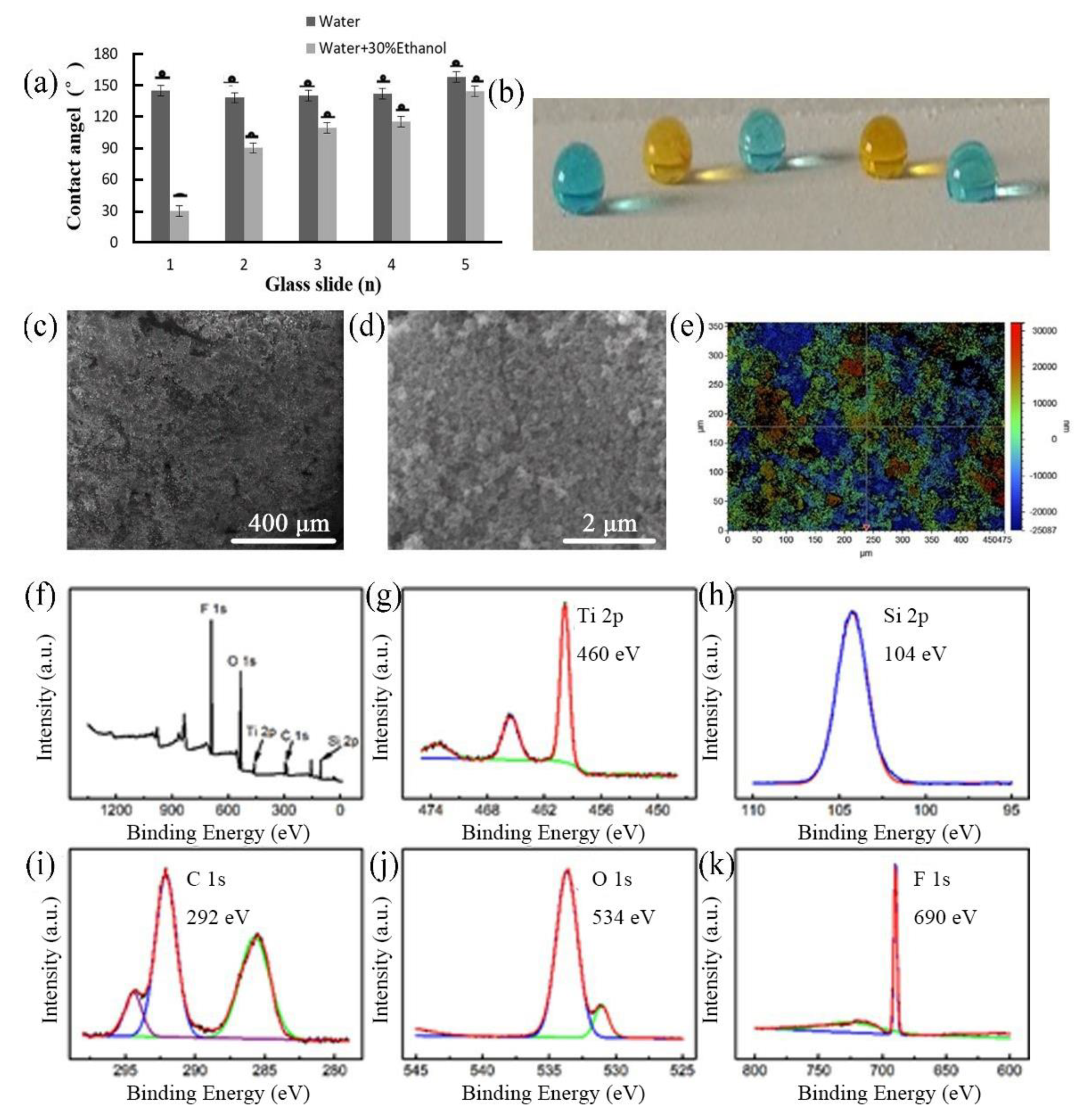
2.1. Preparation of Quasi-Superamphiphobic Surfaces
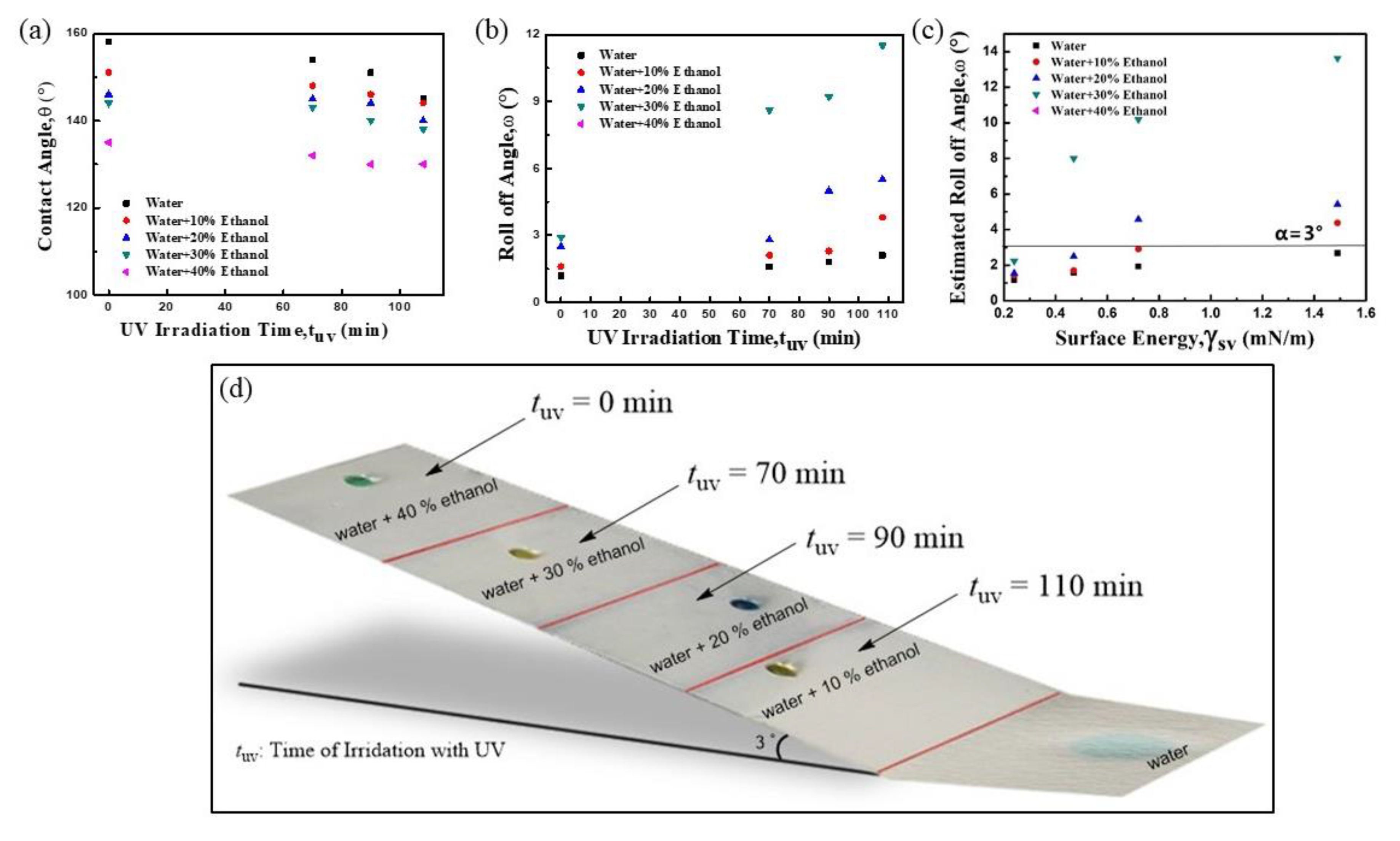
2.2. Measurement of Surface Tension, Contact Angles, and Roll Off Angles
2.3. Tuning Surface Chemistry and Solid Surface Energy via UV Irradiation
2.4. Characterization of Surface Morphology, Roughness, and Chemical Composition
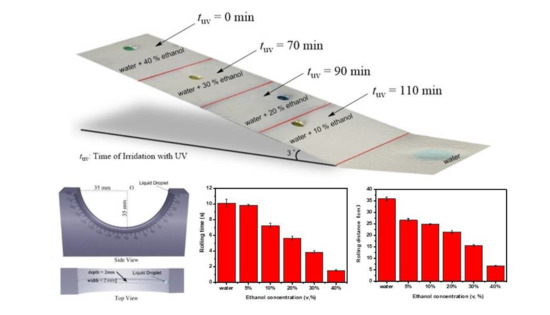
2.5. Calculation of the Rolling Distance
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and Distribution of Water-repellent, Self-cleaning Plant Surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Bhushan, B.; Her, E.K. Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Chan, B.; Bush, J.W.M. The hydrodynamics of water strider locomotion. Nature 2003, 424, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef] [PubMed]
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef]
- Vahabi, H.; Wang, W.; Movafaghi, S.; Kota, A.K. Free-Standing, Flexible, Superomniphobic Films. ACS Appl. Mater. Interfaces 2016, 8, 21962–21967. [Google Scholar] [CrossRef]
- Peng, S.; Deng, W. A simple method to prepare superamphiphobic aluminum surface with excellent stability. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 143–150. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Yang, J.-H.; Li, L.-L.; Cui, C.; Li, Y.; Liu, S.-Q.; Zhou, X.-M.; Qu, L.-B. Facile Fabrication of Superhydrophobic Copper-Foam and Electrospinning Polystyrene Fiber for Combinational Oil⁻Water Separation. Polymers 2019, 11, 97. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.; Zhou, X.; Ge, B.; Chen, S.; Wu, W. A facile way to fabricate a superamphiphobic surface. Appl. Phys. A 2014, 115, 765–770. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Dong, J.; Zhang, J. Roles of silanes and silicones in forming superhydrophobic and superoleophobic materials. J. Mater. Chem. A 2016, 4, 13677–13725. [Google Scholar] [CrossRef]
- Xi, J.; Feng, L.; Jiang, L. A general approach for fabrication of superhydrophobic and superamphiphobic surfaces. Appl. Phys. Lett. 2008, 92, 053102–053103. [Google Scholar] [CrossRef]
- Li, T.; Paliy, M.; Wang, X.; Kobe, B.; Lau, W.-M.; Yang, J. Facile One-Step Photolithographic Method for Engineering Hierarchically Nano/Microstructured Transparent Superamphiphobic Surfaces. ACS Appl. Mater. Interfaces 2015, 7, 10988–10992. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, M.; Zheng, Q. Dopamine/Silica Nanoparticle Assembled, Microscale Porous Structure for Versatile Superamphiphobic Coating. ACS Nano 2016, 10, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef]
- Wu, X.; Wyman, I.; Ganwei, Z.; Lin, J.; Liu, Z.; Wang, Y.; Hu, H. Preparation of superamphiphobic polymer-based coatings via spray- and dip-coating strategies. Prog. Org. Coat. 2016, 90, 463–471. [Google Scholar] [CrossRef]
- Ge, D.; Yang, L.; Zhang, Y.; Rahmawan, Y.; Yang, S. Transparent and Superamphiphobic Surfaces from One-Step Spray Coating of Stringed Silica Nanoparticle/Sol Solutions. Part. Part. Syst. Charact. 2014, 31, 763–770. [Google Scholar] [CrossRef]
- Huang, J.; Lai, Y.; Pan, F.; Yang, L.; Wang, H.; Zhang, X.; Fuchs, H.; Chi, L. Multifunctional Superamphiphobic TiO2 Nanostructure Surfaces with Facile Wettability and Adhesion Engineering. Small 2014, 10, 4865–4873. [Google Scholar] [CrossRef]
- Milionis, A.; Fragouli, D.; Martiradonna, L.; Anyfantis, G.C.; Cozzoli, P.D.; Bayer, I.S.; Athanassiou, A. Spatially Controlled Surface Energy Traps on Superhydrophobic Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 1036–1043. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shimizu, K.; Kaizuma, Y.; Konishi, S. Novel combination of hydrophilic/hydrophobic surface for large wettability difference and its application to liquid manipulation. Lab Chip 2011, 11, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Movafaghi, S.; Wang, W.; Metzger, A.; Williams, D.D.; Williams, J.D.; Kota, A.K. Tunable superomniphobic surfaces for sorting droplets by surface tension. Lab Chip 2016, 16, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, Y.; Wang, Y.; Xia, F. Tunable superamphiphobic surfaces: A platform for naked-eye ATP detection. Anal. Bioanal. Chem. 2018, 411, 4721–4727. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, H.; Yang, W.; Zhao, Y.; Fang, J.; Lin, T. Selective, Spontaneous One-Way Oil-Transport Fabrics and Their Novel Use for Gauging Liquid Surface Tension. ACS Appl. Mater. Interfaces 2015, 7, 22874–22880. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, C.; Li, J.; Guan, Z. Transparent, Superhydrophobic Surface with Varied Surface Tension Responsiveness in Wettability Based on Tunable Porous Silica Structure for Gauging Liquid Surface Tension. ACS Appl. Mater. Interfaces 2017, 9, 4142–4150. [Google Scholar] [CrossRef]
- Liu, K.-F.; Li, P.-P.; Zhang, Y.-P.; Liu, P.-F.; Cui, C.-X.; Wang, J.-C.; Li, X.-J.; Qu, L.-B. Laboratory filter paper from superhydrophobic to quasi-superamphiphobicity: Facile fabrication, simplified patterning and smart application. Cellulose 2019, 26, 3859–3872. [Google Scholar] [CrossRef]
- Mi, Y.X.; Zhou, Z.H.; Wang, Z.; Wang, G.W.; Yao, J. Superhydrophobic Surface Prepared by Organic-Inorganic Hybrid Material on Plasma Spraying Al2O3+13wt. %TiO2 Ceramic Coating. Mater. Sci. Forum 2015, 817, 76–81. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Stępień, M.; Haapanen, J.; Mäkelä, J.M.; Saarinen, J.J.; Toivakka, M.; Kuusipalo, J. Wettability conversion on the liquid flame spray generated superhydrophobic TiO2 nanoparticle coating on paper and board by photocatalytic decomposition of spontaneously accumulated carbonaceous overlayer. Cellulose 2013, 20, 391–408. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 12, 40–52. [Google Scholar]
- Chhatre, S.S.; Guardado, J.O.; Moore, B.M.; Haddad, T.S.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Fluoroalkylated Silicon-Containing Surfaces−Estimation of Solid-Surface Energy. ACS Appl. Mater. Interfaces 2010, 2, 3544–3554. [Google Scholar] [CrossRef]
- Babu, S.R. An absolute method for the determination of surface tension of liquids using pendent drop profiles. Bull. Mater. Sci. 1986, 8, 217–224. [Google Scholar] [CrossRef]
- Hansen, F.; Rødsrud, G. Surface tension by pendant drop. J. Colloid Interface Sci. 1991, 141, 1–9. [Google Scholar] [CrossRef]
- Maximino, B.R. Surface Tension and Density of Binary Mixtures of Monoalcohols, Water and Acetonitrile: Equation of Correlation of the Surface Tension. Phys. Chem. Liq. 2009, 47, 475–486. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Ali, H.; Al-Sharafi, A.; Al-Aqeeli, N. Droplet dynamics on a hydrophobic surface coated with N-octadecane phase change material. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 546, 28–39. [Google Scholar] [CrossRef]
- Ahmed, G.; Sellier, M.; Jermy, M.C.; Taylor, M. Modeling the effects of contact angle hysteresis on the sliding of droplets down inclined surfaces. Eur. J. Mech.B/Fluids 2014, 48, 218–230. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-P.; Fan, D.; Bai, X.-Z.; Cui, C.-X.; Chen, J.; Li, R.-L.; Liu, P.-F.; Qu, L.-B. Sorting Liquid Droplets by Surface Tension Using Devices with Quasi-Superamphiphobic Coatings. Polymers 2020, 12, 820. https://doi.org/10.3390/polym12040820
Zhang Y-P, Fan D, Bai X-Z, Cui C-X, Chen J, Li R-L, Liu P-F, Qu L-B. Sorting Liquid Droplets by Surface Tension Using Devices with Quasi-Superamphiphobic Coatings. Polymers. 2020; 12(4):820. https://doi.org/10.3390/polym12040820
Chicago/Turabian StyleZhang, Yu-Ping, Di Fan, Xiu-Zhi Bai, Cheng-Xing Cui, Jun Chen, Ren-Long Li, Peng-Fei Liu, and Ling-Bo Qu. 2020. "Sorting Liquid Droplets by Surface Tension Using Devices with Quasi-Superamphiphobic Coatings" Polymers 12, no. 4: 820. https://doi.org/10.3390/polym12040820
APA StyleZhang, Y.-P., Fan, D., Bai, X.-Z., Cui, C.-X., Chen, J., Li, R.-L., Liu, P.-F., & Qu, L.-B. (2020). Sorting Liquid Droplets by Surface Tension Using Devices with Quasi-Superamphiphobic Coatings. Polymers, 12(4), 820. https://doi.org/10.3390/polym12040820