Synthesis and Conformational Characteristics of Thermosensitive Star-Shaped Six-Arm Polypeptoids
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymer Star Synthesis
2.1.1. Preparing of Hexa-N-(11-bromoundecanoyl) Trianglamine (2)
2.1.2. Polymerization of Oxazolines(Oxazines) Using 2 As Initiator. Typical Procedure
2.2. Solution Investigation
3. Results and Discussion
3.1. Polymer Synthesis And Structural Characteristics
3.2. Hydrodynamic Characteristics And Conformation of CPh6-PAlOx And CPh6-PAlOz Macromolecules
3.3. LCST Behavior of CPh6-PAlOx And CPh6-PAlOz
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, F.; Bronich, T.K.; Kabano, A.V.; Rauh, R.D.; Roovers, J. Synthesis and characterization of star poly(ε-caprolactone)-b-poly(ethylene glycol) and poly(L-lactide)-b-poly(ethylene glycol) copolymers: Evaluation as drug delivery carriers. Bioconjugate Chem. 2008, 19, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, W.; Li, J. Star polymers: Advances in biomedical applications. Prog. Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Lou, L.; Jiang, L.; Liu, J.; Sun, W.; Shen, Z. Synthesis and characterization of optically active star-shaped poly (N-phenylmaleimide)s with a calixarene core. Polym. Int. 2007, 56, 796–802. [Google Scholar] [CrossRef]
- Terashima, T.; Kojima, H.; Sawamoto, M. Core-imprinted star polymers via living radical polymerization: Precision cavity microgels for selective molecular recognition. Chem. Lett. 2014, 43, 1690–1692. [Google Scholar] [CrossRef]
- Granata, G.; Paterniti, I.; Geraci, C.; Cunsolo, F.; Esposito, E.; Cordaro, M.; Blanco, A.R.; Cuzzocrea, S.; Consoli, G.H.L. Potential eye drop based on a calix[4]arene nanoassembly for curcumin delivery: Enhanced drug solubility, stability, and antiInflammatory effect. Mol. Pharm. 2017, 14, 1610–1622. [Google Scholar] [CrossRef]
- Drakalska, E.; Momekova, D.; Manolova, Y.; Budurova, D.; Momekov, G.; Genova, M.; Antonov, L.; Lambov, N.; Rangelov, S. Hybrid liposomal PEGylated calix[4]arene systems as drug delivery platforms for curcumin. Int. J. Pharm. 2014, 472, 165–174. [Google Scholar] [CrossRef]
- Guillerm, B.; Monge, S.; Lapinte, V.; Robin, J.-J. Novel investigations on kinetics and polymerization mechanism of oxazolines initiated by iodine. Macromolecules 2010, 43, 5964–5970. [Google Scholar] [CrossRef]
- Obeid, R.; Maltseva, E.; Thunemann, A.F.; Tanaka, F.; Winnik, F.M. Temperature response of self-assembled micelles of telechelic hydrophobically modified poly(2-alkyl-2-oxazoline)s in water. Macromolecules 2009, 42, 2204–2214. [Google Scholar] [CrossRef]
- Borisova, N.E.; Reshetova, M.D.; Ustynyuk, Y.A. Metal-free methods in the synthesis of macrocyclic schiff bases. Chem. Rev. 2007, 107, 46–79. [Google Scholar] [CrossRef]
- Gajewy, J.; Szymkowiak, J.; Kwit, M. Fine tuning of molecular and supramolecular properties of simple trianglimines—The role of the functional group. Rsc Adv. 2016, 6, 53358–53369. [Google Scholar] [CrossRef]
- Gawronski, J.; Gawronska, K.; Grajewski, J.; Kwit, M.; Plutecka, A.; Rychlewska, U. Trianglamines—Readily prepared, conformationally flexible inclusion forming chiral hexamines. Chem. Eur. J. 2006, 12, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, A.; Grilli, S.; Savoia, D.; Kwit, M.; Gawronski, J. C-hexaphenyl-substituted trianglamine as a chiral solvating agent for carboxylic acids. Org. Biomol. Chem. 2011, 9, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Mouchaham, G.; Shkurenko, A.; Hoang, P.; Moosa, B.; Bhatt, P.M.; Adil, K.; Salama, K.N.; Eddaoudi, M.; Khashab, N.M. Trianglamine-based supramolecular organic framework with permanent intrinsic porosity and tunable selectivity. J. Am. Chem. Soc. 2018, 140, 14571–14575. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Huang, W.; Wang, L.; Gou, S. Chiral 27-membered [3 + 3] schiff-base macrocycles and their reactivity with first-row transition metal ions. Polyhedron 2008, 27, 1079–1092. [Google Scholar] [CrossRef]
- Tenkovtsev, A.V.; Dudkina, M.M.; Scherbinskaya, L.I.; Aseyev, V.; Tenhu, H. Star-shaped macromolecules with calixarene core and neutral amphiphilic block copolymer arms: New hosts for ions. Polymer 2010, 51, 3108–3115. [Google Scholar] [CrossRef]
- Kempe, K. Chain and step growth polymerizations of cyclic imino ethers: From poly(2-oxazoline)s to poly(ester amide)s. Macromol. Chem. Phys. 2017, 218, 1700021. [Google Scholar] [CrossRef]
- Park, J.-S.; Kataoka, K. Precise control of lower critical solution temperature of thermosensitive poly(2-isopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline as a hydrophilic comonomer. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Weber, C.; Wagner, M.; Baykal, D.; Hoeppener, S.; Paulus, R.M.; Festag, G.; Altuntas, E.; Schacher, F.H.; Schubert, U.S. Easy access to amphiphilic heterografted poly(2-oxazoline) comb copolymers. Macromolecules 2013, 46, 5107–5116. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Bezen, M.; Jordan, R. Kinetic investigations on the polymerization of 2-oxazolines using pluritriflate initators. Macromol. Rapid Commun. 2008, 29, 1509–1513. [Google Scholar] [CrossRef]
- Tenkovtsev, A.V.; Amirova, A.I.; Filippov, A.P. Temperature-Responsive Polymers: Chemistry, Properties and Applications; Chapter 3; Khutoryanskiy, V., Georgiou, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 67. [Google Scholar]
- Dworak, A.; Trzebicka, B.; Kowalczuk, A.; Tsvetanov, C.; Rangelov, S. Polyoxazolines–mechanism of synthesis and solution properties. Polymery 2014, 59, 88–94. [Google Scholar] [CrossRef]
- Glassner, M.; D’hooge, D.R.; Park, J.; Van Steenberge, P.H.; Monnery, B.D.; Reyniers, M.-F.; Hoogenboom, R. Systematic investigation of alkyl sulfonate initiators for the cationic ring-opening polymerization of 2-oxazolines revealing optimal combinations of monomers and initiators. Eur. Polym. J. 2015, 65, 298–304. [Google Scholar] [CrossRef]
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties. Polym. Int. 2018, 67, 32–45. [Google Scholar] [CrossRef]
- Oleszko-Torbus, N.; Wałach, W.; Utrata-Wesołek, A.; Dworak, A. Control of the crystalline properties of 2-isopropyl-2-oxazoline copolymers in condensed state and in solution depending on the composition. Macromolecules 2017, 50, 7636–7645. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef]
- Grube, M.; Leiske, M.N.; Schubert, U.S.; Nischang, I. POx as an alternative to PEG? A hydrodynamic and light scattering study. Macromolecules 2018, 51, 1905–1916. [Google Scholar] [CrossRef]
- Tu, C.; Zhu, L.; Li, P.; Chen, Y.; Su, Y.; Yan, D.; Zhu, X.; Zhou, G. Supramolecular polymeric micelles by the host–guest interaction of star-like calix[4]arene and chlorin e6 for photodynamic therapy. Chem. Commun. 2011, 47, 6063–6065. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Kronek, J.; Bosowska, K.; Trzebicka, B.; Dworak, A. Star poly(2-ethyl-2-oxazoline)s—Synthesis and thermosensitivity. Polym. Int. 2011, 60, 1001–1009. [Google Scholar] [CrossRef]
- Amirova, A.I.; Golub, O.V.; Kirila, T.U.; Razina, A.B.; Tenkovtsev, A.V.; Filippov, A.P. Influence of arm length and number on star-shaped poly(2-isopropyl-2-oxazoline) aggregation in aqueous solutions near cloud point. Soft Mater. 2016, 14, 15–26. [Google Scholar] [CrossRef]
- Amirova, A.; Golub, O.; Kirila, T.; Razina, A.; Tenkovtsev, A.; Filippov, A. Influence of arm length on aqueous solution behavior of thermosensitive poly(2-isopropyl-2-oxazoline) stars. Colloid Polym. Sci. 2017, 295, 117–124. [Google Scholar] [CrossRef]
- Kobayashi, S.; Igarashi, T.; Moriuchi, Y.; Saegusa, T. Block copolymers from cyclic imino ethers: A new class of nonionic polymer surfactant. Macromolecules 1986, 19, 535–541. [Google Scholar] [CrossRef]
- Bassiri, T.G.; Levy, A.; Litt, M. Polymerization of cyclic imino ethers. I. Oxazolines. J. Polym. Sci. B Polym. Lett. 1967, 5, 871–879. [Google Scholar] [CrossRef]
- Bloksma, M.M.; Paulus, R.M.; van Kuringen, H.P.; van der Woerdt, F.; Lambermont-Thijs, H.M.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive poly(2-oxazine)s. Macromol. Rapid Commun. 2012, 33, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Lambermont-Thijs, H.M.L.; Fijten, M.W.M.; van der Linden, A.J.T.; van Lankvelt, B.M.; Bloksma, M.M.; Schubert, U.S.; Hoogenboom, R. Efficient cationic ring-opening polymerization of diverse cyclic imino ethers: Unexpected copolymerization behavior. Macromolecules 2011, 44, 4320–4325. [Google Scholar] [CrossRef]
- Sedlacek, O.; Hoogenboom, R. Drug delivery systems based on poly(2-oxazoline)s and poly(2-oxazine)s. Adv. Ther. 2019, 1900168. [Google Scholar] [CrossRef]
- Kurlykin, M.P.; Dudkina, M.M.; Tenkovtsev, A.V. Star-shaped thermosensitive poly(2-ethyl-2-oxazines) with the calixarene core. Polym. Sci. B 2019, 61, 51–55. [Google Scholar] [CrossRef]
- Giulia, M.; Verbraeken, B.; Ramakrishna, S.N.; Gombert, Y.; Cavalli, E.; Rosenboom, J.-G.; Zenobi-Wong, M.; Spencer, N.D.; Hoogenboom, R.; Benetti, E.M. Chemical design of non-ionic polymer brushes as biointerfaces: Poly(2-oxazine)s outperform both poly(2-oxazoline)s and PEG. Angew. Chem. Int. Ed. 2018, 57, 11667–11672. [Google Scholar]
- Lübtow, M.M.; Hahn, L.; Haider, M.S.; Luxenhofer, R. Drug specificity, synergy and antagonism in ultrahigh capacity poly(2-oxazoline)/poly(2-oxazine) based formulations. J. Am. Chem. Soc. 2017, 139, 10980–10983. [Google Scholar] [CrossRef]
- Bloksma, M.M.; Schubert, U.S.; Hoogenboom, R. Poly(cyclic imino ether)s beyond 2-substituted-2-oxazolines. Macromol. Rapid Commun. 2011, 32, 1419–1441. [Google Scholar] [CrossRef]
- Kirila, T.U.; Smirnova, A.V.; Filippov, A.S.; Razina, A.B.; Tenkovtsev, A.V.; Filippov, A.P. Thermosensitive star-shaped poly-2-ethyl-2-oxazine. Synthesis, structure characterization, conformation, and self-organization in aqueous solutions. Eur. Polym. J. 2019, 120, 109215. [Google Scholar] [CrossRef]
- Witte, H.; Seeliger, W. Cyclische imidsäureester aus nitrilen und aminoalkoholen. Justus Liebigs Ann. Chem. 1974, 6, 996–1009. [Google Scholar] [CrossRef]
- Ye, X.D.; Yang, J.X.; Ambreen, J. Scaling laws between the hydrodynamic parameters and molecular weight of linear poly(2-ethyl-2-oxazoline). RSC Adv. 2013, 3, 15108–15113. [Google Scholar] [CrossRef]
- Chen, C.H.; Wilson, J.; Chen, W.; Davis, R.M.; Riffle, J.S. A light-scattering study of poly(2-alkyl-2-oxazoline)s: Effect of temperature and solvent type. Polymer 1994, 35, 3587–3591. [Google Scholar] [CrossRef]
- Kirila, T.Y.; Kurlykin, M.P.; Tenkovtsev, A.V.; Filippov, A.P. Behavior of aqueous solutions of thermosensitive starlike polyalkyloxazolines with different arm structures. Polym. Sci. A 2017, 59, 826–838. [Google Scholar] [CrossRef]
- Sezonenko, T.; Qiu, X.-P.; Winnik, F.M.; Sato, T. Dehydration, micellization, and phase separation of thermosensitive polyoxazoline star block copolymers in aqueous solution. Macromolecules 2019, 52, 935–944. [Google Scholar] [CrossRef]
- Amirova, A.; Rodchenko, S.; Makhmudova, Z.; Cherkaev, G.; Milenin, S.; Tatarinova, E.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Synthesis, characterization, and investigation of thermosensitive star-shaped poly(2-isopropyl-2-oxazolines) based on carbosilane dendrimers. Macromol. Chem. Phys. 2017, 218, 1600387. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers; Plenum: New York, NY, USA, 1989. [Google Scholar]
- Rueb, C.J.; Zukoski, C.F. Rheology of suspensions of weakly attractive particles: Approach to gelation. J. Rheol. 1998, 42, 1451–1476. [Google Scholar] [CrossRef]
- Yamakawa, H. Modern Theory of Polymer Solutions; Harper and Row: New York, NY, USA, 1971. [Google Scholar]
- Filippov, A.P.; Belyaeva, E.V.; Tarabukina, E.B.; Amirova, A.I. Behavior of hyperbranched polymers in solutions. Polym. Sci. Ser. C 2011, 53, 107–117. [Google Scholar] [CrossRef]
- Bodnár, I.; Silva, A.S.; Deitcher, R.W.; Weisman, N.E.; Kim, Y.H.; Wagner, N.J. Structure and rheology of hyperbranched and dendritic polymers. I. Modification and characterization of poly(propyleneimine) dendrimers with acetyl groups. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 857–873. [Google Scholar]
- Jeong, M.; Mackay, M.E.; Vestberg, R.; Hawker, C.J. Intrinsic viscosity variation in different solvents for dendrimers and their hybrid copolymers with linear polymers. Macromolecules 2001, 34, 4927–4936. [Google Scholar] [CrossRef]
- Simonova, M.A.; Tarasova, E.V.; Dudkina, M.M.; Tenkovtsev, A.V.; Filippov, A.P. Synthesis and hydrodynamic and conformation properties of star-shaped polystyrene with calix[8]arene core. Int. J. Polym. Anal. Charact. 2019, 24, 87–95. [Google Scholar] [CrossRef]
- Terao, K.; Hokajo, T.; Nakamura, Y.; Norisuye, T. Solution properties of polymacromonomers consisting of polystyrene. 3. Viscosity behavior in cyclohexane and toluene. Macromolecules 1999, 32, 3690–3694. [Google Scholar] [CrossRef]
- Wales, M.; Rehfeld, S.J. Molecular weight distribution by velocity ultracentrifugation. J. Polym. Sci. 1962, 62, 179–196. [Google Scholar] [CrossRef]
- Chadim, M.; Budesınsky, J.; Hodacova, J.; Zavada, P.C. Junk (3+3)-cyclocondensation of the enantiopure and racemic forms of trans-1,2-diaminocyclohexane with terephthaldehyde. Formation of diastereomeric molecular triangles and their stereoselective solid-state stacking into microporous chiral columns. Tetrahedron Asymmetry 2001, 12, 127–133. [Google Scholar] [CrossRef]
- Ten’kovtsev, A.V.; Trofimov, A.E.; Shcherbinskaya, L.I. Thermoresponsive star-shaped poly(2-isopropyl-2-oxazolines) based on octa-tert-butylcalix[8]arene. Polym. Sci. Ser. B 2012, 54, 142–148. [Google Scholar] [CrossRef]
- Plet, L.; Delecourt, G.; Hanafi, M.; Pantoustier, N.; Pembouong, G.; Midoux, P.; Bennevault, V.; Guégan, P. Controlled star poly(2-oxazoline)s: Synthesis, characterization. Eur. Polym. J. 2020, 122, 109323. [Google Scholar] [CrossRef]
- Gubarev, A.S.; Monnery, B.D.; Lezov, A.A.; Sedlacek, O.; Tsvetkov, N.V.; Hoogenboom, R.; Filippov, S.K. Conformational properties of biocompatible poly(2-ethyl-2-oxazoline)s in phosphate buffered saline. Polym. Chem. 2018, 9, 2232–2237. [Google Scholar] [CrossRef]
- Zimm, B.H.; Kilb, R.W. Dynamics of branched polymer molecules in dilute solution. J. Polym. Sci. 1959, 37, 19–42. [Google Scholar] [CrossRef]
- Burchard, W. Solution properties of branched macromolecules. In Branched Polymers II; Roovers, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 113–194. [Google Scholar]
- Kurata, M.; Abe, M.; Iwama, M.; Matsushima, M. Randomly branched polymers. I. Hydrodynamic properties. Polym. J. 1972, 3, 729–738. [Google Scholar] [CrossRef]
- Weissmuller, M.; Burchard, W. Molar mass distributions of end-linked polystyrene star macromolecules. Polym. Int. 1997, 44, 380–390. [Google Scholar] [CrossRef]
- Stockmayer, W.H.; Fixman, M. Dilute solutions of branched polymers. Ann. N. Y. Acad. Sci. 1953, 57, 334–352. [Google Scholar] [CrossRef]
- Zimm, B.H.; Stockmayer, W.H. The Dimensions of Chain Molecules Containing Branches and Rings. J. Chem. Phys. 1949, 17, 1301–1314. [Google Scholar] [CrossRef]
- Burchard, W. Particle scattering factors of some branched polymers. Macromolecules 1977, 10, 919–927. [Google Scholar] [CrossRef]
- Burchard, W. Static and dynamic light scattering from branched polymers and biopolymers. Adv. Polym. Sci. 1983, 48, 1–124. [Google Scholar]
- Daoud, M.; Cotton, J.P. Star shaped polymers: A model for the conformation and its concentration dependence. J. Phys. 1982, 43, 531–538. [Google Scholar] [CrossRef]
- Sung, J.H.; Lee, D.C. Molecular shape of poly(2-ethyl-2-oxazoline) chains in THF. Polymer 2001, 42, 5771–5779. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. A hydrodynamic invariant of polymeric molecules. Russ. Chem. Rev. 1982, 51, 975–993. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic invariant of polymer-molecules. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 3447–3486. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Roy, R.; Michailova, N.A.; Ortega, P.C.; Perez, M.A. Sedimentation, translational diffusion, and viscosity of lactosylated polyamidoamine dendrimers. Progr. Colloid Polym. Sci. 1999, 113, 150–157. [Google Scholar]
- Lin, P.; Clash, C.; Pearce, E.M.; Kwei, T.K.; Aponte, M.A. Solubility and miscibility of poly(ethyl-oxazoline). J. Polym. Sci. Part B Polym. Phys. 1988, 26, 603–619. [Google Scholar] [CrossRef]
- Christova, D.; Velichkova, R.; Loos, W.; Goethals, E.J.; Prez, F.D. New thermo-responsive polymer materials based on poly(2-ethyl-2-oxazoline) segments. Polymer 2003, 44, 2255–2261. [Google Scholar] [CrossRef]
- Diab, C.; Akiyama, Y.; Kataoka, K.; Winnik, F.M. Microcalorimetric study of the temperature-induced phase separation in aqueous solutions of poly(2-isopropyl-2-oxazolines). Macromolecules 2004, 37, 2556–2562. [Google Scholar] [CrossRef]
- Kirile, T.Y.; Tobolina, A.I.; Elkina, A.A.; Kurlykin, M.P.; Tenkovtsev, A.V.; Filippov, A.P. Self-assembly processes in aqueous solutions of eat-sensitive star-shaped poly-2-ethyl-2-oxazoline. Fiber Chem. 2018, 50, 248–251. [Google Scholar] [CrossRef]
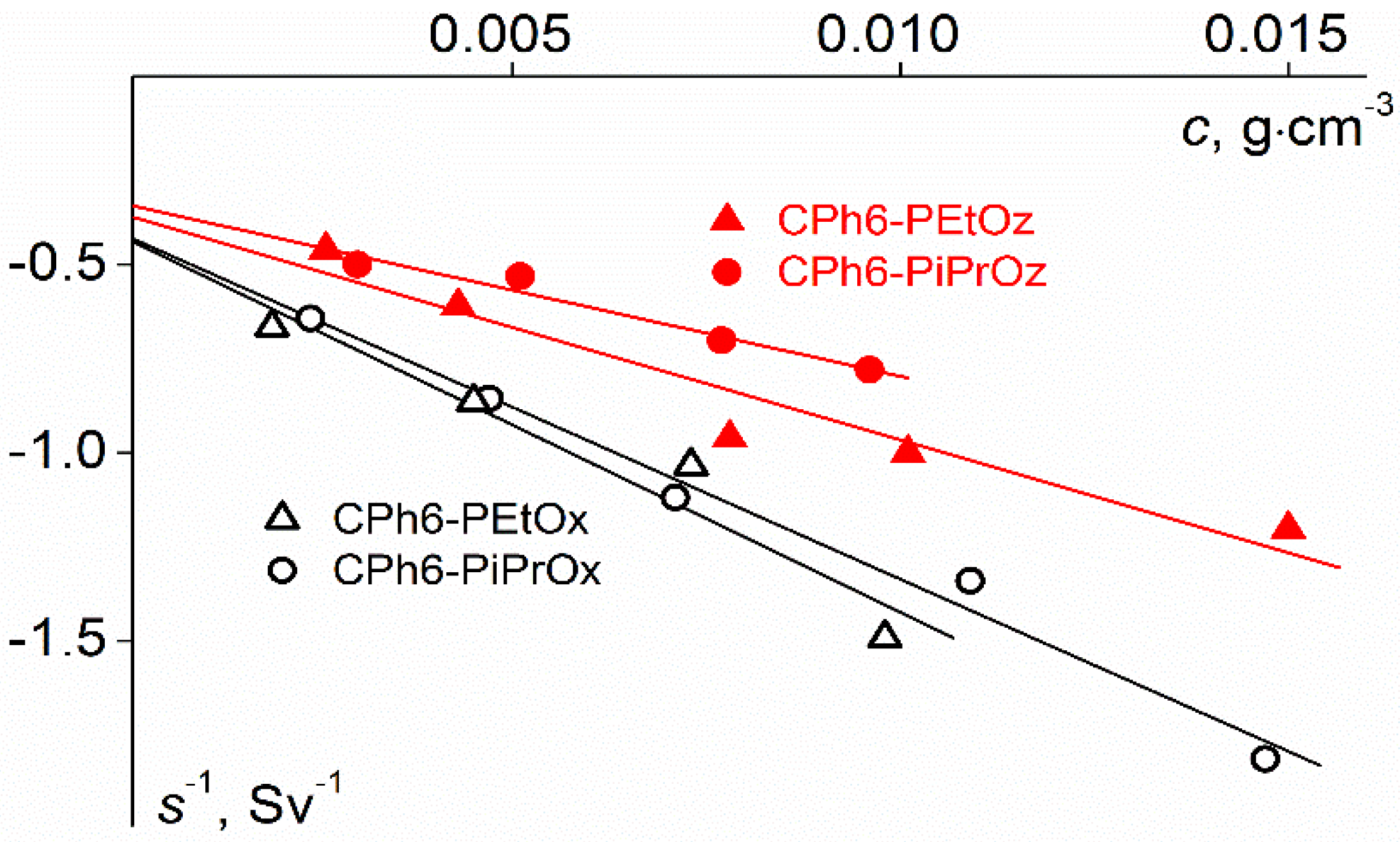
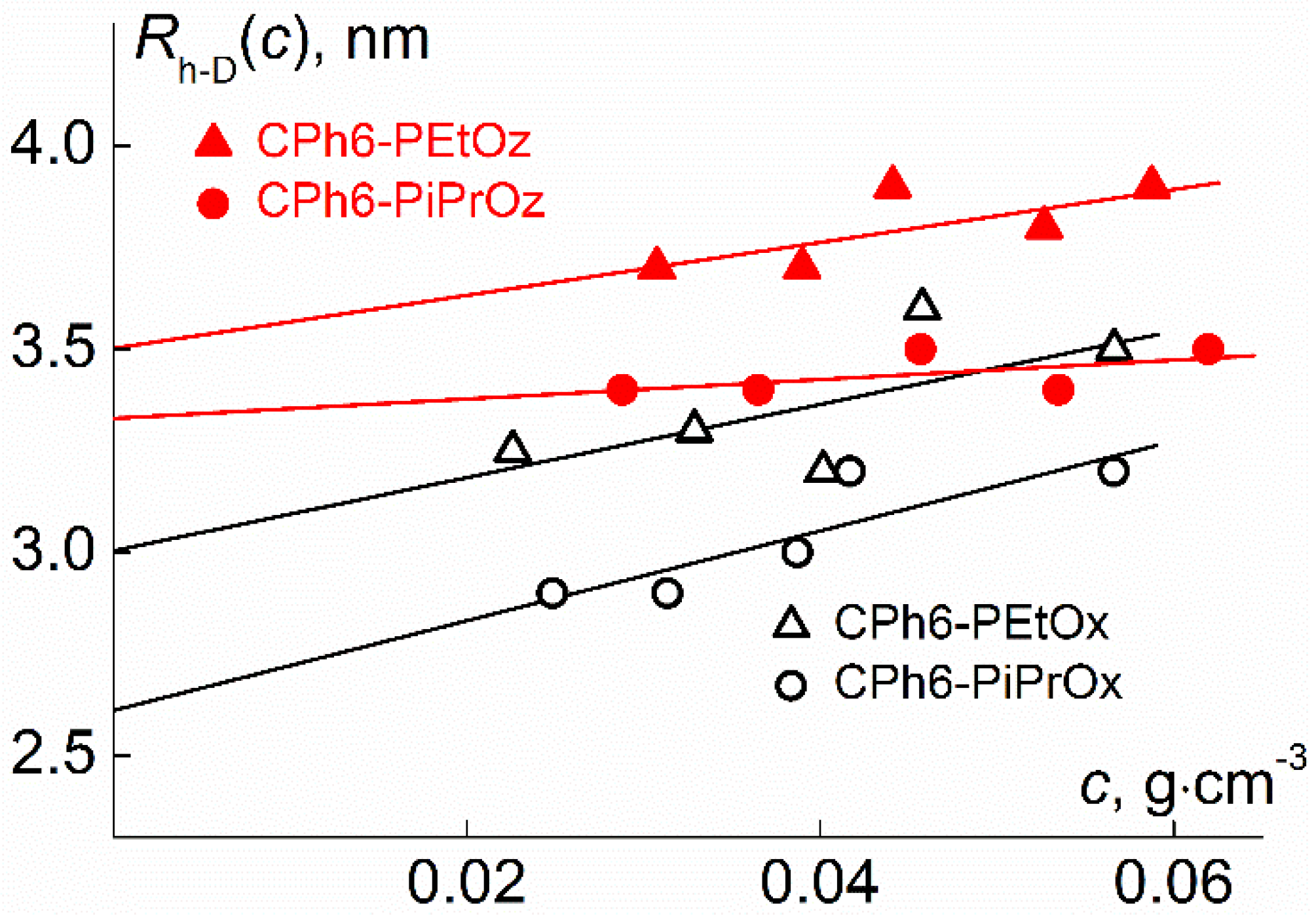
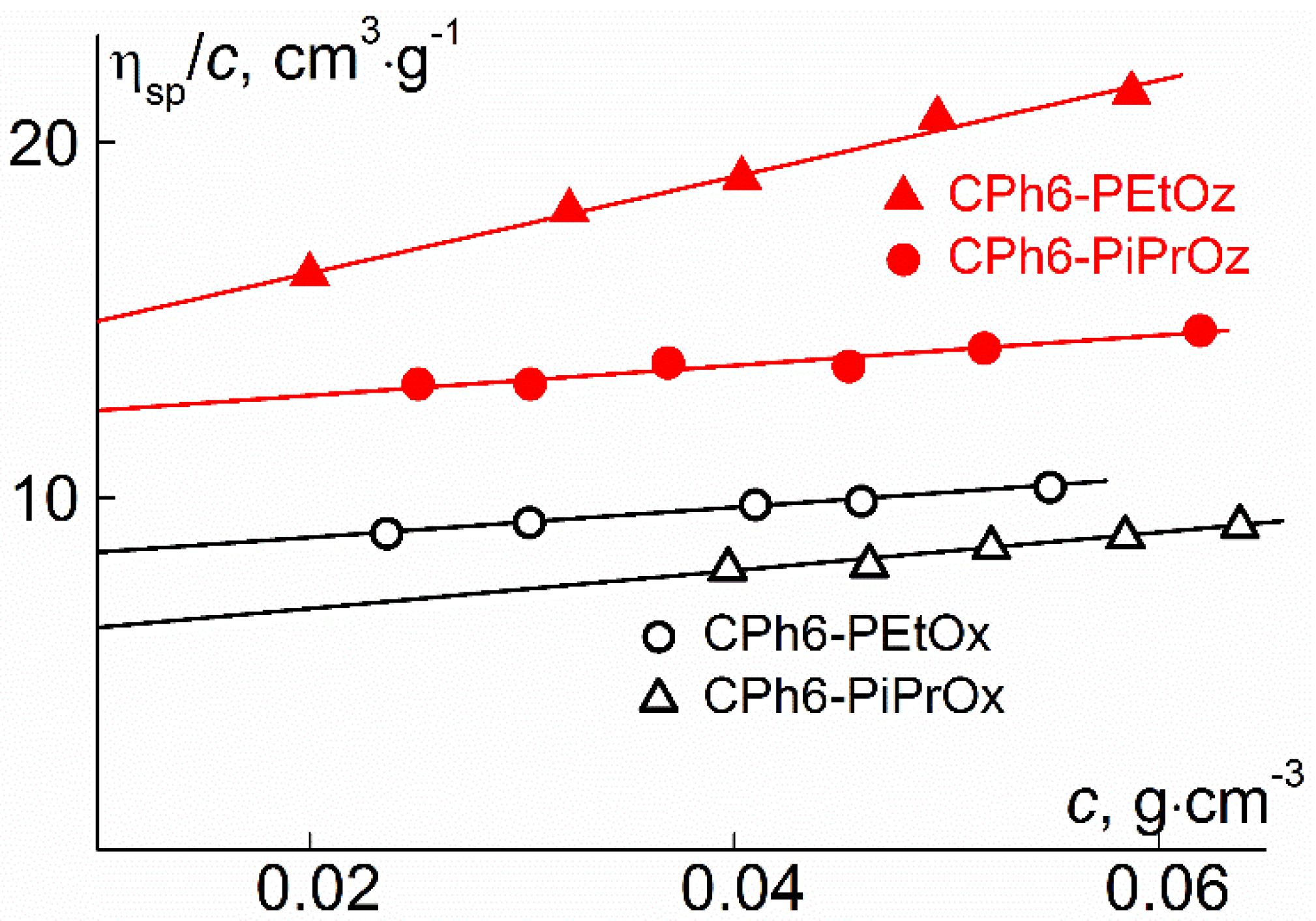



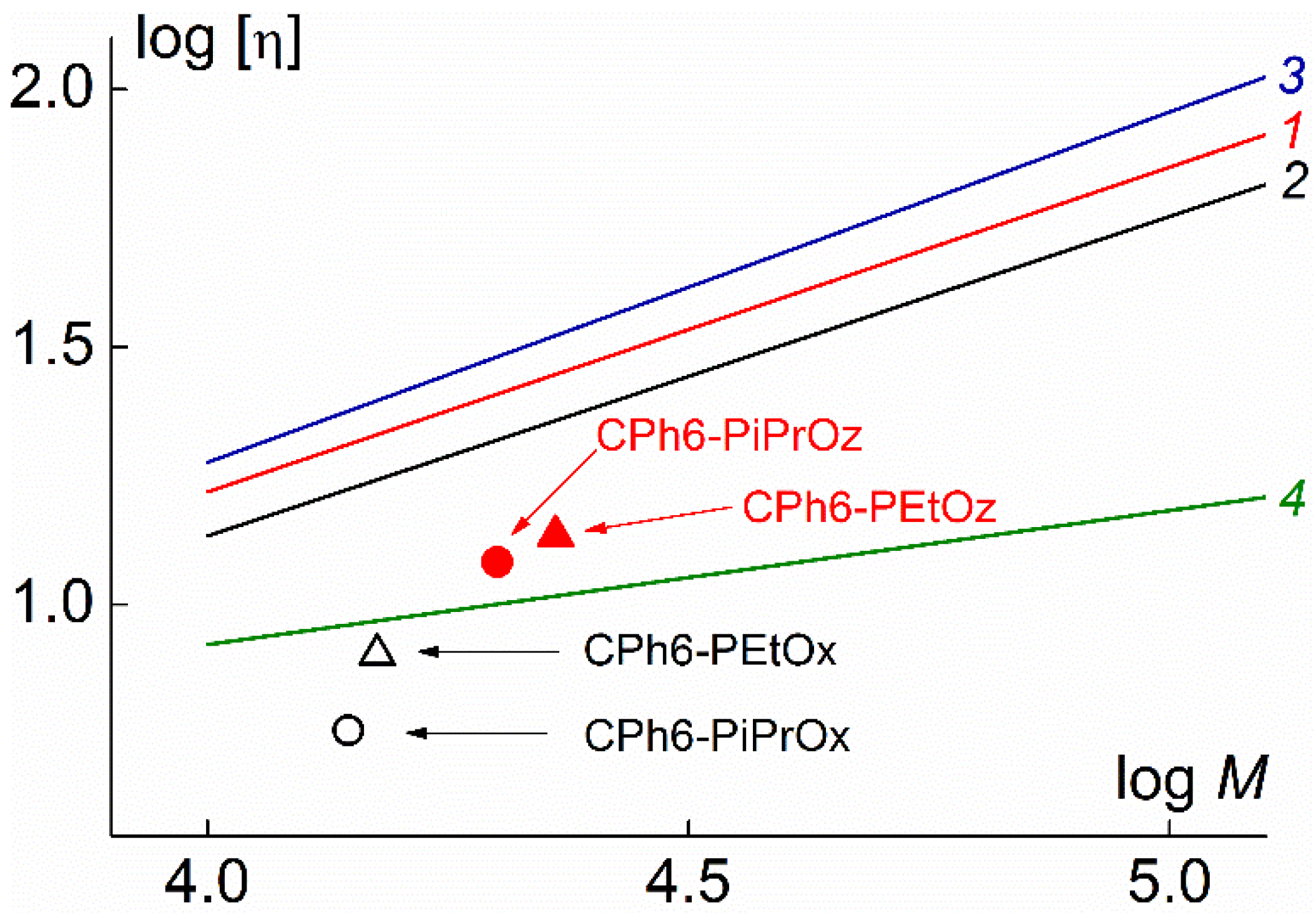
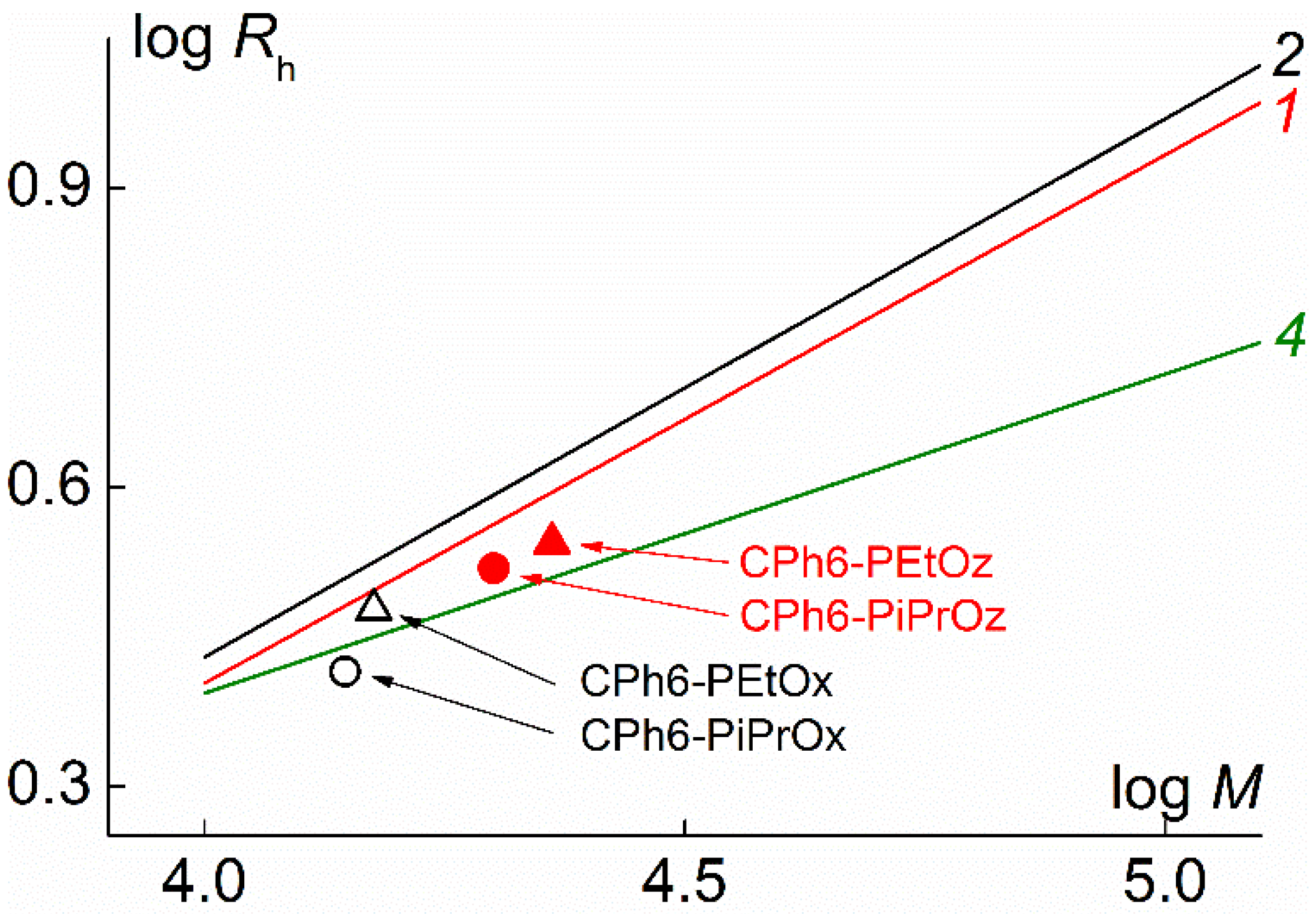
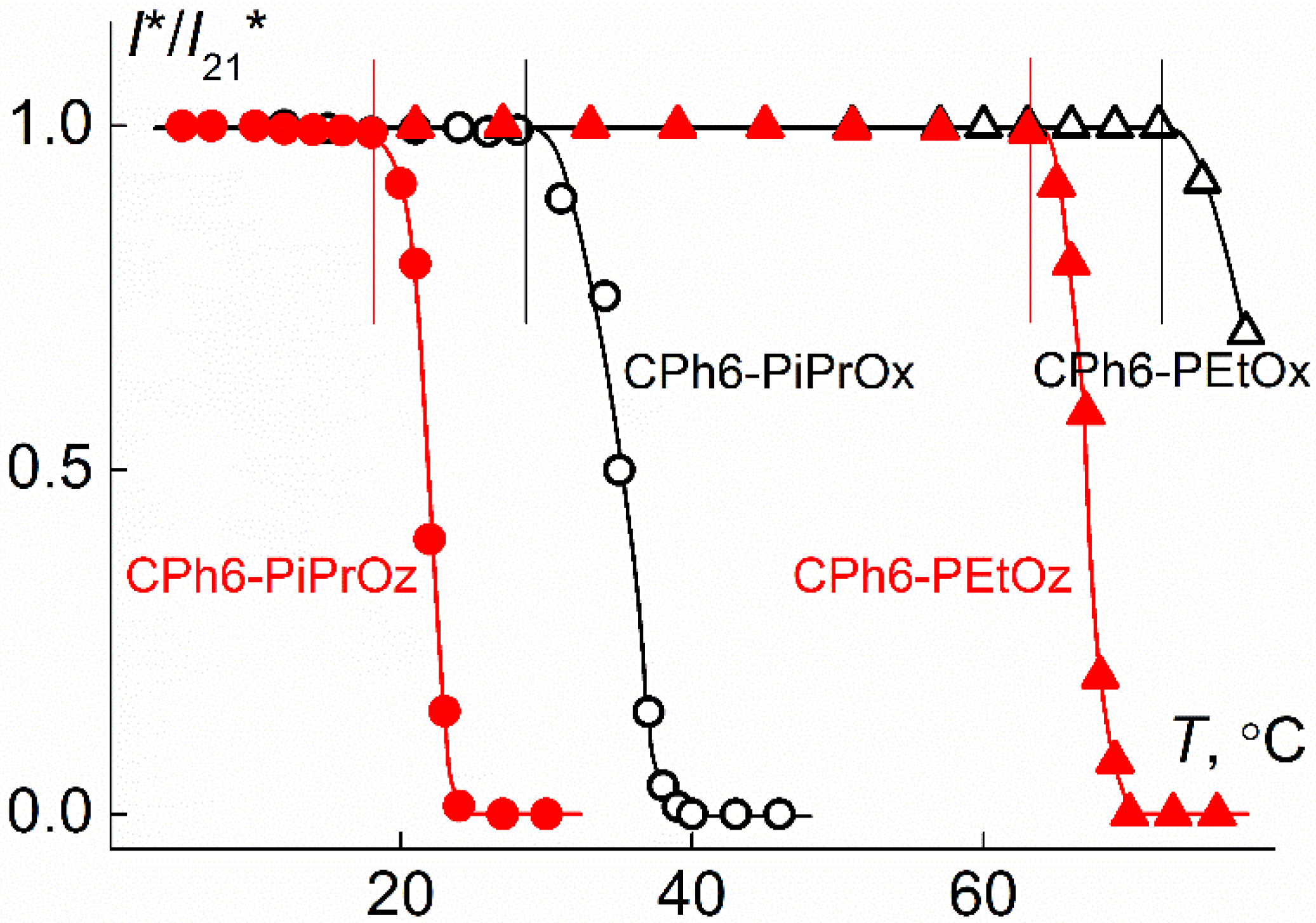
| Polymer | s0, Sv | Rh-D, nm | cm3g−1 | MsD, g⋅mol−1 | [η], cm3g−1 | dn/dc, cm3g−1 |
|---|---|---|---|---|---|---|
| CPh6-PEtOz | 2.7 | 3.5 | 0.843 | 23,000 | 13.6 | 0.088 |
| CPh6-PiPrOz | 3.6 | 3.3 | 0.890 | 20,000 | 12.1 | 0.073 |
| CPh6-PEtOx | 2.3 | 3.0 | 0.861 | 15,000 | 8.0 | 0.091 |
| CPh6-PiPrOx | 2.3 | 2.6 | 0.851 | 14,000 | 5.7 | 0.084 |
| Polymer | MsD, g⋅mol−1 | ω, mol % | M0, g⋅mol−1 | Ntsc | Ltsc, nm | Larm, nm |
|---|---|---|---|---|---|---|
| CPh6-PEtOz | 23,000 | 7.2 | 113 | 31 | 15.9 | 17.1 |
| CPh6-PiPrOz | 20,000 | 8.3 | 127 | 24 | 12.1 | 13.4 |
| CPh6-PEtOx | 15,000 | 11.0 | 99 | 22 | 8.5 | 9.8 |
| CPh6-PiPrOx | 14,000 | 11.8 | 113 | 18 | 6.9 | 8.1 |
| Polymer | Larm/Rh-D | Larm/APEtOx | A0⋅1010, erg⋅K−1mol−1/3 |
|---|---|---|---|
| CPh6-PEtOz | 4.9 | 11 | 3.3 |
| CPh6-PiPrOz | 3.8 | 8 | 3.2 |
| CPh6-PEtOx | 3.3 | 6 | 2.8 |
| CPh6-PiPrOx | 3.2 | 5 | 2.8 |
| Polymer | g′ | h | gη | gh | Larm/APEtOx |
|---|---|---|---|---|---|
| CPh6-PEtOz | 0.60/0.49 | 0.88/0.94 | 0.51/0.41 | 0.58/0.74 | 11 |
| CPh6-PiPrOz | 0.58/0.47 | 0.87/0.93 | 0.49/0.39 | 0.57/0.71 | 8 |
| CPh6-PEtOx | 0.38/0.46 | 0.81/0.86 | 0.31/0.38 | 0.44/0.54 | 6 |
| CPh6-PiPrOx | 0.28/0.34 | 0.71/0.76 | 0.22/0.27 | 0.29/0.36 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirila, T.; Smirnova, A.; Razina, A.; Tenkovtsev, A.; Filippov, A. Synthesis and Conformational Characteristics of Thermosensitive Star-Shaped Six-Arm Polypeptoids. Polymers 2020, 12, 800. https://doi.org/10.3390/polym12040800
Kirila T, Smirnova A, Razina A, Tenkovtsev A, Filippov A. Synthesis and Conformational Characteristics of Thermosensitive Star-Shaped Six-Arm Polypeptoids. Polymers. 2020; 12(4):800. https://doi.org/10.3390/polym12040800
Chicago/Turabian StyleKirila, Tatyana, Anna Smirnova, Alla Razina, Andrey Tenkovtsev, and Alexander Filippov. 2020. "Synthesis and Conformational Characteristics of Thermosensitive Star-Shaped Six-Arm Polypeptoids" Polymers 12, no. 4: 800. https://doi.org/10.3390/polym12040800
APA StyleKirila, T., Smirnova, A., Razina, A., Tenkovtsev, A., & Filippov, A. (2020). Synthesis and Conformational Characteristics of Thermosensitive Star-Shaped Six-Arm Polypeptoids. Polymers, 12(4), 800. https://doi.org/10.3390/polym12040800






